Migration and wintering of vulnerable adult Chinese Egrets (Egretta eulophotes) revealed by GPS tracking
2022-10-03ZhijunHuangXiaopingZhouWenzhenFangXiaolinChen
Zhijun Huang,Xiaoping Zhou,Wenzhen Fang ,Xiaolin Chen
Key Laboratory of the Ministry of Education for Coastal and Wetland Ecosystems,College of the Environment and Ecology,Xiamen University,Xiamen,361102,China
Keywords:Egretta eulophotes Habitat selection Migration route Mortality Wintering area
ABSTRACT Knowledge of migratory bird requirements is critical to developing conservation plans for vulnerable migratory species.This study aimed to determine the migration routes,wintering areas,habitat uses,and mortalities of adult Chinese Egrets (Egretta eulophotata).Sixty adult Chinese Egrets (31 females and 29 males) on an uninhabited offshore breeding island in Dalian,China were tracked using GPS satellite transmitters.GPS locations recorded at 2 h intervals from June 2019 to August 2020 were used for analysis.A total of 44 and 17 tracked adults completed their autumn and spring migrations,respectively.Compared with autumn migration,tracked adults displayed more diverse routes,higher number of stopover sites,slower migration speed,and longer migration duration in the spring.Results indicated that migrant birds had different behavioral strategies during the two migratory seasons.The spring migration duration and stopover duration for females were significantly longer than those for males.A positive correlation existed between the spring arrival and spring departure dates,as well as between the spring arrival date and stopover duration.This finding indicated that the egrets that arrived early at the breeding grounds left the wintering areas early and had a shorter stopover duration.Adult birds preferred intertidal wetlands,woodlands,and aquaculture ponds during migration.During the wintering period,adults preferred offshore islands,intertidal wetlands,and aquaculture ponds.Adult Chinese Egrets showed a relatively low survival rate compared with most other common ardeid species.Dead specimens were found in aquaculture ponds,indicating human disturbance as the main cause of death of this vulnerable species.These results highlighted the importance of resolving conflicts between egrets and human-made aquaculture wetlands and protecting intertidal flats and offshore islands in natural wetlands through international cooperation.Our results contributed to the hitherto unknown annual spatiotemporal migration patterns of adult Chinese Egrets,thereby providing an important basis for the conservation of this vulnerable species.
1.Introduction
Advanced tracking techniques are extensively used to study the spatial ecology of birds and account for variations in their movement and habitat selection (Naef-Daenzer and Gruebler,2016;Pang et al.,2020).Tracking migratory birds throughout their annual life cycle aids the understanding of their biology such as migration routes,nonbreeding areas,home ranges,and habitat uses(Webster et al.,2002;Marra et al.,2015;Ng et al.,2018).Full annual-cycle animal tracking can reveal when and where threats occur (Alerstam,2011;Jorge et al.,2011;Lok et al.,2015),provide clues to population decline (Kirby et al.,2008;Hewson et al.,2016),and disclose the habitat details in breeding,wintering,and migratory seasons (Monti et al.,2018;Zhang et al.,2018a);thus,it can thus guide species conservation more efficiently to prevent and reverse population decline.Knowledge of migratory patterns(Strandberg et al.,2010;Harrison et al.,2011;Cooper et al.,2017),migratory connectivity(Cresswell,2014;Dhanjal-Adams et al.,2017;Rotics et al.,2018),survival rate and causes of death (Bates et al.,2015;Fox et al.,2021),and habitat selection in stopover areas,breeding,and wintering area during life-history stages are urgently required,especially in the current environment that is changing quickly (Leu and Thompson,2002;Koczur et al.,2018;Hadjikyriakou et al.,2020).
The Chinese Egret(Egretta eulophotes;Pelecaniformes:Ardeidae)is a vulnerable species with a declining population of fewer than 10,000 individuals (BirdLife International,2020).The egret is a long-distance migratory waterbird that breeds primarily on offshore islands in eastern China,North Korea,South Korea,and eastern Russia,migrating during autumn and spring through coastal regions and then wintering in the coastal areas of Southeast Asia(Kushlan and Hancock,2005;BirdLife International,2020).The Chinese Egret’s population has decreased because of the reclamation of tidal mudflats,estuarine habitats,and offshore breeding islands for agriculture and industry (BirdLife International,2020).Thus far,the Chinese Egret is considered to be of conservation concern owing to lack of information (Kushlan,2018).Some studies describe the juvenile autumn migration of this species (Zhang et al.,2018b;Huang et al.,2021;Xu et al.,2022),but little is known about the migration and wintering of adult Chinese Egrets,which may differ from those of juveniles (Strandberg et al.,2010;McKinnon et al.,2014).
In the present study,we tracked the annual migration and wintering of adult Chinese Egrets by using GPS tags.The questions addressed here are as follows: (1) do the migratory routes of adult Chinese Egrets vary between autumn and spring migration? (2) what are the migratory movement traits including migration duration,migration distance,flight speed,and number of stopovers of adult Chinese Egrets during annual migrations? (3) where are the wintering areas of adult Chinese Egrets located? (4) what habitats do adult egrets use in stopover sites and wintering areas? (5) what are the mortalities of adult Chinese Egrets in annual migration and wintering areas?(6)finally,where do threats occur and what is the main cause of death of this vulnerable species in migration and wintering?
2.Methods
2.1.Study area and transmitter attachment
In June 2019,60 adult Chinese Egrets were captured on Fantuozi Island (39°9′12.16′′N,122°18′19.84′′E) in Dalian,Liaoning Province,China(Huang et al.,2021).The egrets were captured on the ground near their nests in the evening using a hand-held net,and their weights and body length were measured(Huang et al.,2021).Feathers were collected for DNA extraction and subsequent sex determination (Wang et al.,2011).These egrets were then fitted with solar-charged GPS transmitters(HQB-2512S with weight=14 g or HQB-2009P with weight 9 g;maximum location accuracy,5 m;Hunan Global Messenger Technology,China).The weight of the transmitter and backpacks with a Teflon ribbon harness amounted to 2-4%of the average egret’s weight(Hadjikyriakou et al.,2020).Both types of transmitters relayed data at 2 h intervals via the GSM network.All birds were released within 2 h at the capture site after no abnormal behavior or death was observed following transmitter attachment (Huang et al.,2021).A total of 67,251 GPS locations were used to analyze the migration and wintering of the egrets.
2.2.Migration parameters
The annual life cycle of the adult Chinese Egret was divided into five life-history stages,i.e.,breeding,premigration,autumn migration,wintering,and spring migration based on the recorded GPS locations.The end of the breeding stage in the colony was determined from transmitter information,and results showed that nest visits stopped(van Der Winden et al.,2012).The premigration stage was calculated as the time between the first record of an adult leaving the breeding ground(starting date) and the first record of autumn migration (end date;van Der Winden et al.,2012;Rappole,2013).Departure for autumn or spring migration was considered to have occurred when tag locations showed a persistent southward or northward movement(Huschle et al.,2013).The autumn migration of Chinese Egrets was defined as the last point recorded from the vicinity of its breeding island before departure until the first point reached in the wintering area,and the spring migration stage was defined as the last point from the wintering area to the first point reached in the breeding area.The wintering area or breeding area was based on the International Union for Conservation of Nature publication or determined by discontinued movements to the north in winter or the south in spring (Huschle et al.,2013).Using the World Geodetic System 1984 (WGS-84,used by all GPS devices) coordinate system,migration distance was calculated by summing all the distances between consecutive migration locations between the last point and the first point of autumn migration or spring migration,excluding the variation in stopover site locations (Li et al.,2020).A stopover site was defined as an area at a speed of 0 km/h where an egret stayed for at least 4 h(two GPS positions and intervals recorded with a transmitter)during the migration journey (Alonso et al.,2020;Huang et al.,2021).The inland and coastal migration routes were defined as more than two-thirds of the egret stopover sites occurring in freshwater and coastal wetlands,respectively (Huang et al.,2021).Autumn migration duration was defined as the total number of days between the last date in the vicinity of the breeding island before departure and the first date on which successfully arriving individuals reached the wintering area,including the periods spent at stopover sites (Huschle et al.,2013).Spring migration duration was defined as the total number of days between the last date in the wintering area and the arrival date at the breeding ground.Migration speed was calculated as the migration distance divided by the migration duration(Monti et al.,2018;Li et al.,2020).
The death of a tracked egret was identified based on three indicators(Monti et al.,2018;Huang et al.,2021):the transmission data indicated that the transmitter was functioning normally,GPS locations confirmed that the transmitter was stationary,or the egret was not moving,and terminal temperature values followed the environmental temperature fluctuations.However,this indirect death identification sometimes cannot be distinguished from transmitter loss and might therefore overestimate egret mortality.Therefore,retrieval of carcasses or transmitters in the field was used to further confirm death and determine the cause of death if possible.Transmitter failure(signal loss)was assumed to occur when the GPS signal was suddenly interrupted,and no new GPS data were received.This transmitter failure identification procedure sometimes included death that occurred over the sea,as no carcass or transmitter could be found and might therefore underestimate mortality.
2.3.Habitat selection
Habitat classification followed the Ramsar Convention on Wetlands(Gardner et al.,2018) and included natural wetlands,human-made wetlands (human-influenced wetlands),and “other land” types.In the study area,the natural wetlands included offshore islands,intertidal flats(<6 m deep),and rivers.Human-made wetlands included aquaculture ponds,reservoirs (>8 ha in area),and irrigated land.“Other lands”included reclamation areas and woodlands.Overall home ranges (95%utilization distribution) and core areas (50% utilization distribution) of adult Chinese Egrets during the wintering stage were estimated using kernel-density estimators (Cohen et al.,2018;Monti et al.,2018;Calenge,2019).
2.4.Data analyses
The migration routes of adult Chinese Egrets were mapped by GPS positions.Their movements and habitat types were visualized using Google Earth remote-sensing images (Google Inc,2013),and their wintering areas were identified by ArcGIS 10.3 (Klaassen et al.,2010;Page et al.,2014;Monti et al.,2018;Hadjikyriakou et al.,2020).Home range and habitat selection were analyzed in R version 3.5.1 by using the R packages “adehabitatHR” and “adehabitatHS” (Calenge,2019;R Development Core Team,2019;Jourdan et al.,2021).The geographic information system ArcGIS 10.3 was used to calculate the size of a given habitat (Klaassen et al.,2010;Monti et al.,2018;Hadjikyriakou et al.,2020).Data are shown as the mean ± standard deviation.Generalized linear model(Gaussian distribution)was applied to test how the date of arrival at the breeding grounds was influenced by the date of spring departure from the wintering areas,stopover duration,migration distance,migration speed,and stopover number (Bai et al.,2021).A chi-square test was used to compare mortality or stopover-site habitat selection,andt-test was used to evaluate differences between sexes or migration seasons.All statistical analyses were conducted using R 3.5.1(Urbanek et al.,2018).
3.Results
3.1.Tracking results
Sixty adult Chinese Egrets (31 females and 29 males)were captured and tracked in 2019.Forty-nine of them survived into the autumn migration stage and were successfully tracked through autumn migration.However,data of 5 of these 49 egrets were lost(signal loss)during the migration journey(Table 1),and finally 44 egrets(20 females and 24 males)completed autumn migration and arrived in the wintering area.In 2020,24 egrets (11 females and 13 males) were successfully tracked through spring migration,but data of 2 of them were lost,1 of them returned to the wintering area during the migration journey,4 of them died,and finally 17 of them(6 females and 11 males)completed spring migration and arrived in the breeding area.In different life-history stages,the mortalities of tracked adult Chinese Egrets in premigration or autumn migration were significantly lower than that in the wintering,spring migration,or breeding(allp<0.05;Table 1).A total of 54.55%of dead tracked Chinese Egrets were found in aquaculture ponds.Overall,the annual mortality of tracked adult Chinese Egrets was 36.67%,and the annual data-loss ratio was 33.33%.
3.2.Migration routes
The range of autumn migration departure of adult Chinese Egrets(N=49)was between August 29th and October 14th,lasting 46 d.Among the 44 birds that completed autumn migration,the range of autumn migration departure of adult Chinese Egrets was between September 10th and October 14th,lasting 34 d;93.18%(N=41)of these departure dates occurred between September 18th and October 14th (Fig.1;Appendix Table S1).The range of autumn migration arrival of these 44 adults was between September 14th and December 11th,lasting 88 d;93.18%(N=41)of these arrival dates occurred between September 14th and October 31st (Fig.1;Appendix Table S1).In spring migration,the range of departure of adult Chinese Egrets(N=24)was between March 22nd and June 9th,lasting 79 d.Among the 17 birds that completed spring migration,the range of departure of adult Chinese Egrets was also between March 22nd and June 9th,lasting 79 d;82.35% (N=14) of these departure dates occurred between April 6th and May 30th(Fig.1;Appendix Table S1).The range of spring migration arrival of these 17 adults was between May 5th and August 19th,lasting 106 d;88.24%(N=15) of these arrival dates occurred between May 5th and June 28th(Fig.1;Appendix Table S1).
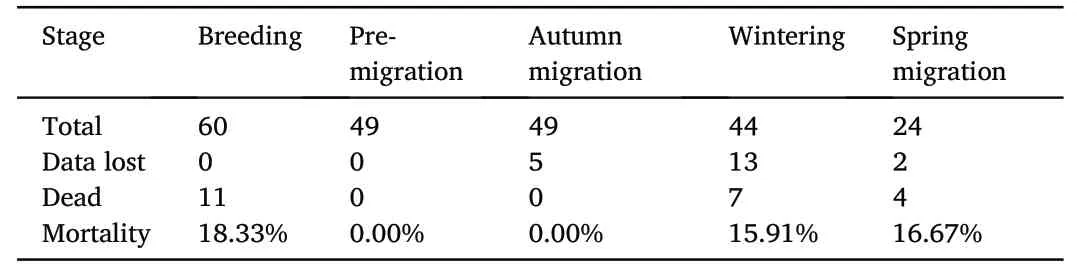
Table 1 Mortality of tracked adult Chinese Egrets at different life-history stages.
In 49 recorded autumn(southward)migration routes,the adult Chinese Egrets migrated via the coastal route(Fig.2),utilizing stopover sites in the coastal wetlands of Jiangsu,Zhejiang,Fujian,Taiwan,Guangdong,and Guangxi provinces in China.Two birds selected a few stopover sites in the inner wetlands of Jiangsu,Zhejiang,and Fujian provinces.
In 22 recorded spring (northward) migration routes,the adult Chinese Egrets returned to the breeding area via 2 migration routes: thecoastal route and the inland route.These egrets exhibited a high degree of fidelity (100%) to breeding areas between successive years.Twenty egrets(90.91%)migrated along the coastal route by using stopover sites distributed in the coastal wetlands of the Philippines and China(Hainan,Guangdong,Fujian,Taiwan,Zhejiang,Jiangsu,and Shandong provinces;Fig.3).One bird selected a few stopover sites in the inner wetlands of Guangdong,Fujian,Jiangxi,Zhejiang,and Jiangsu provinces.Two egrets(9.09%)returned to the breeding area via the inland route (Fig.3),primarily using stopover sites in the inner wetlands of Thailand,Laos,Vietnam,and China (Guangxi,Hunan,Hubei,Anhui,Henan,and Shandong provinces).Both of these egrets traveled a shorter migration distance in spring migration via the inland route than that in autumn migration via the coastal route (ID-E19-30,4509.59 km in spring,5032.7 km in autumn;ID-E19-47,5475.71 km in spring,6046.24 km in autumn).
3.3.Migration movements
The autumn migration duration was 3-70 d,averaging 16.20±16.85 d (20 females,15.75 ± 16.58 d;24 males,16.58 ± 17.42 d;p>0.05;Appendix Table S1).The average migration distance of these egrets was 4047.63 ± 775.66 km (females,4093.45 ± 758.24 km;males,4009.45±804.08 km;p>0.05).During the autumn migration,the average flight speed was 59.95 ± 12.23 km/h,the maximum flight speed was 119.9 km/h,and the average number of stopovers was 3.46±1.39.
The spring-migration duration was 17-79 d,averaging 43.47 ±20.17 d(Appendix Table S1).The spring-migration duration(6 females,57.17 ± 22.09 d) and stopover duration (50.57 ± 22.92 d) for females were significantly longer than that for males (11 males,36.36 ± 15.40 d and 28.94 ± 14.51 d;p<0.05).The average migration distance was 4767.59 ± 668.47 km (females,4900.31 ± 324.29 km;males,4741.27± 807.81 km;p>0.05).During spring migration,the average flight speed was 46.22 ± 7.87 km/h (females,48.51 ± 8.23 km/h;males,44.98±7.77 km/h;p>0.05),the maximum flight speed was 110.7 km/h,and the average number of stopovers was 8.65±2.57(females,9.67±2.73;males,8.09±2.43;p>0.05).
Comparison of the spring and autumn migration parameters of 17 egrets that completed their full annual migration revealed that the migration distance (4553.75 ± 768.08 km) during autumn did not significantly differ from that during spring (p>0.05).However,spring migration had longer migration duration (autumn,21.41 ± 22.82;p<0.05),more stopovers (autumn,4.76 ± 1.35;p<0.05),and slower migration speed (autumn,61.04 ± 10.59 km/h;p<0.05).Analyses of the linear relationship between the date of arrival at the breeding grounds and the date of spring departure from the wintering areas,stopover duration,migration distance,migration speed,or stopover number showed a significant relationship between the spring arrival date and spring departure date (R=0.73;p<0.05) and between the spring arrival date and stopover duration(R=0.65;p<0.05).
3.4.Wintering areas
Among the 44 egrets that completed autumn migration (Fig.4),83.36%(N=38;16 females,22 males)wintered in the Philippines,East Malaysia,and Indonesia,and 16.64% (N=6;female=4,male=2)wintered in the South China Sea,Vietnam,Myanmar,Singapore,and West Malaysia.The latitude and longitude of the egret wintering areas ranged from 3.18°S to 18.06°N and from 98.52°E to 124.89°E,respectively.The wintering duration of 17 egrets that completed their full annual migration was 166-242 d,averaging 200.71 ± 19.76 d (6 females,194.33 ± 24.91 d;11 males,204.18 ± 16.63 d;p>0.05;Appendix Table S1)which was significantly longer than autumn migration or spring migration(allp<0.05).
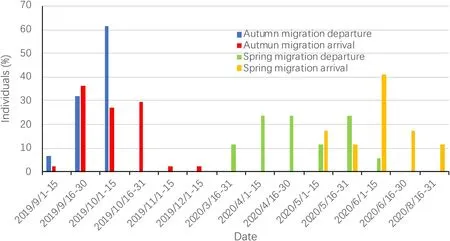
Fig.1.Migration departure and arrival times of adult Chinese Egrets that completed autumn migration (N=44) or spring migration (N=17).
3.5.Habitat selections
During migration,adult Chinese Egrets primarily used coastal wetland habitats,including natural and human-made wetlands.In their autumn migration,55.56% of the adult birds stopped over in intertidal flats,13.58%in woodlands,7.41%in rivers,7.41%in aquaculture ponds,6.17% in reclamation lands,and less than 5% in each of the other habitats(Fig.5).In their spring migration,62.69%of the adults stopped over in intertidal flats,13.61% in aquaculture ponds,10.88% in woodlands,and less than 5% in each of the other habitats (Fig.5).Comparison of habitat selections in stopover sites during spring and autumn migration indicated no significant difference between seasons(p>0.05).
The overall home range and core home range of adult Chinese Egrets during the winter period were 92.63 ± 164.35 km2(range=2.86-758.06 km2) and 10.36± 20.52 km2(range=0.01-105.01 km2),respectively.No significant differences existed between females and males in the overall home range(females,134.78±202.65 km2;males,59.84±123.50 km2;p>0.05)or the core home range(females,11.47±14.20 km2;males,9.50± 24.73 km2;p>0.05).
During winter,adult Chinese Egrets relied on coastal wetland habitats,including natural wetlands and human-made wetlands.In the core home range,habitat selection by adults was as follows: offshore islands(64.72%),intertidal flats (29.65%),and aquaculture ponds (5.63%;Fig.6).In the overall home range,adult Chinese Egrets also displayed a preference for offshore islands(46.01%),followed by aquaculture ponds(28.16%),and intertidal flats(25.83%;Fig.6).
4.Discussion
This study presented for the first time a comprehensive flyway map of the autumn and spring migration corridors of adult Chinese Egrets by using satellite tracking data.Our tracking data showed that the coastal route was a frequently used flyway for the autumn and spring migration of adult Chinese Egrets.During autumn migration,100% of adult Chinese Egrets migrated southward via the coastal route.During spring migration,90.91% and 9.09% of egrets migrated northward via the coastal and inland routes,respectively.Compared with juvenile Chinese Egrets that select the coastal,inland,and oceanic routes for autumn migration(Huang et al.,2021;Xu et al.,2022),adult Chinese Egrets had fewer routes.Autumn migratory routes may differ between adults and juveniles,possibly through learning or social interactions in the growth.The reason is that most migrating birds are adults repeating their migrations and improving their migration skills over time,whereas juveniles migrate for the first time(Crysler et al.,2016;Åkesson et al.,2021).Comparison of the spring and autumn migration parameters of 17 egrets with their full annual cycle revealed that the spring migration had more stopovers,slower migration speed,and longer migration duration.The slower migration speed of adult Chinese Egrets during spring migration was in contrast to many studies showing a higher speed of spring migration than autumn migration because of the competition for arrival order at breeding grounds and environmental factors such as increased daylight (Nilsson et al.,2013).However,recent evidence suggests that some bird species tend to migrate faster in autumn than in spring.White-fronted Geese (Anser albifrons) has a considerably longer migration duration for spring(83 d)than for autumn(42 d),and the difference is primarily determined by the time spent at stopovers (Kölzsch et al.,2016).Icelandic Whimbrel (Numenius phaeopus islandicus),a long-distance migratory wader has shorter migration duration in autumn than in spring and has higher migration speed in autumn,with all individuals undertaking a direct flight to the wintering areas.Conversely most of them make a stopover in spring (Carneiro et al.,2019).The slower migration in spring may minimize energy expenditure and result in arrival under good physiological condition because faster migration speed costs more energy per distance covered(Hedenström,2008).The migrants may also avoid encountering unfavorable weather events(e.g.,winds,cold spells,or snowfall) during migration and unfavorable conditions upon arrival at the breeding site(Carneiro et al.,2019).Notably,a higher number of stopovers and longer spring migration may allow migrants more time to rest and refuel,thereby benefiting their breeding(Kölzsch et al.,2016).Our results suggested that migration routes may differ between ages,and migration parameters may differ between seasons or species.
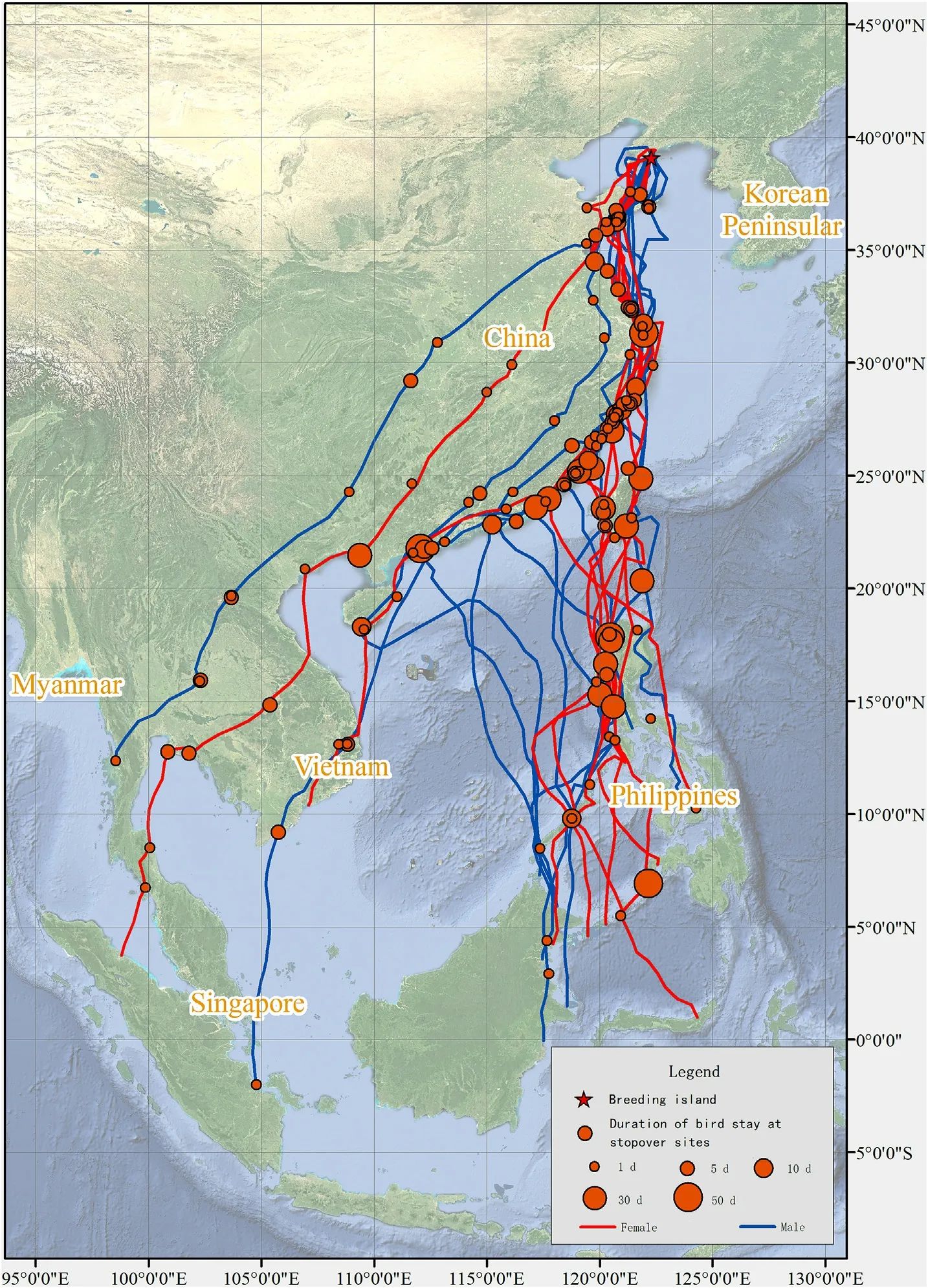
Fig.2.Autumn migration routes of adult Chinese Egrets.
The autumn migration strategy of adult Chinese Egrets is similar to that of Night Herons(Nycticorax nycticorax;Ledwoń and Betleja,2015),traveling slowly with several long stopoversen route,but differs from that of Purple Herons (Ardea purpurea;van Der Winden et al.,2010).Adult Chinese Egrets completed their autumn migration journey in 4.76±1.35 stopovers lasting 10.25 ± 12.77 d (range=3-70 d),whereas Night Herons have 4 stopovers lasting 17 d on average(range=9-20 d)in an autumn migration journey of around 4000 km (Ledwoń and Betleja,2015).Conversely,Purple Herons cover an autumn migration distance of about 4000 km in just 5-7 d,with only a few short stops(van Der Winden et al.,2010).The autumn migration speed of adult Chinese Egrets(59.95±12.23 km/h)was similar to that of Night Herons(54.5 km/h;range=44-65 km/h) but was slower than that of Purple Herons (45.9 km/h;range=43-50 km/h).The autumn migration strategies of Chinese Egrets and Night Herons that differ from that of Purple Herons could be attributed to the differences in wing loadings and foraging ecology(Ledwoń and Betleja,2015).Our results revealed that the autumn migration strategy of adult Chinese Egrets was highly consistent with that of adult Night Herons.
Our data contributed to the limited knowledge on migration,especially on the spring migration,of the vulnerable Chinese Egret.Previous migration information for this species suggests that the birds migrate southward in September and October and northward in March and April(Kushlan and Hancock,2005).Previous studies have found that juvenile Chinese Egrets begin their autumn migration journeys between July and November,with a departure peak in October,arriving in wintering areas between October and December(Zhang et al.,2018b;Huang et al.,2021;Xu et al.,2022).The present results indicated that the range of autumn migration departure and arrival of adult Chinese Egrets highly overlapped with that of juvenile Chinese Egrets.The long spring and autumn migratory journeys of the Chinese Egret,as evidenced by the results from this study,were not different between sexes and supported the notion that this egret was a long-distance migratory species and a seasonal migrant among ardeid birds(Pineau,2000;Kushlan and Hancock,2005).Our findings also emphasized the importance of international cooperation in conserving this vulnerable species and the wetland habitats on which it depends.
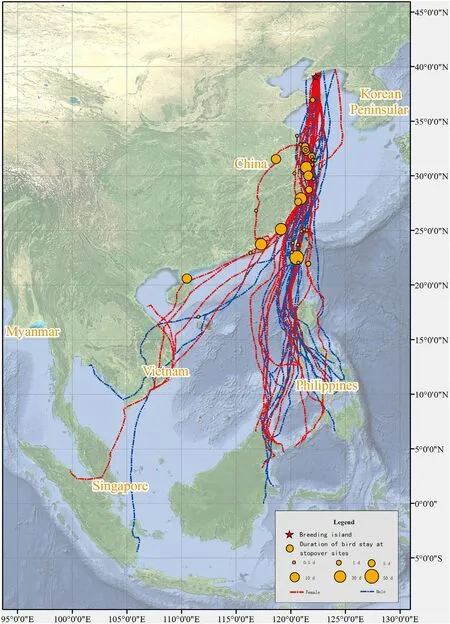
Fig.3.Spring migration routes of adult Chinese Egrets.
For a long time,many studies have focused on the timing of arrival at breeding areas in long-distance migratory bird species (Rotics et al.,2018;Pearman et al.,2020;Schwemmer et al.,2021).Our results showed a significant relationship between the spring arrival and departure dates and between the spring arrival date and stopover duration.The Chinese Egret that arrived early in the breeding grounds left the wintering areas earlier and had shorter stopovers.Likewise,positive correlations exist between arrival dates at breeding grounds and departure from wintering grounds in Great Reed Warblers(Acrocephalus arundinaceus;Lemke et al.,2013),Red-eyed Vireos (Vireo olivaceus;Callo et al.,2013),Western Kingbirds (Tyrannus verticalis;Jahn et al.,2013),and Pied Flycatchers(Ficedula hypoleuca;Ouwehand and Both,2017).The departure and arrival decisions for migration in various species may be explained by multiple mechanisms,such as weather,resources,and genetics (Ouwehand and Both,2017;Pearman et al.,2020;Schwemmer et al.,2021).
Our data demonstrated the importance of coastal wetlands in the Philippines and Malaysia to the wintering of Chinese Egret(54.55% and 31.82% of tracked adults,respectively) breeds in Northeast China.Among coastal wetlands,the habitats selected by wintering adult Chinese Egrets were dominated by offshore islands,intertidal flats,and aquaculture ponds.During spring migration,adult Chinese Egrets displayed a preference for intertidal flats and aquaculture ponds at their stopover sites.During autumn migration,intertidal flats,woodlands,rivers,and aquaculture ponds were the preferred habitats.These data emphasized the significant role of natural wetlands,including offshore islands and intertidal flats,in providing the most important feeding and roosting grounds for adult Chinese Egrets.Aquaculture ponds and human-made wetlands provided important feeding and roosting wetlands for the wintering and spring migration of these adults.The preference for natural coastal wetlands and human-made wetlands observed in this study was in accordance with other reports on Ardeidae and Threskiornithidae birds.The endangered Black-faced Spoonbill (Platalea minor) prefers intertidal flat habitats for feeding and resting(Yu and Swennen,2004).A satellite-tracking study on the Great Egret (Ardea alba) has shown that natural wetlands provide the majority of foraging habitats,but some human-made wetlands (aquaculture ponds or lakes) are also strongly selected(Fidorra et al.,2016).Satellite-tracking studies on the space use of adult Reddish Egrets (Egretta rufescens),a near-threatened coastal species,have shown that they exhibit a preference for unvegetated tidal flats for foraging and roosting during winter (Koczur et al.,2018).However,in Hong Kong,China,nearly all GPS-tracked Little Egrets(E.garzetta)prefer commercial fishponds to other habitats in all seasons.This finding suggests that human-made wetlands could provide complementary habitats for waterbirds,compensating for the loss of natural wetlands(Pang et al.,2020).
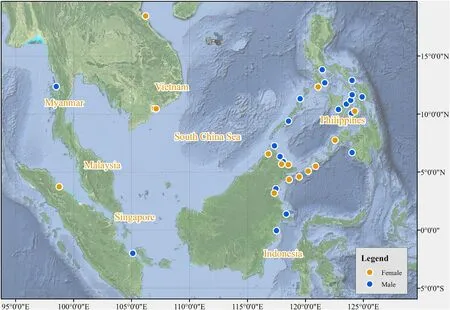
Fig.4.Wintering areas of adult Chinese Egrets.
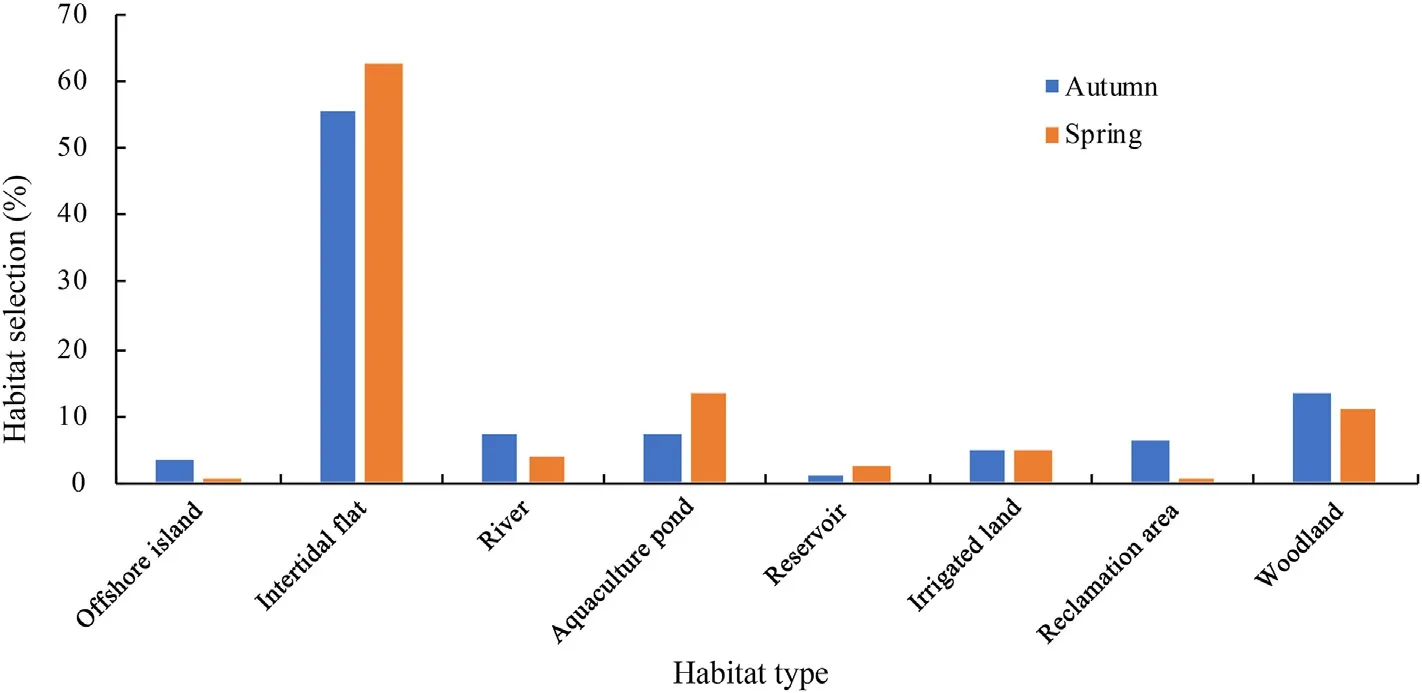
Fig.5.Stopover-site habitat selection of adult Chinese Egrets during migrations.
The survival rate of adult Chinese Egrets tracked between June 2019 and June 2020 was 63.33%,which was the first estimate of adult survival in this vulnerable species.The survival rate in Chinese Egrets was markedly lower than that (annual survival 75.6%) of adult Reddish Egrets tracked with satellite transmitters(Koczur et al.,2017).Previous survival-rate estimates of egret and heron species,despite being not entirely reliable estimations from recoveries,may provide some references for comparison (Cezilly,1997;Hafner et al.,1998).Our survival estimates of adult Chinese Egrets were relatively low,in contrast with those found in most other common ardeid species.Estimates of annual survival rates using capture-mark-recapture models are 71.4% (Hafner et al.,1998)and 78%(Galarza and Arizaga,2014)for adult Little Egrets(E.garzetta)and 85% for adult Great Egrets(A.alba)(Wlodarczyk et al.,2020),as well as 78.1% and 70.5%,respectively,for adult Great Blue Herons (A.herodias) and adult Grey Herons (A.cinerea) (Hafner et al.,1998).These data provide important evidence that the Chinese Egret remains vulnerable,and that more appropriate conservation interventions are needed in the future.Moreover,the mortality of tracked adult Chinese Egrets was significantly lower than that of juvenile Chinese Egrets in autumn premigration (juveniles,23.08%),autumn migration(juveniles,38.46%),and wintering(juveniles,23.08%)life-history stages(Huang et al.,2021;p<0.05,respectively).Therefore,the first autumn migration and wintering are critical periods affecting the population dynamics of inexperienced juvenile Chinese Egrets,supporting the conclusion that juvenile birds have higher mortality than adults on an annual and a seasonal basis (Cresswell,2014;Naef-Daenzer and Gruebler,2016;Wlodarczyk et al.,2020).
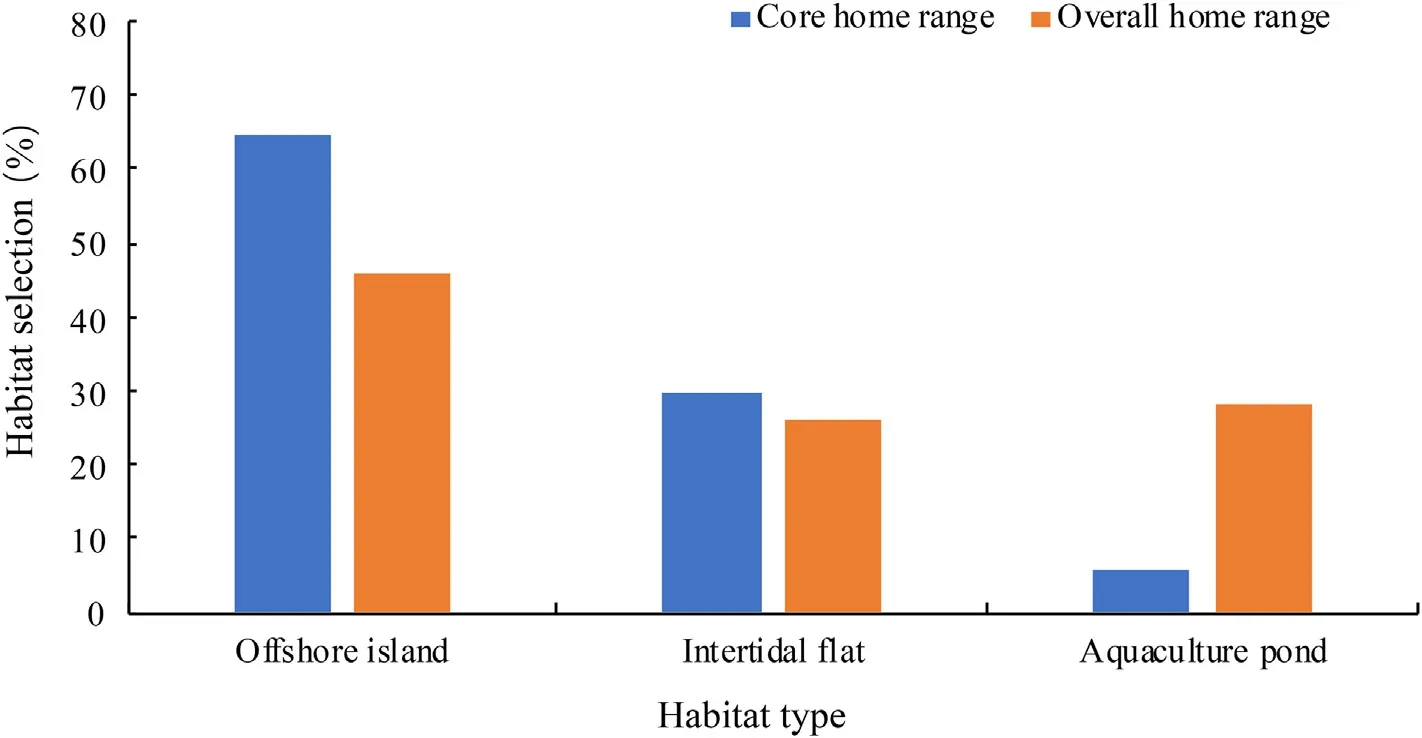
Fig.6.Habitat selection of adult Chinese Egrets in wintering areas.
Understanding the potential threats to survival during life history is important particularly for the conservation of threatened species (Bates et al.,2015).The mortality of adult Chinese Egrets was higher during spring migration or wintering stage than autumn migration.Longer duration was found during spring migration or wintering stage than autumn migration,and more stopovers were found during spring migration than autumn migration.These results indicated that stopovers benefited egret refueling but also threatened their survival,and the longer the threatened,the more the deaths.In this study,most dead tracked Chinese Egrets were found in aquaculture ponds,implying that the main cause of death of this vulnerable species was human disturbance.In adult Reddish Egrets,depredation by predators and senescence are the possible causes of mortality(Koczur et al.,2017).Previous studies on the recovery of dead birds from 1956 to 1976 found that shooting snowy egrets in North America was an important cause of their mortality(35.2% of recoveries;Hafner et al.,1998).Our present findings regarding the main cause of Chinese Egret deaths emphasized that the conflict between herons and aquaculture remains a serious concern for heron conservation(Marion,2000).
5.Conclusions
This study was the first to reveal that the coastal route was a frequently used flyway for the migration of adult Chinese Egrets.Their most important wintering areas were the coastal wetlands in the Philippines,followed by Malaysia.Their selected habitat during migration and wintering primarily included natural wetlands and human-made wetlands.Our data further demonstrated that adult Chinese Egrets had different strategies for autumn and spring migration.Adult Chinese Egrets showed a relatively lower survival rate than most other common ardeid species.The main cause of death of this vulnerable species was human disturbance,thereby emphasizing the urgency of resolving the conflict between heron and aquaculture for heron conservation at present,in addition to natural-wetland conservation.
Authors' contribution
ZH designed and performed the experiments,completed the analyses,and wrote the manuscript;XC and WF conceived,directed,and coordinated this study,helped with writing,and revised the manuscript.XZ collected some samples,assisted with analyses and lab work.All authors read and approved the final manuscript.
Ethics statement
The license for this research in the study site was issued by the Forestry and Grassland Administration,Liaoning Province,China(Permission Number: 201810).This research and all procedures were approved by the Animal Ethics Committee in Xiamen University,China.Field work was conducted in the evening,and visits to a breeding colony were restricted to a maximum of 2 h per day.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We thank Jingtao Liu,Danxia Xia,Ying Yin,and Yihuang Chen who helped attach transmitters and collect samples in field.We also thank Kunyu Zhang,Yuwei Huang,Haoran Luo,and Wei Xu for assistance in laboratory work of this study.This research was supported by the National Natural Science Foundation of China (Grant Nos.42076107,41676123,and 41476113).The funder had no role in the data collection and analysis,publish decision,or manuscript preparation of this study.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.avrs.2022.100055.
Appendix ASupplementary data
The datasets used in the present study are available from the corresponding author on reasonable request.
杂志排行
Avian Research的其它文章
- Functional and phylogenetic structures of pheasants in China
- Thermoregulatory function and sexual dimorphism of the throat sack in Helmeted Guineafowl (Numida meleagris) across Africa
- Distribution pattern and driving factors of genetic diversity of passerine birds in the Mountains of Southwest China
- Multiple lines of evidence confirm that the critically endangered Blue-crowned Laughingthrush(Garrulax courtoisi)is an independent species
- Corrigendum to “Multiple lines of evidence confirm that the critically endangered Blue-crowned Laughingthrush (Garrulax courtoisi) is an independent species” [Avian Res.13 (2022) 100022]
- Altitudinal seasonality as a potential driver of morphological diversification in rear-edge bird populations
