Amorphous transformation of ternary Cu45Zr45Ag10 alloy under microgravity condition
2022-09-24MingHuaSu苏明华FuPingDai代富平andYingRuan阮莹
Ming-Hua Su(苏明华), Fu-Ping Dai(代富平), and Ying Ruan(阮莹)
School of Physical Science and Technology,Northwestern Polytechnical University,Xi’an 710072,China
Keywords: Cu-Zr-Ag,rapid solidification,microgravity,glass transformation
1. Introduction
Rapid solidification is a liquid-solid transition process far from the equilibrium condition with the characteristics of containerless, microgravity, and high vacuum. These conditions directly affect the microstructure, phase transformation mechanism, and solidification path, further influencing the mechanical properties of the alloy, such as crystal grain refinement, metastable phase formation, solid solubility extension, even amorphous formation under this condition. Therefore, rapid solidification provides a method for preparing advanced metal materials and has attracted the extensive attention of researchers.[1-10]In the drop tube, the alloy melt falls freely at an acceleration rate of 9.8 m·s-2and rapidly solidifies into droplets of different diameters. Compared with the other rapid solidification methods, the alloy obtains microgravity conditions,deep undercooling,and a high cooling rate by drop tube.[11-13]Consequently,we use a drop tube to investigate the glass transformation during the solidification.
Cu-Zr-based alloy is a kind of typical amorphous alloy system and has an extensive application in mechanical structural materials, mold materials, precision optical materials,and sports equipment due to its excellent mechanical,physical,and chemical properties.[14-17]The addition of the Ag element will improve the glass-forming ability(GFA)of the Cu-Zr-Ag alloy and exhibits excellent properties such as high strength,good ductility,and thermal stability.[18,19]The current studies of ternary Cu-Zr-Ag alloy mainly focus on the GFA,thermal stability,and liquid phase separation.[20-22]However,there are seldom researches focusing on the phase composition and microstructural characteristics of ternary Cu-Zr-Ag alloy during rapid solidification. In this work, we use the drop tube and single-roller melt spinning to study the microstructural evolution of ternary Cu45Zr45Ag10alloy. Furthermore,the possibility of metallic glass formation and the effects of cooling rate and undercooling on the amorphous transformation are also investigated.
2. Experimental methods
Cu45Zr45Ag10alloy ingots were prepared from highpurity Cu(99.999%),Zr(99.99%),and Ag(99.9999%)by arc melting under an argon atmosphere,each sample being about 2 g in weight. We performed the microgravity solidification of the alloy in a 3-m-long drop tube. The sample was placed in a Φ16 mm×150 mm quartz tube with a nozzle of Φ0.3 mm at the bottom and then melted by induction heating for several seconds. The casting temperature (1360 K) was about 200 K higher than the liquidus temperature. And then high purity argon gas was filled into the quartz tube. In the process of falling,the liquid alloy was dispersed into fine droplets with diameters of about 100 µm-1000 µm under the action of gas pressure. To make a comparative experiment, we fabricated the ribbon samples by single-roller melt spinning and the wheel speed of 40 m·s-1. The sample was placed in a Φ16 mm×150 mm quartz tube with a nozzle of 1-mm size at its bottom.The casting temperature was aboutT=1360 K and the resulting ribbons had a width of about 3 mm and a thickness of about 40µm. The phase composition and microstructure of the samples were identified by x-ray diffraction with Cu Kαradiation (XRD, Rigaku D/max 2500 V), a scanning electron microscope(SEM,FEI Sirion Verios G4),a transmission electron microscope (TEM) (FEI Talos F200X TEM), a focused ion/electron double beam electron microscope (FIB,Helios G4 CX)and an electron dispersive spectrometer(EDS,INCA Energy 300). Thermodynamic properties were examined by differential scanning calorimetry(DSC)(Netzsch DSC 404C)at a heating rate of 10 K·min-1.
3. Results and discussion
3.1. Solidification of master alloy

Fig.1. (a)X-ray diffraction pattern;(b)DSC curve;(c)microstructure of alloy;(d)partial enlarged view of panel(c)of Cu45Zr45Ag10 master alloy.
Figure 1(a)shows the XRD pattern of the arc melted sample. The alloy is composed of Cu10Zr7phase and AgZr phase.Compared with the diffraction peaks of the AgZr phase, the strongest diffraction peak of Cu10Zr7at 2θ=42.36°and the largest peak sum of Cu10Zr7show that they may be the primary phase and dominant phase,respectively. To identify the solidification pathways of the Cu45Zr45Ag10alloy, we perform the DSC thermal analysis at a heating-cooling rate of 10 K·min-1as shown in Fig. 1(b). The sample melts completely at 1160 K in the heating process,which is determined to be the liquidus temperature. According to the DSC cooling curve, the first exothermic peak appears at 1111 K which means that the primary Cu10Zr7phase starts to solidify from the alloy melt. Then the second exothermic peak occurs at 1099 K corresponding to the solidification of the AgZr phase.The microstructure of the master alloy is shown in Figs. 1(c)and 1(d). The Cu10Zr7phase grows in the form of coarse dendrites,and the lamellar(Cu10Zr7+AgZr)eutectic is distributed in the dendrite gap.
3.2. Solidification of alloy ribbons
To make a comparative experiment,the solidification of alloy ribbons is studied by single-roller melt spinning. Figure 2(a)shows the XRD pattern of the ribbon sample. The broad diffraction and the absence of any crystalline reflection indicate that the ribbon is in a completely amorphous state. The cooling rate of the ribbon can reach about 105K·s-1,indicated by calculation.When the alloy liquid is undercooled to the glass transition temperature, the alloy forms an amorphous state. We perform the DSC analysis to determine the thermal behavior of the ribbon,and the obtained results are shown in Fig.2(b).

Fig.2. (a)X-ray diffraction pattern and(b)DSC curve of rapidly quenched alloy ribbon.
With the increase in temperature, a glass transition endothermic peak and an obvious crystallization exothermic peak appear in the DSC curve successively. The starting temperatures of the two peaks are respectively designated as glass transition temperature (Tg) and crystallization temperature (Tx), and the maximum of the crystallization exothermic peak is designated asTp. The glass transition temperatureTg=674 K, crystallization temperatureTx=717 K, undercooled liquid phase ΔTx=43 K,and crystallization peak temperatureTp=723 K are obtained.
3.3. Solidification of alloy droplets
3.3.1. Undercooling and cooling rate
The heat transfer inside the alloy melt will directly affect the microstructural characteristics of the droplets and it is difficult to measure the temperature variation during free fall. We use Newton’s cooling model[23]to calculate the cooling rateRcas follows:

whereρLis the density of alloy droplets,CPLis the specific heat,Dis the diameter of alloy droplets,εis the radiation coefficient,σis the Stefan-Boltzmann constant,Tis the ambient temperature, andhis the heat transfer coefficient. Furthermore, we calculate the undercooling ΔTof alloy droplets according to the heat transfer model proposed by Lee and Ahn[24]as follows:

whereTNis the nucleation temperature,f(D) the heterogeneous nucleation factor,TLthe liquidus temperature,σSLthe liquid-solid interface energy,kBthe Boltzmann constant,ΔHthe melting enthalpy,KVthe kinetic parameter,andkgthe thermal conductivity of the gas. The calculation results obtained from Eqs. (1)-(5) are shown in Fig. 3. The related physical parameters used in the calculations are listed in Table 1. TheRcincreases from 0.93×103K·s-1to 4.46×104K·s-1and ΔTincreases from 42 K to 264 with the decrease of diameter.

Fig.3.Cooling rate and undercooling rate versus droplet diameter of ternary Cu45Zr45Ag10 alloy.
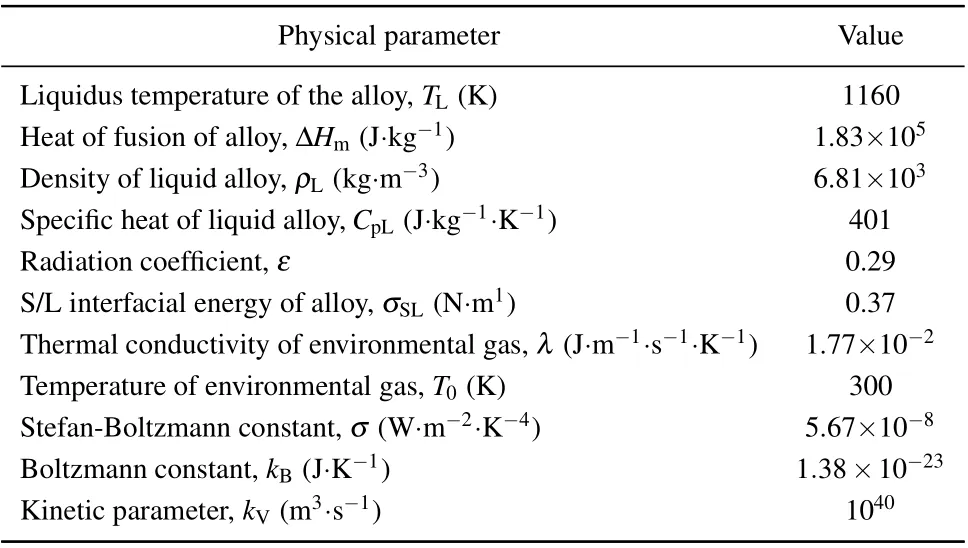
Table 1. Physical parameters of Cu45Zr45Ag10 alloy.[25]
3.3.2. Microstructural characteristics
Figure 4 shows the morphologies of the alloy droplets with different diameters. ForD= 850 µm, the primary Cu10Zr7phase nucleates directly from the melt as a dendrite structure,and the(Cu10Zr7+AgZr)eutectic phase is distributed around dendrites (Fig. 4(a)). The cooling rate isRc=1.18×103K·s-1, and the undercooling is ΔT=52 K.The alloy droplets radiate heat from the inside to the environment, and the sample surface is preferentially cooled. Thus the primary Cu10Zr7dendrites first nucleate on the surface and then grow inward. Figure 4(b)shows the solidification structure of alloy droplet with a diameter ofD=570 µm. With the decrease in diameter, the coarse dendrites are broken and the grains are refined. The cooling rateRcof alloy droplets reaches 2.09×103K/s and the undercooling is ΔT=76 K.As the diameter decreases toD=320 µm, the undercooling degree of alloy droplets is as high as ΔT=120 K,and the cooling rate reachesRc=5.28×103K·s-1(Fig.4(c)). A comparison of the sample with diameterD=850µm with that with diameterD=570µm shows that the former has larger undercooling and cooling rate. During solidification, the primary Cu10Zr7phase first precipitates from the alloy,and then rapidly nucleates and grows up. There is not enough time for eutectic reaction due to the high cooling rate and undercooling. Therefore,the eutectic reaction does not yet occur,and the alloy droplets are solidified. So,only the primary Cu10Zr7phase is observed in the solidified microstructure, but no eutectic phase is observed(Fig.4(d)).

Fig.4. Solidified microstructure of Cu45Zr45Ag10 alloy droplets with diameter D=850 µm (a), 570 µm (b), and 320 (c) µm, (d) magnified part of panel(c).
The Cu45Zr45Ag10is an amorphous component, and it is reported that in most of researches of this system the traditional quenching method was used.[26,27]Under the microgravity condition,the alloy droplets are in a high vacuum environment and do not contact the container wall during the falling,thus obtaining a high cooling rate and deep undercooling. Therefore,to explore whether the amorphous transformation of the Cu45Zr45Ag10alloy can take place in the drop tube,we further analyze the alloy droplets with a diameter ofD=180µm.In the case of the droplets withD=180µm,the cooling rate and undercooling reach 1.48×104K·s-1and 178 K respectively. The cooling rate of alloy ribbon under singleroller quenching condition can reach about 105K·s-1. We analyze the microstructural characteristics of theD=180µm alloy droplets by TEM and focused ion/electron double beam electron microscope (FIB). As shown in Figs. 5(a) and 5(b),a small sample is cut from the alloy droplet,where the thickness of the thin area of the sample is about 53.96 nm. The atoms are randomly arranged without any ordered structure,and the selective diffraction pattern shows wide and dispersed halo rings, the pattern which is a typical amorphous diffraction pattern as shown in Figs. 5(c) and 5(d). Therefore, the alloy droplets withD=180µm can be determined to have an amorphous structure, and the EDS analysis give the evidence of a homogeneous glassy structure in Fig. 6. Figure 7 shows the microstructural evolution of the alloy.
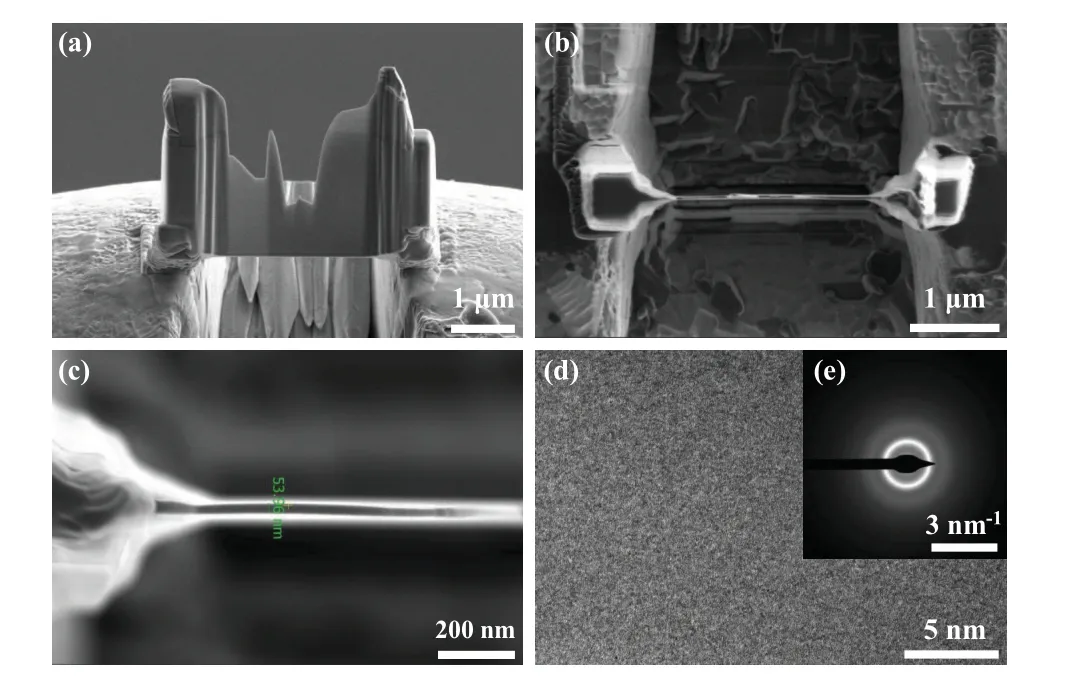
Fig. 5. Preparing [(a)-(c)] TEM and FIB sample of solidified microstructure of Cu45Zr45Ag10 alloy droplet with a diameter of 180 µm; (d) highresolution TEM image;(e)selected area electron diffraction map.
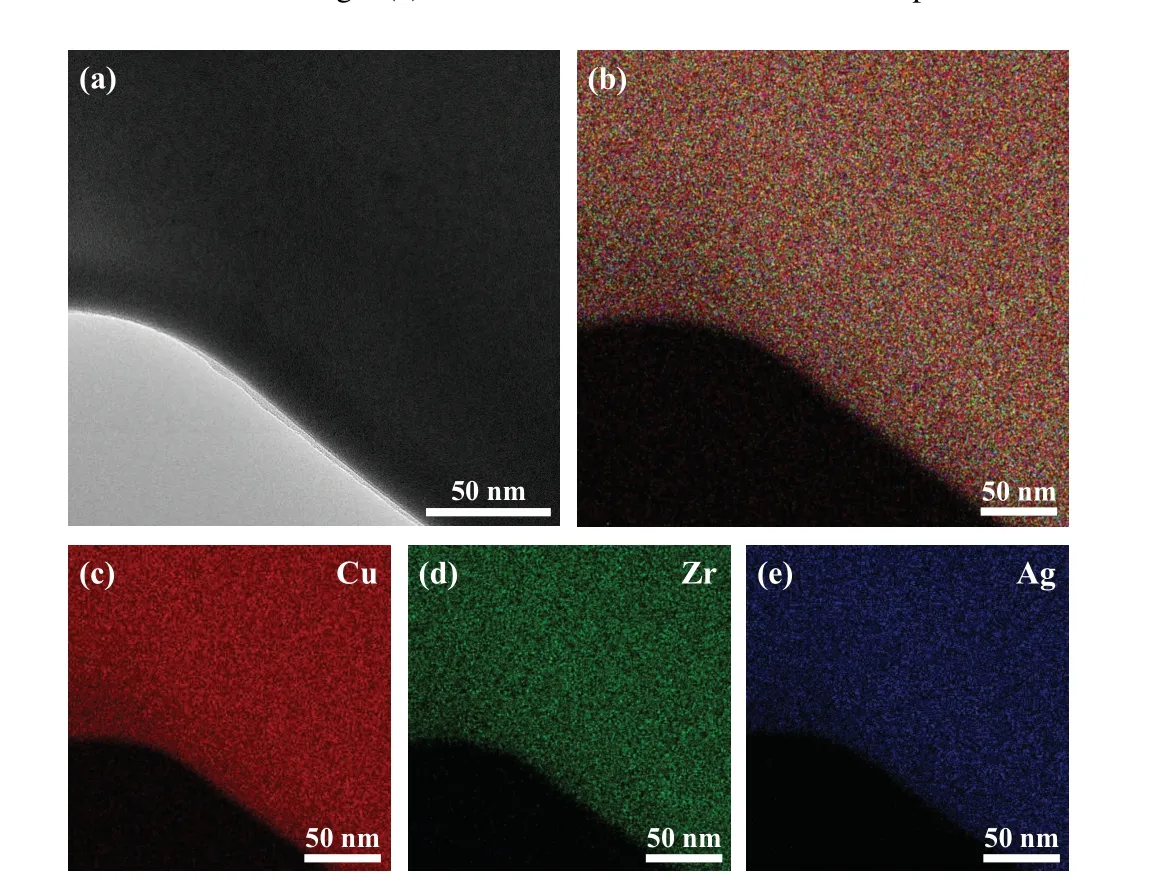
Fig.6. EDS analyses of 180-µm-diameter Cu45Zr45Ag10 alloy droplets.
To sum up,with the decrease in diameter,the undercooling and cooling rate increase, and the ternary Cu45Zr45Ag10alloy realizes the transformation from crystal into an amorphous structure under the microgravity condition. According to the calculation results, the cooling rate and the undercooling degree of alloy ribbon under single-roller quenching conditions are about 105K·s-1and 43 K, respectively. And the cooling rate and the undercooling degree of the droplets withD=180µm are about 104K·s-1and 178 K,respectively. By comparison,we find that the cooling rate of the droplets withD=180µm is smaller than that of the alloy ribbon,but the undercooling is greater.Under the condition of a low cooling rate and a high undercooling degree,the alloy droplets can still undergo amorphous transformation. Therefore, it is reasonable to believe that undercooling also plays an important role in the amorphous transformation of the alloy through comparative experiments.
Fig.7. Microstructural evolution of Cu45Zr45Ag10 alloy droplet.
4. Conclusions
In conclusion,the microstructural evolution of the ternary Cu45Zr45Ag10alloy is investigated by the drop tube and single-roller melt spinning. The characteristic temperatures of the ternary Cu45Zr45Ag10alloy are confirmed, which are the glass transition temperatureTg=674 K, crystallization temperatureTx=717 K, supercooled liquid phase ΔTx=43 K,and crystallization peak temperatureTp=723 K. The alloy ribbons are completely amorphous.But the droplets during the free-falling present different crystalline states. As the droplet diameter decreases from 850µm to 180µm, the cooling rate and undercooling reach 1.48×104K·s-1and 178 K respectively. The microstructure of the droplet transforms from the mixture of primary Cu10Zr7dendrites and (Cu10Zr7+AgZr)binary eutectic into Cu10Zr7dendrites,eventually into a homogeneous metallic glass. Through comparative study, we find that under the condition of low cooling rate and high undercooling,the alloy droplets can still undergo amorphous transformation. Therefore, undercooling also plays an important role in the amorphous transformation of the alloy.
Acknowledgements
The authors would like to thank Prof. Wei B of the Key Laboratory of Space Materials Science and Technology for his support. We also thank Chen F C and Si Y F for their help in the experiment.
Project supported by the National Natural Science Foundation of China (Grant Nos. 51671161, u1806219, and 52088101) and the Natural Science Foundation Research Project of Shanxi Province,China(Grant No.2020JZ-08).
杂志排行
Chinese Physics B的其它文章
- Characterizing entanglement in non-Hermitian chaotic systems via out-of-time ordered correlators
- Steering quantum nonlocalities of quantum dot system suffering from decoherence
- Probabilistic quantum teleportation of shared quantum secret
- Spin–orbit coupling adjusting topological superfluid of mass-imbalanced Fermi gas
- Improvement of a continuous-variable measurement-device-independent quantum key distribution system via quantum scissors
- An overview of quantum error mitigation formulas
