Study of preoperative diagnostic modalities in Chinese patients with superficial esophageal squamous cell carcinoma
2022-10-11YaTingZengYuYingSunWenChengTanShuAiLuoBiHuiZouGuangYuLuoChunYuHuang
Ya-Ting Zeng, Yu-Ying Sun, Wen-Cheng Tan, Shu-Ai Luo, Bi-Hui Zou, Guang-Yu Luo, Chun-Yu Huang
Ya-Ting Zeng, Yu-Ying Sun, Wen-Cheng Tan, Shu-Ai Luo, Bi-Hui Zou, Guang-Yu Luo, Chun-Yu Huang, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China
Ya-Ting Zeng, Yu-Ying Sun, Wen-Cheng Tan, Shu-Ai Luo, Bi-Hui Zou, Guang-Yu Luo, Chun-Yu Huang, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China
Ya-Ting Zeng, Wen-Cheng Tan, Shu-Ai Luo, Bi-Hui Zou, Guang-Yu Luo, Chun-Yu Huang, Department of Endoscopy, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China
Yu-Ying Sun, Cancer Prevention Center, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China
Abstract BACKGROUND Endoscopic ultrasonography (EUS) and magnifying endoscopy (ME) reliably determine indications for endoscopic resection in patients with superficial esophageal squamous cell carcinoma (SESCC). ME is widely accepted for predicting the invasion depth of superficial esophageal cancer with satisfying accuracy. However, the addition of EUS is controversial.AIM To evaluate the diagnostic efficiency of ME vs EUS for invasion depth prediction and investigate the influencing factors in patients with SESCC to determine the best diagnostic model in China.METHODS We retrospectively analyzed patients with suspected SESCC who completed both ME and EUS and then underwent endoscopic or surgical resection at Sun Yat-Sen University Cancer Center between January 2018 and December 2021. We evaluated and compared the diagnostic efficiency of EUS and ME according to histological results, and investigated the influencing factors.RESULTS We included 152 lesions from 144 patients in this study. The diagnostic accuracies of ME and EUS in differentiating invasion depth were not significantly different (73.0% and 66.4%, P = 0.24); both demonstrated moderate consistency with the pathological results (ME: kappa = 0.58, 95% confidence interval [CI]: 0.48-0.68, P < 0.01; EUS: kappa = 0.46, 95%CI: 0.34-0.57, P < 0.01). ME was significantly more accurate in the diagnosis of high-grade intraepithelial (HGIN) or carcinoma in situ (odds ratio [OR] = 3.62, 95%CI: 1.43-9.16, P = 0.007) subgroups. Using a miniature probe rather than conventional EUS can improve the accuracy of lesion depth determination (82.3% vs 49.3%, P < 0.01). Less than a quarter of circumferential occupation and application of a miniature probe were independent risk factors for the accuracy of tumor invasion depth as assessed by EUS (< 1/4 circumferential occupation: OR = 3.07, 95%CI: 1.04-9.10; application of a miniature probe: OR = 5.28, 95%CI: 2.41-11.59, P < 0.01). Of the 41 lesions (41/152, 27.0%) that were misdiagnosed by ME, 24 were corrected by EUS (24/41, 58.5%).CONCLUSION Preoperative diagnosis of SESCC should be conducted endoscopically using white light and magnification. In China, EUS can be added after obtaining patient consent. Use of a highfrequency miniature probe or miniature probe combined with conventional EUS is preferable.
Key Words: Superficial esophageal squamous cell carcinoma; Endoscopic ultrasound; Magnifying endoscopy; Endoscopic resection; Japan Esophageal Society classification
lNTRODUCTlON
Esophageal cancer is the leading malignancy in China, with national morbidity and mortality rankings of third and fourth, respectively, among all malignancies[1]. In China, esophageal squamous cell carcinoma accounts for 90% of esophageal carcinomas[2].
Due to its mild and atypical clinical manifestations, most patients with esophageal carcinoma are diagnosed with advanced-stage disease. This results in a poor prognosis, reduced treatment effectiveness, and low quality of life. This situation underscores the need for better methods for detecting and treating esophageal squamous cell carcinoma during the early disease stages.
Superficial esophageal squamous cell carcinoma (SESCC), considered early-stage cancer, is defined as a tumor confined within the mucosa and submucosa layers of the esophagus, regardless of lymph node metastasis[3]. There are several treatment options for SESCC including traditional surgery or endoscopic resection (ER). Compared to surgery, ER can be curative and less invasive, is generally well tolerated, and is associated with fewer postoperative complications[4]. Identifying patients with SESCC who are ER candidates is, therefore, critical. ER is indicated based on the tumor infiltration depth because the risk of lymph node metastasis increases with the depth of invasion. Lesions confined to the epithelium/lamina propria mucosa (EP/LPM) are rarely accompanied by lymph node metastasis (0-3.3%)[5-7]; in these cases, ER may be curative[8]. Despite their association with an elevated risk of lymph node metastasis, lesions confined to the muscularis mucosa/superficial submucosa (MM/SM1) are also suitable for ER, potentially followed by additional treatments[4,8]. Lesions deeper than the SM1 are contraindicated for ER because of the high rate of lymph node and distant metastases (> 20%)[5-7,9]; surgery is recommended for these lesions[8].
Accurate determination of tumor infiltration depth before resection is important. To estimate the lesion invasion depth, conventional endoscopy combined with magnification (ME) and endoscopic ultrasound (EUS) are considered the best approaches[10-12]. Currently, ME is more widely accepted than EUS for predicting the invasion depth of SESCC with satisfying accuracy, but the addition of EUS is controversial[13-15].
The endoscopists, access environment, and medical policies differ markedly between China and foreign countries. Chinese physicians require a preoperative diagnosis model that maximizes patient benefit. We, therefore, sought to evaluate the diagnostic efficiency of MEvsEUS for invasion depth prediction, to determine the most suitable preoperative diagnostic modality for Chinese patients with SESCC.
MATERlALS AND METHODS
Patients and lesions
We retrospectively analyzed patients with suspected SESCC who underwent examination, including both ME and EUS, and then underwent surgery or ER at Sun Yat-Sen University Cancer Center between January 2018 and December 2021. We included patients with suspected SESCC following white light imaging (WLI) screening or other modalities. All patients were pathologically diagnosed with atypical esophageal hyperplasia or SESCC. We excluded patients who received chemotherapy or radiotherapy as an initial treatment after diagnosis and those who were suspected of having lymph node or organ metastases by imaging. The institutional review board of Sun Yat-Sen University Cancer Center approved this study.
Resected complete specimens obtained during surgery or ER were processed and diagnosed by our Center’s pathology department. According to the Paris Endoscopic Classification of Superficial Neoplastic Lesions[16] and the 11thEdition of the Japanese Classification of Esophageal Cancers[3], in the esophageal mucosa (T1a), lesion involvement included the epithelium (EP) (including high-grade intraepithelial neoplasia (HGIN) and carcinomain situ), the lamina propria mucosa (LPM), and the muscularis mucosa (MM). Submucosal (SM, T1b) lesions were divided into SM1, SM2, and SM3. These lesion layers featured equivalent thickness and were ordered from shallower (SM1) to deeper (SM3). Since the submucosal thickness remained unknown in endoscopically resected specimens, lesions involving the submucosa to 200 µm or less from the MM were classified as T1b-SM1. Those deeper than 200 µm were considered T1b-SM2/SM3. Thus, in our study, lesion invasion depths were categorized pathologically as pEP/LPM, pMM/SM1, and pSM2/SM3.
Examination procedure
The examination procedure was identical to that used in our daily practice. All lesions included were initially examined by conventional endoscopy with WLI. Suspicious lesions were further assessed using magnifying endoscopy with narrow-band or blue laser imaging (ME-NBI/BLI) using a GIF-H260Z (Olympus Corporation, Tokyo, Japan) or EG-L590ZW gastroscope (Fujifilm Corporation, Tokyo, Japan). EUS followed, utilizing 7.5MHz, 10MHz, or 12MHz radical scanning probes (SU 9000, EG-530UR2, Fujifilm; EU-ME2, Olympus) or a 20-MHz miniature probe (UM-DP20-25R, Olympus). Six certified and experienced endoscopists at our center performed all these examinations. The involved endoscopists were divided into junior and senior groups according to their seniority. The senior endoscopist was defined as having a title of Associate Professor or higher with at least 12 years of experience in endoscopy. The junior endoscopist is defined as having a title of Attending Physician or above, with more than 6 years of experience in endoscopy. Residents and trainees did not participate in this study. Each patient's ME-NBI/BLI and EUS were conducted on the same day. The endoscopic findings were later extracted from the electronic medical record.
ME, combined with image-enhanced endoscopy, NBI, or BLI, allows visualization of micro-vessels on the esophageal surface. Intra-papillary capillary loops (IPCL) are basic microvasculature units on the squamous mucosal surface. IPCL forms are used to characterize lesions and predict invasion depth for SESCC. We applied the Japan Esophageal Society (JES) classification scheme, which integrates previous Inoue and Arima classification schemes, presently in widespread clinical use[7,17]. Here, micro-vessels observed by ME were divided into type A and type B. Type A vessels were non-cancerous lesions; type B vessels were abnormal micro-vessels characterized by dilatation, meandering, caliber change, and uneven morphology. These abnormal features were suggestive of cancerous lesions and include three subtypes: B1 (vessels with loop-like formations), B2 (without loops but appearing stretched and markedly elongated), and B3 (highly dilated vessels with calibers more than three times those of B2 vessels). To predict invasion depth, type B1, B2, and B3 vessels corresponded with depths of EP/LPM, MM/SM1, and SM2/SM3, respectively. The subclassification of type B vessels was based upon the indication for ER: Lesions with B1 were absolutely indicated, B2 vessels were relatively indicated, and B3 vessels were contraindicated.
During EUS, a cross-sectional image of the esophageal wall structure was obtained and divided into five layers using a 7.5 MHz radical conventional probe[18]. When using a high-frequency (≥ 20 mHz) miniature probe, the canal wall was depicted as a nine-layer structure if the distance between the probe and mucosa was appropriate. Specifically, the mucosa and submucosa were sonographically divided into an additional four layers. The first and second layers corresponded to the EP/LPM, the third layer to the MM, and the fourth layer to the SM. Specifically, lesions confined to the first and second layers were categorized as EP/LPM; lesions involving the third layer were MM/SM1; lesions that invaded the fourth layer were SM2/SM3. Esophageal cancer usually appears as a hypoechoic lesion that disrupts the normal structure of the esophageal wall, forming images with defects, irregularities, and interruptions.
Statistical analysis
The diagnostic efficiencies of EUS and ME-NBI/BLI for determining exact invasion depth were evaluated by sensitivity, specificity, and accuracy. A pairedχ2test (McNamar's) was used to assess their differences.Pvalues < 0.05 were considered statistically significant. We applied Cohen's kappa to evaluate the consistency of EUS and ME-NBI/BLI with the final pathological result for determining the depth of tumor infiltration[19,20]. The accuracy of ME-NBI/BLI or EUS concerning the clinicopathologic features was assessed using theχ2test or Fisher's exact test. Multivariate logistic regression analysis was performed to identify variables that significantly influenced the performance of ME-NBI/BLI or EUS. SPSS version 25 for Windows software (IBM Inc, Armonk, United States) was used for statistical analyses.
RESULTS
Clinicopathological features of patients and lesions
Of the 146 patients who met our enrollment criterion, two were excluded from the analyses. One was because of hemorrhage during ER, which was later converted to surgical resection; this resulted in an incomplete pathological specimen. Another patient was excluded because we could not obtain a clear view during ME-NBI, preventing micro-vessel characterization.
Ultimately 152 lesions in 144 patients were included in this study. Of these, 108 were male (75%), and 36 were female (25%), with a mean age of 61.3 ± 7.5 years. Most tumors were located in the middle thoracic esophagus (82/152, 53.9%), and the main macroscopic type was flat (90/152, 59.2%). The mean tumor size was 22.9 mm (range 5-60 mm). The average time interval between examinations and resection treatment was 18 d (1-82 d). As for treatment selection, 71 lesions were treated by ER, and 81 were treated by surgery. Pathologically, 78 lesions (51.3%) were diagnosed as pEP/LPM lesions, 28 (22.4%) as pMM/SM1, and 46 (30.3%) as pT1b-SM2/SM3. Detailed clinicopathological features of the patients and lesions are shown in Table 1.
Diagnostic efficiency of ME-NBI/BLI and EUS in estimating invasion depth
The relationships between ME-NBI/BLI or EUS diagnosis and the final pathological result after treatment are listed in Table 2 and Figures 1-3. The overall accuracy of ME-NBI/BLI, based upon the JES classification for determining invasion depth, was 73.0% (111/152), moderately consistent with the pathological results (kappa = 0.58, 95% confidence interval [CI]: 0.48-0.68,P< 0.01). The overall accuracy of EUS for determining invasion depth was 66.4% (101/152), also moderately consistent with the pathological results (kappa = 0.46, CI: 0.34-0.57,P< 0.01).
We also compared the diagnostic efficiency of ME-NBI/BLI and EUS for determining the invasion layer according to the indication for ER (Table 3). There was no significant difference in overall accuracy between ME-NBI/BLI and EUS (73.0%vs66.4%,P= 0.24). For pEP/LPM lesions, ME-NBI/BLI had a higher sensitivity, specificity, and accuracy than EUS (sensitivity 84.6%vs73.1%; specificity 91.9%vs81.1%; accuracy 88.2%vs77.0%), with a significant difference in accuracy (P< 0.01). For pMM/SM1 lesions, ME-NBI/BLI was more sensitive, and EUS had a better specificity (sensitivity 92.9%vs35.7%; specificity 73.4%vs91.1%;P< 0.01 for both); the two techniques demonstrated equivalent accuracy (77.0%vs80.9%,P= 0.51). For pSM2/SM3, ME-NBI/BLI was more specific and EUS was more sensitive (sensitivity 41.3%vs73.9%,P< 0.01; specificity 98.1%vs75.4%,P< 0.01); the techniques had equivalent accuracy (80.9%vs75.0%,P= 0.22). Lastly, of the 41 lesions (41/152, 27.0%) misdiagnosed by MENBI/BLI, 24 were corrected by EUS (24/41, 58.5%).
Clinicopathological factors that influence diagnostic accuracy
For ME-NBI/BLI, diagnostic accuracy did not vary significantly according to the tumor location, macroscopic type, circumferential occupation, tumor size, or endoscopist grade (Table 4). The accuracy of ME-NBI/BLI increased significantly for HGIN or carcinomain situsubgroups (P= 0.03). During the multivariate analysis, HGIN and carcinomain situwere independent risk factors for the accuracy of tumor invasion depth, as assessed by ME-NBI/BLI (odds ratio [OR] = 3.62, 95%CI: 1.43-9.16,P= 0.007).
As for EUS, the overall diagnostic accuracy did not vary significantly according to the tumor location, macroscopic type, differentiation degree, and endoscopist grade (Table 4). Increased circumferential occupation and tumors larger than 3 cm were mostly associated with decreased accuracy (P= 0.06 andP= 0.05, respectively). Using a miniature probe instead of conventional EUS improved accuracy (82.3%vs49.3%,P< 0.01). In the multivariate analysis, less than a quarter of circumferential occupation and application of a miniature probe were independent risk factors for the accuracy of tumor invasion depth, as assessed by EUS (< 1/4 circumferential occupation: OR = 3.07, 95%CI: 1.04-9.10; application of a miniature probe: OR = 5.28, 95%CI: 2.41-11.59,P< 0.01).
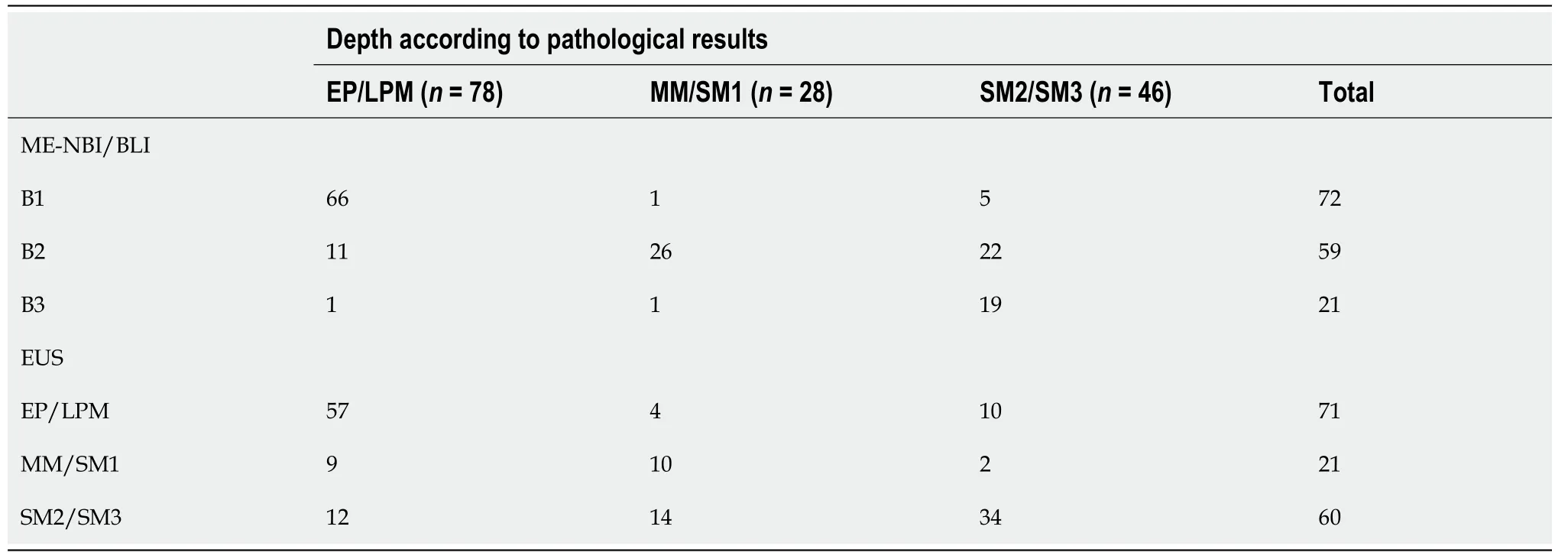
Table 2 Relationship between magnifying endoscopy or endoscopic ultrasound diagnosis and final pathological results
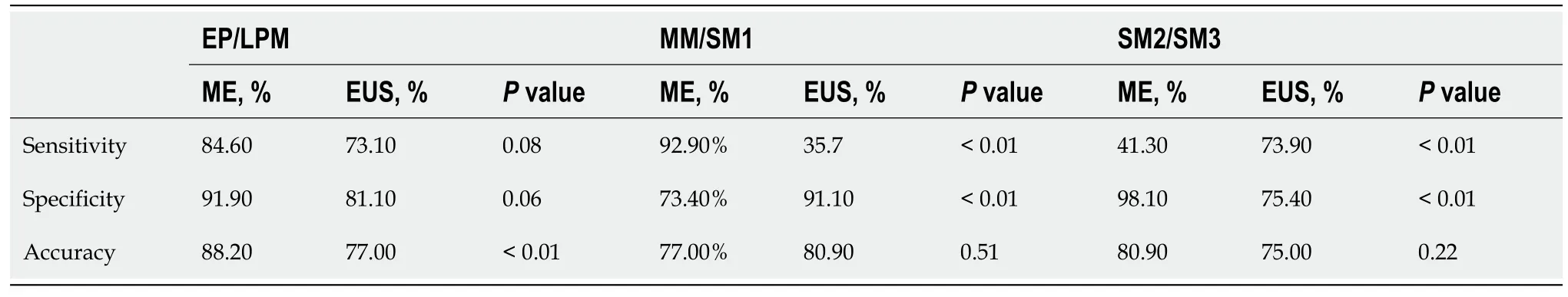
Table 3 Diagnostic efficiency of magnifying endoscope or endoscopic ultrasound in dividing specific invasion layer
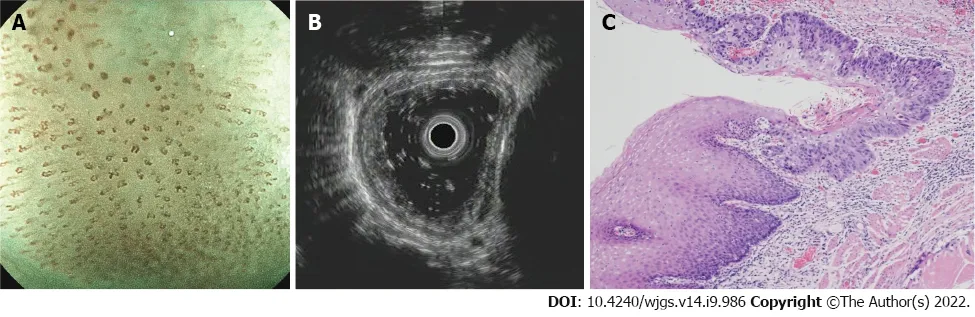
Figure 1 A typical case of carcinoma in situ. A: ME-BLI image shows micro-vessels with a loop-like formation (type B1); B: Ultrasonography image shows hypoechoic thickening confined to the first two layers; C: Hematoxylin-eosin staining (× 40) of an endoscopic resection specimen shows that the squamous cell carcinoma is limited to the epithelium, without invasion.
DlSCUSSlON
In daily practice, SESCC invasion depth can be diagnosed by observing the micro-vessels using MENBI/BLI and is unaffected by biopsy, inflammation,etc.However, sometimes visualization is impeded. In contrast, EUS can image deeper lesions and collect vital information that differs from that obtainable by ME. The objective of this study was to evaluate the diagnostic efficiency of ME-NBI/BLIvsEUS for diagnosing invasion depth in patients with SESCC based on the indication for ER. We also investigated influencing factors to determine the best model for use during preoperative diagnosis in Chinese patients with SESCC.
We applied accuracy, sensitivity, and specificity to evaluate diagnostic efficiency. Of these parameters, accuracy is widely used because it combines sensitivity and specificity. We found no significant differences in the diagnostic accuracy of ME-NBI/BLI and EUS for determining invasion depth (73%vs66.4%,P= 0.24), and both demonstrated moderate consistency with pathological findings (ME-NBI/BLI: kappa=0.58; EUS: kappa = 0.46). However, both had advantages and limitations for differentiating distinct invasion layers.
We grouped patients according to the indications for ER to optimize clinical decision-making for patients. ME-NBI/BLI presented better diagnostic efficiency than EUS in the prediction of pEP/LPM layer. In addition, tumors confined to EP—including HGIN and carcinomain situ—were more accurately assessed by ME-NBI/BLI than other subgroups (OR = 3.62, 95%CI: 1.43-0.16,P= 0.007). Thus, ME-NBI/BLI performed better than EUS for distinguishing EP/LPM invasion; this finding was consistent with current clinical practice and previous research[7,21,22].
Figure 2 A typical muscularis mucosal lesion. A: ME-BLI image shows type B2 vessels without loop-like formations but with a stretched and markedly elongated transformation; B: Ultrasonography image shows a hypoechoic lesion invading the third layer with continuous submucosa; C: Hematoxylin-eosin staining (× 40) of a surgical specimen shows a moderately differentiated squamous cell carcinoma invading the muscularis mucosa.
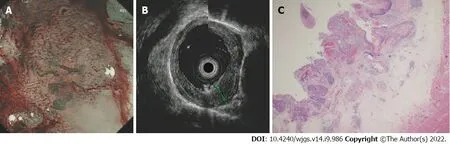
Figure 3 A typical submucosal lesion. A: ME-NBI image shows micro-vessels dilated more than three times that of B2 vessels (type B3); B: Ultrasonography image shows a hypoechoic lesion invading the fourth layer; C: Hematoxylin-eosin staining (× 20) of a surgical specimen shows a moderately differentiated squamous cell carcinoma infiltrated to the middle third of the submucosa without muscularis propria involvement.
For pT1b-SM2/SM3 lesions, B3 vessels were highly specific for diagnosis (98.1%) but less sensitive (41.3%), consistent with previous reports. Type B3 vessels were negative for 43.1% of the pT1b-SM2/SM3[23]; however, according to our data, EUS can compensate for this deficiency with a significantly higher specificity than ME-NBI/BLI (EUS 73.9%vsNBI 41.3%,P< 0.01). Therefore, EUS can be a useful supplementary tool to determine if a lesion has invaded the submucosa. Combining MENBI/BLI and EUS enables the most comprehensive assessment of lesion infiltration depth.
Considering the lesser diagnostic accuracy for B2 and B3 vessels (77.0% and 80.9%, respectively), the criteria for B2 and B3 vessel characteristics required further refinement[24,25] to improve the accuracy of JES classification. However, this violated the original intention of the JES classification to simplify the items set by previous Inoue and Arima classifications[17], thus increasing the difficulty of memorization and impeding widespread use. Therefore, we tried to find a model of preoperative diagnosis. Surprisingly, we found that when patients were misdiagnosed by ME-NBI/BLI, EUS often determined the correct invasion depth (24/41, 58.5%). These findings may assist clinicians with treatment decisionmaking and maximize the benefit to the patient.
In our study, EUS was performed using either a miniature probe or conventional EUS. Some lesions were visualized using both probe types according to different detection purposes. Except for depth prediction, EUS can determine the presence of malignant regional lymph nodes with better sensitivity than CT and PET-CT[26], and can sample the suspected lymph nodes to gain pathological confirmation. We compared the accuracy of conventional EUS and the miniature probe for determining lesion infiltration depth. The miniature probe was significantly more accurate than conventional EUS (82.3%vs49.3%,P< 0.01). This finding answers questions unanswered by previous data and is consistent with previous study findings[11,27,28]. Because of higher frequencies, the miniature probe can clearly visualize esophageal wall structures. However, as frequency increases, the detection range becomes shallower and more limited, potentially preventing comprehensive exploration of large lesions[29]. Therefore, the miniature probe seems more suitable for small, superficial, and early-stage lesions[27]. This conclusion was further confirmed by our findings. We observed that increased circumferential occupation (P= 0.06) and larger (P= 0.05) tumors were less accurately assessed using EUS. In our clinical practice, we mainly use miniature probes to determine the infiltration depth of early-stage lesions. Conventional EUS is typically used to detect the apparent advanced-stage lesions and determine the presence of lymph nodes or adjacent organ metastases.
Compared with foreign peers, most Chinese endoscopists in tertiary hospitals are proficient in MENBI/BLI and EUS. From our data, the diagnostic capacities of junior and senior endoscopists in our center were comparable, and the difference was not significant (ME-NBI/BLI,P= 0.21; EUS,P= 0.10). Additionally, in China, the cost of EUS examinations-including general gastroscopy-is around 150 dollars, much lower than that of developed countries, such as Europe, America, Japan,etc.Due to affordability, EUS does not post a substantial financial burden on Chinese patients.
Our findings should be considered within the context of specific limitations. First, all patients were initially examined using ME-NBI/BLI, then EUS. There may be an ordering effect, with ME-NBI/BLI affecting the prediction obtained using EUS. Future studies should alter the order of EUS and MENBI/BLI to control for a potential order effect. Second, this was a retrospective study of extracting patients' medical records at a single cancer center in China. As such, selection bias could not be denied. Future prospective multi-center nationwide double-blinded trials are needed to evaluate the clinical validity of EUS and ME-NBI/BLI in patients with SESCC.
CONCLUSlON
We recommend that preoperative diagnosis of SESCC be conducted based on the finding of WLI and ME-NBI/BLI. EUS can be added after patient consent in China, preferably utilizing a high-frequency miniature probe or miniature probe combined with conventional radical EUS.
ARTlCLE HlGHLlGHTS
Research background
Early-stage detection and treatment of esophageal carcinoma can typically optimize prognosis.Compared with traditional surgery, endoscopic resection is a less invasive and potentially curative treatment for early-stage esophageal cancer. Identification of patients that are candidates for endoscopic resection is crucial. Endoscopic ultrasonography (EUS) and magnifying endoscopy (ME) reliably determine indications for endoscopic resection in patients with superficial esophageal squamous cell carcinoma (SESCC). ME is a widely accepted method for predicting the invasion depth of superficial esophageal cancer with satis fying accuracy. However, the addition of EUS is controversial.
Research motivation
To evaluate the diagnostic efficiency of ME vs EUS for invasion depth prediction, and investigate the influencing factors.
Research objectives
To determine the most suitable preoperative diagnostic modality for Chinese patients with SESCC.
Research methods
We retrospectively analyzed patients with suspected SESCC who completed both ME and EUS and then underwent endoscopic or surgical resection at Sun Yat-Sen University Cancer Center between January 2018 and December 2021. We evaluated and compared the diagnostic efficiency of EUS and ME according to histological results, and investigated the influencing factors.
Research results
EUS and ME demonstrated comparable accuracy for determining the depth of invasion of early-stage esophageal cancers, and EUS can compensate for deficiencies inherent to NBI in some cases. The miniature probe was best suited for detecting early-stage lesions
Research conclusions
Preoperative diagnosis of SESCC should be conducted endoscopically using white light and magnification. In China, EUS can be added after obtaining patient consent. Use of a high-frequency miniature probe or miniature probe combined with conventional EUS is preferable.
Research perspectives
Future studies are required to explore how to combine the findings of ME and EUS to make a compre hensive preoperative evaluation, instead of solely depending on the experience of endoscopists.
FOOTNOTES
Author contributions:Zeng Y designed and performed the study and wrote the manuscript; Sun Y and Huang C contributed to the conception of the study; Tan W and Luo G helped perform the analysis with constructive discussions; Luo S and Zhou B collected the data;
Supported bythe Guangdong Esophageal Cancer Institute Science and Technology Program, No. M202013.
lnstitutional review board statement:This study was approved by the Ethics Committee of the Sun Yat-Sen University Cancer Center.
lnformed consent statement:Informed consent from patients was waived by the Ethics Committee of the Sun Yat-Sen University Cancer Center.
Conflict-of-interest statement:All authors report no relevant conflict of interest for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Ya-Ting Zeng 0000-0002-2654-5979; Yu-Ying Sun 0000-0002-5789-256X; Wen-Cheng Tan 0000-0002-6724-9949; Shu-Ai Luo 0000-0002-7473-8361; Bi-Ηui Zou 0000-0003-1166-8850; Guang-Yu Luo 0000-0002-8335-1986; Chun-Yu Ηuang 0000-0002-1346-5961.
S-Editor:Wu YXJ
L-Editor:Wang TQ
P-Editor:Wu YXJ
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Oncologic aspects of the decision-making process for surgical approach for colorectal liver metastases progressing during chemotherapy
- Research progress on the immune microenvironment of the gallbladder in patients with cholesterol gallstones
- Central pancreatectomy for benign or low-grade malignant pancreatic tumors in the neck and body of the pancreas
- lrinotecan- vs oxaliplatin-based regimens for neoadjuvant chemotherapy in colorectal liver metastasis patients: A retrospective study
- Predictors of difficult endoscopic resection of submucosal tumors originating from the muscularis propria layer at the esophagogastric junction
- Liver transplantation with simultaneous splenectomy increases risk of cancer development and mortality in hepatocellular carcinoma patients
