低NOx环境异戊二烯促进甲苯生成甲基丁烯二醛的模拟实验
2022-09-20韩士杰李勤勤王文路王伯光
张 扬,韩士杰,李勤勤,王文路,肖 扬,郭 严,王 好,2,3*,王伯光,2,3**
低NO环境异戊二烯促进甲苯生成甲基丁烯二醛的模拟实验
张 扬1,韩士杰1,李勤勤1,王文路1,肖 扬1,郭 严1,王 好1,2,3*,王伯光1,2,3**
(1.暨南大学环境与气候研究院,广东 广州 511443;2.中澳空气质量科学与管理广东国际联合研究中心,广东 广州 511443;3.粤港澳环境质量创新联合实验室,广东 广州 511443)
在低NO浓度条件下开展甲苯和异戊二烯复合体系的烟雾箱模拟实验,使用高时间分辨率的在线质子转移反应飞行时间质谱(PTR-TOF-MS)实时监测混合体系中反应物与产物的浓度变化情况,探究人为源与天然源交汇过程中, 自然源挥发性有机物(BVOCs)对人为挥发性有机物(AVOCs)化学降解的影响.结果表明,异戊二烯与甲苯竞争OH自由基,从而抑制了甲苯的化学降解,该竞争反应开始得越早,抑制效果越显著.研究还发现异戊二烯会增强甲苯RO2降解途径产物的产量,生成更多1,4不饱和-二羰基化合物(如丁烯二醛和甲基丁烯二醛)与二羰基化合物(如乙二醛和甲基乙二醛),其中甲基丁烯二醛增量最高可达38.6%.此外,异戊二烯快速氧化生成的RO2自由基碳数更少,可能与甲苯氧化生成的RO2自由基发生了快速的交叉反应,有利于甲苯RO自由基的生成及裂解,最终导致甲苯RO2途径裂解产物的增加.
甲苯;异戊二烯;烟雾箱模拟;人为源-天然源交汇作用;RO2途径;甲基丁烯二醛;挥发性有机物
与大气中的羟基自由基(OH)反应是甲苯化学降解的主要途径[13-14],多项研究对甲苯的氧化降解反应展开了烟雾箱和计算机模型模拟研究[15-18].最新的准特定化学机理(MCM)表明,甲苯与OH自由基的反应包含4个途径,分别为醛途径、酚途径、双环过氧自由基(RO2)途径和环氧化物途径[19].其中RO2途径是甲苯氧化降解过程中的最关键反应途径,分支比高达65%,亦是甲苯氧化生成O3和SOA的关键途径[20].甲苯RO2产物进一步裂解开环,生成甲基丁烯二醛和丁烯二醛等不饱和二羰基化合物.这些物质是羧酸类、氢过氧化物类、O3和过氧乙酰硝酸酯(PAN)等的重要前体物,也是大气自由基的重要来源之一[21].然而,由于甲苯氧化产生的不饱和二羰基化合物化学性质高度活泼[22],不同研究得出的产率结论差异较大[23-26],甲苯氧化产物在不同条件下的生成情况需要进一步研究.
随着我国森林蓄积面积增加和城镇绿化程度上升,生物源挥发性有机物(BVOCs)对城市大气环境的影响不容忽视[27-29],但目前针对BVOCs影响人为污染物降解的研究较少.人为源与天然源交汇过程中,不仅大气氧化性以及O3和SOA的生成会发生改变[30-34],BVOCs和AVOCs的降解产物与反应途径也可能发生变化[35].但BVOCs如何影响AVOCs的降解仍然不太确定.异戊二烯是大气环境中排放量最大且化学性质最活泼的BVOCs[36],现有烟雾箱实验对异戊二烯如何影响甲苯降解的认识差异较大. Jaoui等[37]在其设定的甲苯/异戊二烯混合体系中发现,异戊二烯会抑制甲苯光化学降解反应,降低甲苯氧化产物的产量.相反,Chen等[38]在不同异戊二烯/甲苯浓度比值的混合体系下发现异戊二烯促进了甲苯的降解,提高了O3和SOA等二次产物的产量;甲苯初始浓度越低,二次产物的增长率越大.综上所述,在人为源与天然源交汇过程中,异戊二烯等BVOCs如何影响甲苯等AVOCs的降解仍需要进一步研究.
本研究使用烟雾箱开展了甲苯-异戊二烯混合体系的模拟实验研究,通过分析甲苯和甲基丁烯二醛浓度的变化情况,探讨异戊二烯对甲苯降解及其RO2途径产物的影响.
1 材料与方法
1.1 烟雾箱
模拟实验采用暨南大学环境与气候研究院搭建的烟雾箱系统JNU-VMDSC.该烟雾箱以Teflon- FEP薄膜(DuPont,0.05mm)制成,体积为8m3,置于温度湿度稳定的室内,顶部和两侧均匀布设有120盏黑光灯(GE F40BLB,365nm)提供光源,最大光解速率为no2=0.362min-1.烟雾箱配置有精密的温湿度控制系统及多个传感器,可实时采集和记录烟雾箱内环境参数(如光照、温度、湿度、压强等)的变化.
由零气发生器(热电Model 111)提供背景反应空气.空气在进入零气发生器前先通过硅胶干燥管和颗粒物过滤器,再依次通过硅胶干燥管、Purafil催化剂、活性碳和霍贾拉特催化剂,分别去除水蒸气、NO/SO2等酸性气体以及臭氧和CO等.在正式实验之前,分别以扫描电迁移率粒径谱仪(SMPS, Scanning mobility particle sizer,TSI,3938L75)和质子转移反应飞行时间质谱(PTR-TOF-MS, Ionicon Analytik GmbH, Innsbruck,澳大利亚)实时采样至少0.5h,记录烟雾箱反应本底.甲苯和异戊二烯等前体物初始浓度均小于0.1×10-9,颗粒物初始数浓度小于1个/cm3.
开灯前10min,向烟雾箱内通入NO标气(Air Liqui-de, 99.99%),再以氮气分别将甲苯(99.5%,默克)和过氧化氢(30%,默克)引入烟雾箱.之后,扰流风扇继续工作10min使反应物充分混合;接着关闭风扇,打开黑光灯与制冷机,开始实验.每次实验结束后,开启臭氧发生器(CH-ZTW3g)向烟雾箱内通入300× 10-9的O3,光照反应至少1h,再打开零气发生器和扰流风扇连续清洗48h以上,以去除烟雾箱中的残留前体物和产物.
1.2 监测仪器
实验中全过程开启常规气态污染物监测仪器和PTR-TOF-MS进行在线监测.其中,气态常规污染物O3、CO、SO2、NO、NO2和NO分别使用Thermo 系列的O3分析仪(49i)、CO分析仪(48i)、SO2分析仪(43i)和NO分析仪(42i)实时测量.气态污染物监测仪器定期使用零气发生器(Thermo 111)和动态稀释仪(Thermo 146i)进行零点、跨点校准.
PTR-TOF-MS配备有四级离子向导,每5s记录一次数据,可实时精确测量烟雾箱中VOCs的变化情况.烟雾箱内的气体样品通过全氟烷氧基(PFA)特氟龙管与外部泵(2.0L/min)连接后进入仪器.特氟龙管被保温海绵包裹,防止因烟雾箱与外界环境的温差而导致低挥发性有机物在管内凝结.PTR-TOF- MS以H3O+电离模式运行,漂移管压力为380Pa,温度为50℃,电压为920V,/比率(是电场强度,是气体在漂移管的数密度)为120Td.在此条件下,水簇离子的数量相对较小,大多数VOC产物离子的破碎性不显著,定量结果相对更准确[39-40].
According to the traditional RNN and LSTM,a traditional checking model of Chinese read-backs was proposed in Ref.35.To compare the proposed checking model,the experiments based on traditional model were conducted.The procedure of traditional checking model is shown in Fig.5.
实验过程中,定期使用包含39种VOCs组分的混合标准气体在干燥(RH<1%)条件下对PTR-TOF- MS进行标定校正,以保证仪器运行期间数据的准确性.实验期间,标准气体组分响应因子波动范围均在20%以内.各种仪器测量数据的不确定度在15%~ 20%.考虑到部分物质缺失校准因子和换算过程中产生的误差对灵敏度的影响,本研究中所有物质均采用归一化的每秒计数(cps)信号进行分析.
1.3 实验设计
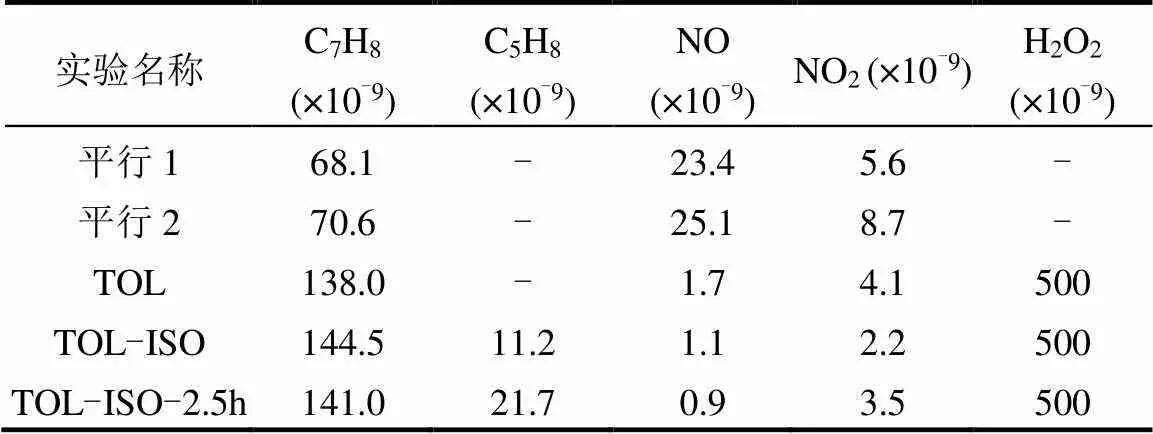
表1 烟雾箱模拟实验初始条件
注:-表示无; TOL代表甲苯,ISO代表异戊二烯.TOL实验表示实验过程中烟雾箱内只有甲苯一种前体物.TOL-ISO表示烟雾箱内同时存在甲苯和异戊二烯两种前体物.
烟雾箱模拟实验的初始条件见表1.本研究NO浓度参考实际大气中低NO浓度设置[26].开灯前10min,向烟雾箱内注射等量过氧化氢(H2O2)溶液,作为每个模拟实验早期的OH自由基来源[41].在甲苯降解实验进行至不同阶段,注入异戊二烯,观测烟雾箱中气态产物的变化.为了探究异戊二烯对甲苯的实际影响,设置TOL-ISO-2.5h实验,表示实验进行2.5h后往烟雾箱内注入异戊二烯;平行1和平行2为一对重复性实验.实验过程中的温度均为25℃、相对湿度均为30%.
2 结果与讨论
2.1 烟雾箱重复性实验
可重复性是衡量烟雾箱实验质量的一个重要指标[18].两组甲苯-NO重复性实验(平行1和平行2)的结果如图1所示.两组实验甲苯、NO等前体物浓度相近,甲苯浓度偏差为3.5%,NO浓度偏差为6.8%,由初始反应条件引起的偏差在可接受的范围之内.两组实验中的甲苯及其产物甲基丁烯二醛的变化曲线相似.平行1与平行2实验中甲苯最终反应量分别为6.7´104cps和6.8´104cps,偏差约为1.9%.甲基丁烯二醛在实验平行1和平行2中的最终产量分别为4.9´103cps和5.0´103cps,对应的偏差为3.4%.前体物及其产物的偏差均小于5%,表明本研究使用的烟雾箱体系中甲苯及其产物变化的重复性较好.

图1 重复实验中甲苯及其典型产物甲基丁烯二醛的时间序列
2.2 异戊二烯对甲苯降解量的影响
Chen等[38]对比了纯甲苯体系和甲苯-异戊二烯混合体系中甲苯的消耗量,发现实验结束后,混合体系中甲苯消耗了22.2µg/m3,为起始浓度的35%.而纯甲苯体系中,甲苯仅消耗了3.1µg/m3,占起始浓度的5.6%,表明异戊二烯对甲苯降解有显著的促进作用.而Jaoui等[37]发现,在甲苯-蒎烯/异戊二烯体系中加入异戊二烯后,甲苯消耗量从1900µg/m3降低到1000µg/m3,甲苯从30%的消耗量降低至15%,异戊二烯的加入显著抑制了甲苯的降解.本研究根据PTR- TOF-MS在线监测数据结果分析异戊二烯加入前后对甲苯降解的影响.如图2所示,相比于纯甲苯体系,甲苯-异戊二烯混合体系中甲苯的消耗量更小.实验至8h时,纯甲苯体系中,甲苯消耗了62%,但在混合体系中,甲苯的消耗量均在50%左右.同时,在甲苯不同反应阶段加入异戊二烯,其对甲苯反应的影响也有所不同.在甲苯反应开始前加入异戊二烯,对甲苯氧化的的抑制效果要高于反应2.5h后加入.结合Jaoui等[37]的研究,表明异戊二烯不但抑制了甲苯的降解,同时也反映出异戊二烯参与反应的时间越早,其抑制作用也越明显.这可能归因于异戊二烯与甲苯的OH竞争反应,异戊二烯与OH的反应速率远大于甲苯,在异戊二烯加入后,OH优先与异戊二烯反应,使得甲苯的消耗量降低.同时,由于甲苯与OH的反应为一级反应,甲苯在反应初期的消耗量高于反应后期,因此异戊二烯参与反应的时间越早,竞争效应带来的抑制效果越显著.

图2 各实验结束时甲苯消耗量占比情况
2.3 异戊二烯对甲苯RO2途径产物的影响

图3 MCM中甲苯氧化机制
改编自文献[19,25]
根据MCM机制,甲苯的RO2降解途径进一步可分为5个,各占20%(图3),主要产物包括乙二醛、甲基乙二醛、丁烯二醛和甲基丁烯二醛等.在纯甲苯和异戊二烯-甲苯实验过程中观测到甲苯RO2途径的产物信号变化如图4所示,相比于纯甲苯实验,加入异戊二烯的实验中虽然观察到的甲苯消耗量减少了,但RO2途径主要产物之一的甲基丁烯二醛信号总量却显著增加.

图4 实验结束时,未加入异戊二烯和加入异戊二烯的甲苯实验中的物质信号对比
在TOL-ISO-2.5h实验中,加入异戊二烯后丁烯二醛和甲基丁烯二醛的信号变化如图5所示,二者信号均出现迅速上升的现象.甲基丁烯二醛信号浓度在短时间内快速上升,然后达到平衡,而丁烯二醛信号则持续上升至实验结束.加入异戊二烯前后1h的甲苯及其产物信号变化量存在明显差异,如图6所示.加入异戊二烯后,产物增加的信号远高于未加异戊二烯时的信号增加结果,加入异戊二烯后1h的甲苯消耗量也小于未加入异戊二烯前1h消耗量.
综上所述,加入异戊二烯后甲苯消耗量降低,而甲苯RO2途径产物产量增大, 表明异戊二烯增强甲苯RO2途径降解生成不饱和二羰基化合物.
Wang等[42]发现异戊二烯会抢夺烟雾箱内OH自由基进行氧化分解,因而本研究中观测到的甲苯氧化产物整体信号上升的原因不能简单排除是异戊二烯降解产物与甲苯降解产物相同,或者产生了相同质荷比产物的可能性.通过汇总文献中已报道的异戊二烯和甲苯主要降解产物及其产率(表2)并做了多方比较分析,部分排除来自异戊二烯产物信号的干扰.
Smith等[24]在甲苯氧化实验中发现甲苯氧化过程中RO2途径会产生/=84的物质,Jang等[43]进一步确定甲苯氧化反应中产生的/为84的物质是丁烯二醛.此外,Fan[44]和Healy等[45]研究发现异戊二烯的主要初级产物为甲基乙烯基甲酮(MVK)和异丁烯醛(MACR),推测初级产物经过进一步氧化分解产生其它化合物,如羰基类化合物等.Pan等[46]在分析异戊二烯氧化实验中产生的气态和颗粒态物质时,发现异戊二烯产物中含有/=84的C5-羰基化合物和2-甲基-3-烯醛.即异戊二烯的部分产物理论上可能会对甲苯氧化产物丁烯二醛的信号造成一定干扰.

Wennberg等[47]确定的异戊二烯氧化产物质谱中未检出甲基丁烯二醛,也未检出其它/=98的物质,这一结果与Kroll等[48]的研究发现相同.然而诸多研究发现甲苯的氧化产物中有3种物质具有相同的/=98,分别是甲基丁烯二醛、4-氧-二戊烯醛和呋喃酮[23-24,49],其中甲基丁烯二醛、4-氧-二戊烯醛互为同分异构体.虽然Smith在高NO实验条件下测到了痕量的呋喃酮[24],但在Seuwen等[50]的低NO实验条件中并未检测出呋喃酮.另外,Zaytsev等[26]最近一项研究认为4-氧-二戊烯醛信号可以归为甲基丁烯二醛.且Mattias等[51]的RO2‘池’反应理论指出实验中的RO2产物可以进行异构化和自氧化,同一途径产出的同分异构体可以视为同一物质,这进一步支持了Zaytsev的结论.Ji等[1]从Schwantes等[52]的研究结果推测羰基化合物也可能是酚途径氧化产物,但Schwantes等研究发现酚途径产物进一步氧化生成的多为保环物质,如二羟基甲苯、三羟基甲苯、四羟基甲苯、五羟基甲苯等,少量的1,4不饱和羰基化合物仅在高NO条件下的颗粒态产物中被检测到.本研究的甲苯实验在低NO条件下开展,呋喃酮和酚途径羰基化合物对研究结果的影响可以忽略不计,因此认为实验中/=98物质信号丰度主要来自RO2途径的甲基丁烯二醛,其信号增强是异戊二烯对甲苯RO2途径降解生成不饱和二羰基化合物的增强结果.
虽然实验中丁烯二醛的实验结果可能会受到异戊二烯产物的影响,但通过对比中途加入异戊二烯的实验中甲基丁烯二醛的信号结果发现,此实验中双环过氧自由基途径(RO2)产物丁烯二醛和甲基丁烯二醛的增速和增量变大,根据公式(5)计算出甲基丁烯二醛增量达38.6%.结合Wang等[42]关于混合体系中不同RO2之间存在的机理,异戊二烯增强甲苯RO2途径产物生成的一个可能原因如公式(1)(2)(3)所示,异戊二烯通过快速提供R2O2,与甲苯降解生成的R1O2之间发生进一步反应,正向促进甲苯的RO2向RO转化[47],而后RO可发生公式(4)的反应,产生甲基丁烯二醛、HO2自由基等产物[19].

图6 在甲苯氧化实验中期加入异戊二烯前后1h的物质信号变化
R1O2+R2O2→R1O+R2O+O2(1)
R1O2+R2O2→R1OH+R2′HO+O2(2)
R1O2+R2O2→R1′HO+R2OH+O2(3)
RO→C2H2O2+C5H6O2+HO2(4)
产物增量=(-)/(5)
式(5)中:为加入异戊二烯前的产物产率,为加入异戊二烯后的产物产率.

表2 已有研究中关于异戊二烯和甲苯的主要氧化产物及其产率的比较
3 结论
3.1 异戊二烯通过竞争OH自由基,抑制了甲苯的降解,且该抑制效果随竞争反应时间的提前而增强.
3.2 同时,根据Hallquist的RO2的‘池’反应理论,发现异戊二烯会增加甲苯RO2途径产物的产量,生成更多1,4不饱和-二羰基化合物(丁烯二醛、甲基丁烯二醛)和二羰基化合物(乙二醛、甲基乙二醛),产物增量最高可达38.6%.
3.3 研究表明,异戊二烯能更快速生成碳数更少的RO2自由基.异戊二烯-RO2自由基可通过与甲苯-RO2自由基进行交叉反应,促进甲苯RO自由基生成,进而增强甲苯RO2途径裂解产物的生成.
3.4 同时,RO2途径产物进一步氧化裂解可生成大量的HO2自由基,显著影响OH自由基的再生浓度,从而导致甲苯二次产物生成量增加.RO2途径产物产量和HO2等自由基的变化,可能是甲苯等芳香烃研究中碳缺失和自由基不闭合问题的一个重要影响因素.
[1] Ji Y, Zhao J, Terazono H, et al. Reassessing the atmospheric oxidation mechanism of toluene [J]. Proceedings of the National Academy of Sciences, 2017,114(31):8169-8174.
[2] Kurtenbach R, Ackermann R, Becker K H, et al. Verification of the contribution of vehicular traffic to the total NMVOC emissions in germany and the importance of the NO3chemistry in the city air [J]. Journal of Atmospheric Chemistry 42:395–411:2002.
[3] Velasco E, Lamb B, Westberg H, et al. Distribution, magnitudes, reactivities, ratios and diurnal patterns of volatile organic compounds in the Valley of Mexico during the MCMA 2002 & 2003 field campaigns [J]. Atmospheric Chemistry and Physics, 2007,7:329-353.
[4] Li M, Zhang Q, Zheng B, et al. Persistent growth of anthropogenic non-methane volatile organic compound (NMVOC) emissions in China during 1990~2017: Drivers, speciation and ozone formation potential [J]. Atmospheric Chemistry and Physics, 2019,19(13):8897-8913.
[5] Karl T, Striednig M, Graus M, et al. Urban flux measurements reveal a large pool of oxygenated volatile organic compound emissions [J]. Proceedings of the National Academy of Sciences, 2018,115(6):1186- 1191.
[6] Li B, Ho S S H, Gong S, et al. Characterization of VOCs and their related atmospheric processes in a central Chinese city during severe ozone pollution periods [J]. Atmospheric Chemistry and Physics, 2019,19(1):617-638.
[7] Zhao Q, Bi J, Liu Q, et al. Sources of volatile organic compounds and policy implications for regional ozone pollution control in an urban location of Nanjing, East China [J]. Atmospheric Chemistry and Physics, 2020,20(6):3905-3919.
[8] Wu R, Xie S. Spatial distribution of secondary organic aerosol formation potential in China derived from speciated anthropogenic volatile organic compound emissions [J]. Environmental Science & Technology, 2018,52(15):8146-8156.
[9] Levine C, Marcillo A. Regarding several points of doubt of the structure of the olfactory bulb: as described by T. Blanes [J]. Anatomical Record-advances in Integrative Anatomy & Evolutionary Biology, 2008,291(7):751-62.
[10] Samet J M, Chiu W A, Cogliano V, et al. The IARC monographs: Updated procedures for modern and transparent evidence synthesis in cancer hazard identification [J]. International Agency for Research on Cancer, 2020,112(1):30-37.
[11] Moolla R, Curtis C J, Knight J. Occupational exposure of diesel station workers to BTEX compounds at a bus depot [J]. International Journal of Environmental Research and Public Health, 2015,12(4): 4101-4115.
[12] 王玉珏,胡 敏,李 晓,等.大气颗粒物中棕色碳的化学组成、来源和生成机制[J]. 化学进展, 2020,32(5):627-641.
Yujue Wang, Min Hu, Xiao Li, et al. Chemical Composition, Sources and Formation Mechanisms of Particulate Brown Carbon in the Atmosphere [J]. Progress in Chemistry, 2020,32(5):627-641.
[13] Le B G, Becker K H. Chemical processes in atmospheric oxidation [J]. Eurotrac, 1997,551.51l-dc20:96-41132.
[14] Bandow H, Washida N. Ring-cleavage reactions of aromatic hydrocarbons studied by FT-IR spectroscopy. II. Photooxidation of o-, m-, and p-xylenes in the NO-air system [J]. Bulletin of the Chemical Society of Japan, 2006,58(9):2541-2548.
[15] Wang S, Newland M J, Deng W, et al. Aromatic photo-oxidation, a new source of atmospheric acidity [J]. Environmental Science & Technology, 2020,54(13):7798-7806.
[16] Arey J, Obermeyer G, Aschmann S M, et al. Dicarbonyl products of the OH radical-initiated reaction of a series of aromatic hydrocarbons [J]. Environmental Science & Technology, 2009,43(3):683-689.
[17] Nehr S, Bohn B, Dorn H P, et al. Atmospheric photochemistry of aromatic hydrocarbons: OH budgets during SAPHIR chamber experiments [J]. Atmospheric Chemistry and Physics, 2014,14(13): 6941-6952.
[18] 贾 龙,徐 福.烟雾箱与数值模拟研究苯和乙苯的臭氧生成潜势[J]. 环境科学, 2014,35(2):495-503.
Long Jia, Yong fu Xu. Studies of Ozone Formation Potentials for Benzene and Ethylbenzene Using a Smog Chamber and Model Simulation [J]. Eevironmental Science, 2014,35(2):495-503.
[19] Lu S, Li X, Liu Y, et al. Advances on atmospheric oxidation mechanism of typical aromatic hydrocarbons [J]. Acta Chimica Sinica, 2021,79(10):18.
[20] Newland M J, Jenkin M E, Rickard A R. Elucidating the fate of the OH-adduct in toluene oxidation under tropospheric boundary layer conditions [J]. Proceedings of the National Academy of Sciences, 2017,114(38):E7856-7857.
[21] Liu X, Jeffries H E, Sexton K G J E S, et al. Atmospheric photochemical degradation of 1,4-Unsaturated dicarbonyls [J]. Environmental Science & Technology, 1999,33(23):4212-4220.
[22] Bierbach A, Barnes I, Becker K H, et al. Atmospheric chemistry of unsaturated carbonyls: Butenedial, 4-Oxo-2-pentenal, 3-Hexene-2, 5-dione, Maleic Anhydride, 3H-Furan-2-one, and 5-Methyl-3H- furan-2-one [J]. Environmental Science & Technology, 1994,28(4): 715-729.
[23] Wu R, Pan S, Li Y, et al. Atmospheric oxidation mechanism of toluene [J]. Journal of Physical Chemistry A, 2014,118(25):4533-4547.
[24] Smith D F, Mciver C D, Kleindienst T E J J O a C. Primary product distribution from the reaction of hydroxyl radicals with toluene at ppb NOmixing ratios [J]. Journal of Atmospheric Chemistry, 1998,30(2): 209-228.
[25] Birdsall A W, Elrod M J. Comprehensive NO-dependent study of the products of the oxidation of atmospherically relevant aromatic compounds [J]. Journal of Physical Chemistry A, 2011,115(21):5397-5407.
[26] Zaytsev A, Koss A R, Breitenlechner M, et al. Mechanistic study of the formation of ring-retaining and ring-opening products from the oxidation of aromatic compounds under urban atmospheric conditions [J]. Atmospheric Chemistry and Physics, 2019,19(23):15117-15129.
[27] Hoyle C R, Boy M, Donahue N M, et al. A review of the anthropogenic influence on biogenic secondary organic aerosol [J]. Atmospheric Chemistry and Physics, 2011,11(1):321-343.
[28] Bryant D J, Dixon W J, Hopkins J R, et al. Strong anthropogenic control of secondary organic aerosol formation from isoprene in Beijing [J]. Atmospheric Chemistry and Physics, 2020,20(12):7531-7552.
[29] Li L, Zhang B, Cao J, et al. Isoprenoid emissions from natural vegetation increased rapidly in eastern China [J]. Environmental Research, 2021,200(12):111462-111462.
[30] Wei D, Fuentes J D, Gerken T, et al. Influences of nitrogen oxides and isoprene on ozone-temperature relationships in the Amazon rain forest [J]. Atmospheric Environment, 2019,206:280-292.
[31] Makar P A, Staebler R M, Akingunola A, et al. The effects of forest canopy shading and turbulence on boundary layer ozone [J]. Nature Communications, 2017,8:15243.
[32] Lv S, Gong D, Ding Y, et al. Elevated levels of glyoxal and methylglyoxal at a remote mountain site in southern China: Prompt in-situ formation combined with strong regional transport [J]. Science of the Total Environment, 2019,672:869-882.
[33] Gong D, Wang H, Zhang S, et al. Low-level summertime isoprene observed at a forested mountaintop site in southern China: implications for strong regional atmospheric oxidative capacity [J]. Atmospheric Chemistry and Physics, 2018,18(19):14417-14432.
[34] Qin M, Hu A, Mao J, et al. PM2.5and O3relationships affected by the atmospheric oxidizing capacity in the Yangtze River Delta, China [J]. Science of the Total Environment, 2022,810:152268.
[35] Chen T, Jang M. Secondary organic aerosol formation from photooxidation of a mixture of dimethyl sulfide and isoprene [J]. Atmospheric Environment, 2012,46:271-278.
[36] 张诗炀,龚道程,王 好,等.南岭国家大气背景站异戊二烯的在线观测研究[J]. 中国环境科学, 2017,37(7):2504-2512.
Shi-yang Zhang, Dao-cheng Gong, et al. Online measurement of isoprene at a national air background monitoring station in the Nanling Mountains, South China [J]. China Environmental Science, 2017, 37(7):2504-2512.
[37] Jaoui M, Edney E O, Kleindienst T E, et al. Formation of secondary organic aerosol from irradiated -pinene/toluene/NOmixtures and the effect of isoprene and sulfur dioxide [J]. Journal of Geophysical Research, 2008,113:D09303.
[38] Chen L, Bao K, Li K, et al. Ozone and secondary organic aerosol formation of toluene/NOirradiations under complex pollution scenarios [J]. Aerosol & Air Quality Research, 2017,17(7):1660-1671.
[39] De Gouw J, Warneke C. Measurements of volatile organic compounds in the earth's atmosphere using proton-transfer-reaction mass spectrometry [J]. Mass Spectrom Reviews, 2007,26(2):223-257.
[40] Yuan B, Koss A R, Warneke C, et al. Proton-transfer-reaction mass spectrometry: Applications in atmospheric sciences [J]. Chemical Reviews, 2017,117(21):13187-13229.
[41] Jorga S D, Kaltsonoudis C, Liangou A, et al. Measurement of formation rates of secondary aerosol in the ambient urban atmosphere using a dual smog chamber system [J]. Environmental Science & Technology, 2020,54(3):1336-1343.
[42] Wang Y, Zhao Y, Li Z, et al. Importance of hydroxyl radical chemistry in isoprene suppression of particle formation from α-pinene ozonolysis [J]. Earth and Space Chemistry, 2021,5(3):487-499.
[43] Jang M, Kamens R M. Characterization of secondary aerosol from the photooxidation of toluene in the presence of NOand 1-propene [J]. Environmental Science & Technology, 2001,35(18):3626-3639.
[44] Fan J W, Zhang R Y. Atmospheric oxidation mechanism of isoprene [J]. Environmental Chemistry, 2004,1:140-149.
[45] Healy R M, Wenger J C, Metzger A, et al. Atmospheric chemistry and physics gas/particle partitioning of carbonyls in the photooxidation of isoprene and 1,3,5-trimethylbenzene [J]. Atmospheric Chemistry and Physics, 2008,8:3215-3230.
[46] Pan G, Hu C J, et al. Direct detection of isoprene photooxidation products by using synchrotron radiation photoionization mass spectrometry [J]. Rapid Communications in Mass Spectrometry Rcm, 2011,26(2):189-194.
[47] Wennberg P O, Bates K H, Crounse J D, et al. Gas-phase reactions of isoprene and its major oxidation products [J]. Chemical Reviews, 2018,118,7:3337–3390.
[48] Kroll J H, Ng N L, Murphy S M, et al. Secondary organic aerosol formation from isoprene photooxidation [J]. Environmental Science & Technology, 2006,40(6):1869-1877.
[49] Birdsall A W, Andreoni J F, Elrod M J. Investigation of the role of bicyclic peroxy radicals in the oxidation mechanism of toluene [J]. Journal of Physical Chemistry A, 2010,114(39):10655-10663.
[50] Seuwen R, Warneck P. Oxidation of toluene in NOfree air: Product distribution and mechanism [J]. International Journal of Chemical Kinetics, 1996,28(5):315-332.
[51] Mattias H, John C. Wenger, et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues [J]. Atmospheric Chemistry and Physics, 2009,9:5155-5236.
[52] Schwantes R H, Schilling K A, Mcvay R C, et al. Formation of highly oxygenated low-volatility products from cresol oxidation [J]. Atmospheric Chemistry and Physics, 2017,17(5):3453-3474.
[53] Baltaretu C O, Lichtman E I, Hadler A B, et al. Primary atmospheric oxidation mechanism for toluene [J]. Journal of Physical Chemistry A, 2009,113(1):221-230.
[54] Jenkin M E, Young J C, Rickard A R. The MCM v3.3.1degradation scheme for isoprene [J]. Atmospheric Chemistry and Physics, 2015, 15(20):11433-11459.
[55] Moschonas N, Glavas S, Danalatos D. The effect of O2and NO2on the ring retaining products of the reaction of toluene with hydroxyl radicals [J]. Atmospheric Environment, 1996,127:875-881.
[56] Jun Zhao, Renyi Zhang, et al. Quantification of hydroxycarbonyls from OH Isoprene reactions [J]. Journal of the American Chemical Society, 2004,126(9):2686–2687.
[57] Tuazon E C, Atkinson R , et al. A product study of the gas-phase reaction of Isoprene with the OH radical in the presence of NO[J]. International Journal of Chemical Kinetics, 1991,21(12):1221-1236.
[58] Sprengnether M, Demerjian K , et al. Product analysis of the OH oxidation of isoprene and 1,3-butadiene in the presence of NO. [J]. Journal of Geophysical Research Atmospheres, 2002,107(D15):ACH 8-1-ACH 8-13.
[59] Carlton A G, Wiedinmyer C, Kroll J H, et al. A review of Secondary Organic Aerosol (SOA) formation from isoprene [J]. Atmospheric Chemistry and Physics Discussions, 2009,9(14):4987-5005.
[60] Baker J, Arey J, Atkinson R, et al. Formation and reaction of hydroxycarbonyls from the reaction of OH radicals with 1,3-butadiene and isoprene [J]. Environmental Science & Technology, 2005,84(11): 4091-4099.
致谢:暨南大学环境与气候研究院袁斌教授为本研究PTR-TOF-MS采样提供了技术指导,PTR-TOF-MS的数据处理由暨南大学环境与气候研究院的陈钰彬和王思行协助完成,在此表示感谢.
A chamber study on isoprene-promoting the production of toluene-derived methylbutenedial in low NOenvironment.
ZHANG Yang1, HAN Shi-jie1, LI Qin-qin1, WANG Wen-lu1, XIAO Yang1, GUO Yan1, WANG Hao1,2,3*, WANG Bo-guang1,2,3**
(1.Institute for Environmental and Climate Research, Jinan University, Guangzhou 511443, China;2.Australia-China Centre for Air Quality Science and Management (Guangdong), Guangzhou 511443, China;3.Guangdong-Hongkong-Macau Joint Laboratory of Collaborative Innovation for Environmental Quality, Guangzhou 511443, China)., 2022,42(9):4401~4408
Toluene and isoprene are typical anthropogenic volatile organic compounds (AVOCs) and biological volatile organic compounds (BVOCs), respectively. In this study, smog chamber experiments were carried out to simulate photochemical reactions of toluene and isoprene at low NOlevels. In order to investigate the effect of BVOCs on the chemical degradation of AVOCs during the interaction between anthropogenic and biogenic emissions, a proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF-MS) was used to monitor the real-time concentration variations of the key gaseous substances in the mixed system. The results show that isoprene inhibited the chemical degradation of toluene, which might be related to the competitive reactions with OH radicals between isoprene and toluene. Moreover, the earlier competitive reaction began, the more significant the inhibition effect was. And isoprene enhanced the production of toluene RO2degradation pathway products, resulting in more unsaturated 1,4-dicarbonyl compounds (Butenedial, Methyl-butenedial) and dicarbonyl compounds (Glyoxal, Methylglyoxal). The increment in methylbutenedial was up to 38.6%. Also, the RO2• generated by the rapid oxidation of isoprene had less carbon number, which may have a rapid cross-reaction with the RO2• generated by toluene oxidation. The cross reaction was conducive to the generation and cleavage of toluene RO• and ultimately led to an increase of the products from toluene RO2• channel. This study can improve an understanding of the impact of BVOCs on AVOCs degradation during the interaction process between anthropogenic and biogenic emissions, and provide insights into regional air pollution prevention and control in the future.
toluene;isoprene;smog chamber simulation;anthropogenic biological source-interaction process;peroxide-bicyclic pathway;methylbutenedial;volatile organic compounds
X511
A
1000-6923(2022)09-4001-08
2022-02-14
国家自然科学基金面上项目(42077190,41877370);广东省科技厅科技创新平台类项目(2019B121202002);广东省“珠江人才计划”引进创新创业团队项目(2016ZT06N263);国家自然科学基金资助项目(42121004)
*责任作者, 教授, wanghao@jnu.edu.cn,** 教授, tbongue@jnu.edu.cn
张 扬(1996-),男,湖北黄冈人,暨南大学硕士研究生,主要从事光化学烟雾箱方面的研究.
