基于羧苄基紫精配体构筑的三种多重响应配合物
2022-09-16刘进剑陆艺炜
刘进剑 刘 娜 陆艺炜
(山西师范大学化学与材料科学学院,太原 030031)
Recently,the exploration of chromic materials has attracted much attention due to their potential applications,including photochromic and electrochromic displays,information storage,solar energy conversion,molecular switches,and inkless printing media[1-3].As a subclass of chromic materials,viologen-derived com-plexes constructed from the self-assembly of viologen ligands and central metal ions have attracted increasing interest[4].It is well known that the electrondeficient viologen derivatives usually display an interesting color change during interactions with electrondonor units[5].The addition of redox viologen groups can confer charge transport properties and response to external stimuli to the synthesized complexes[6].In the past few years,viologen derivatives have been used to consult various chromic materials as functional organic ligands[7-8].Nowadays,assembling suitable viologen derivatives and other structural nodes into the framework to create a suitable electron transfer(ET)pathway is a straightforward approach to constructing such materials.
In the previous study,symmetric viologencarboxylate ligands have been used as building blocks,and introducing these ligands into the framework can significantly tune their physicochemical properties[9].On the one hand,the carboxylate group enhances the coordination ability,adapting to different attachment forms[10].On the other hand,the carboxylate group is beneficial to weaken the effect of positive charges[11].Considering the above aspects,the functional ligand 1,1′-bis(4-carboxybenzyl)-4,4′-bipyridinium dichloride((H2Bpybc)Cl2)is chosen.The methylene group in(H2Bpybc)Cl2connects the phenyl and pyridinium units,deviating the whole ligand from linearity,and imparting flexibility to the molecular framework[12].
In recent years,our research group has been using some viologen-carboxylate ligands to build functionally responsive materials and investigated the role of metal ions[13].According to the previous research of our group,the coordination of metal ions has a great influence on the final structure and performance of chromic response[14-16].Following our recent research aimed at a comparative study,three complexes{[Cd(Bpybc)0.5(HBTC)(H2O)]·0.6H2O}n(1), [Ni(Bpybc)0.5(HBTC)(H2O)4](2), and[Co(Bpybc)0.5(HBTC)(H2O)4](3)have been successfully synthesized by adjustment of the coordination modes between the carboxybenzyl viologen ligand(H2Bpybc)Cl2and auxiliary ligand 1,3,5-benzene tricarboxylic acid(H3BTC)through varying different metal ions.Complex 1 has a 2D layered structure,while complexes 2 and 3 are 0D.Their different structures result in distinct chromic behaviors.Complexes 1-3 all displayed photochromic properties.In addition,complex 2 exhibited thermochromism,which is associated with radical formation by ET,and complex 3 exhibited a color change accompanied by a reversible structural transition with water removal and reabsorption.The effect of metal ions on the structure and chromic property has been verified again.
1 Experimental
All chemicals used in the synthesis were purchased from commercial sources without further purification.(H2Bpybc)Cl2was synthesized according to the literature[17].A Rigaku Ultima Ⅳ-185 diffractometer was used to collect powder X-ray diffraction(PXRD)patterns at 40 kV,40 mA with CuKαradiation(λ=0.154 06 nm)and a graphite monochromator scanning from 5°to 50°.A Vario EL Ⅲ CHNOS elemental analyzer was used to perform elemental analysis of C,H,and N.A Nicolet 5DX spectrometer was used to obtain FT-IR spectra(4 000-500 cm-1)by KBr pellets.A Varian Cary 5000 UV-Vis spectrophotometer was used to perform UV-Vis diffuse reflectance spectrum at room temperature.HTG-3 equipment was used to perform thermogravimetry(TG)experiments in the air in a range of 30-700℃ (10℃·min-1).A Bruker A300-10/12 spectrometer was used to record electron paramagnetic resonance(EPR)spectrum at room temperature.A ThermoFisher ESCALAB 250 X-ray photoelectron spectrometer(powered at 150 W)was used to perform X-ray photoelectron spectroscopy(XPS)by AlKαradiation(λ=0.835 7 nm;spot size,500 m).
1.1 Preparation of complexes 1-3
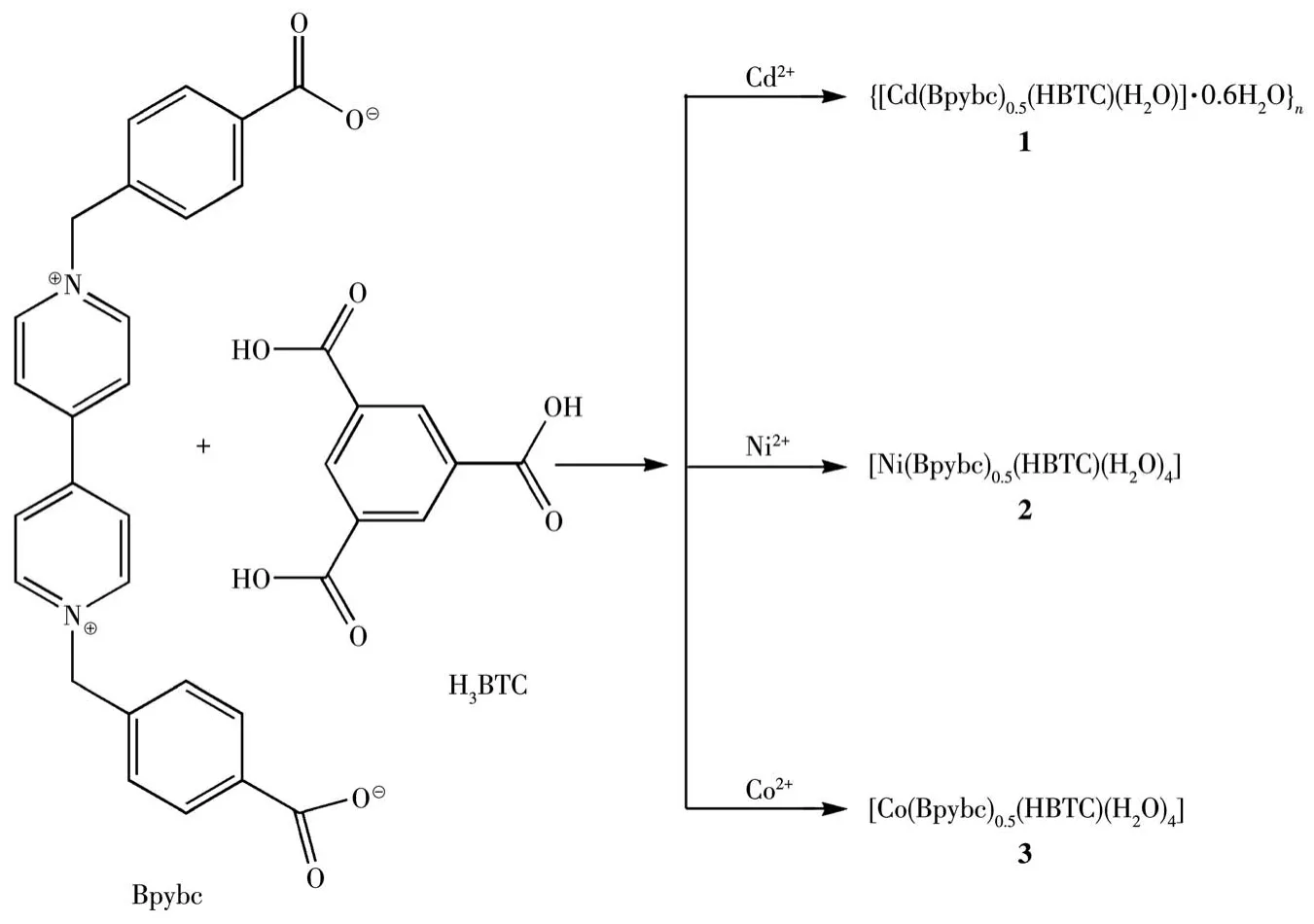
Scheme 1 Preparation of complexes 1-3
The general procedures for preparations of complexes 1-3 were as follows(Scheme 1):H3BTC(21.0 mg,0.1 mmol)was dissolved in a mixture of solvents dimethylformamide(5 mL,DMF),ethanol(5 mL,EtOH),and water(5 mL,H2O).A mixture of(H2Bpybc)Cl2(25.0 mg,0.05 mmol)and Cd(NO3)2·4H2O(31.0 mg,0.1 mmol)for 1/Ni(NO3)2·6H2O(29.0 mg,0.1 mmol)for 2/Co(NO3)2·6H2O(29.0 mg,0.1 mmol)for 3 in 20 mL of water was added to this solution.After the mixture was stirred for 10 min,the residue was filtered.The filtrate was allowed to stand for several days to give block crystals.Yield for 1:27% based on(H2Bpybc)Cl2.Anal.Calcd.for C22H17.2CdNO9.6(%):C,47.02;H,3.09;N,2.49.Found(%):C,47.83;H,3.01;N,2.47.IR(KBr pellet,cm-1):3 413,3 060,2 923,2 026,1 714,1 617,1 556,1 440,1 371,1 278,1 180,1 110,1 020,929,852,771,728,690,632,526.Yield for 2:35% based on(H2Bpybc)Cl2.Anal.Calcd.for C22H22NiNO12(%):C,47.94;H,4.03;N,2.54.Found(%):C,47.86;H,4.08;N,2.63.IR(KBr pellet,cm-1):3 419,3 120,3 052,2 024,1 693,1 612,1 563,1 443,1 366,1 287,1 162,1 109,1 011,854,766,708,640,512.Yield for 3:33% based on(H2Bpybc)Cl2.Anal.Calcd.for C22H22CoNO12(%):C,47.92;H,4.03;N,2.54.Found(%):C,48.04;H,4.11;N,2.51.IR(KBr pellet,cm-1):3 417,3 122,3 052,2 026,1 689,1 610,1 563,1 446,1 365,1 288,1 159,1 106,1 018,852,767,709,642,518.
1.2 X-ray crystallography
X-ray diffraction data of complexes 1-3 were collected on an Oxford Gemini diffractometer at 293 K using graphite monochrome MoKα(λ=0.071 073 nm).The SCALE3 ABSPACK scaling algorithm was used to perform empirical absorption correction of spherical harmonics.The SHELXTL-97 program was used to solve and refine the structure onF2by direct method and full-matrix least-squares technique.All nonhydrogen atoms were anisotropically refined.The crystallographic data of 1-3 are listed in Table 1,and the selected bond lengths and bond angles are listed in Table S1(Supporting information).

Table 1 Crystal data and structure refinement for 1-3 at 293 K
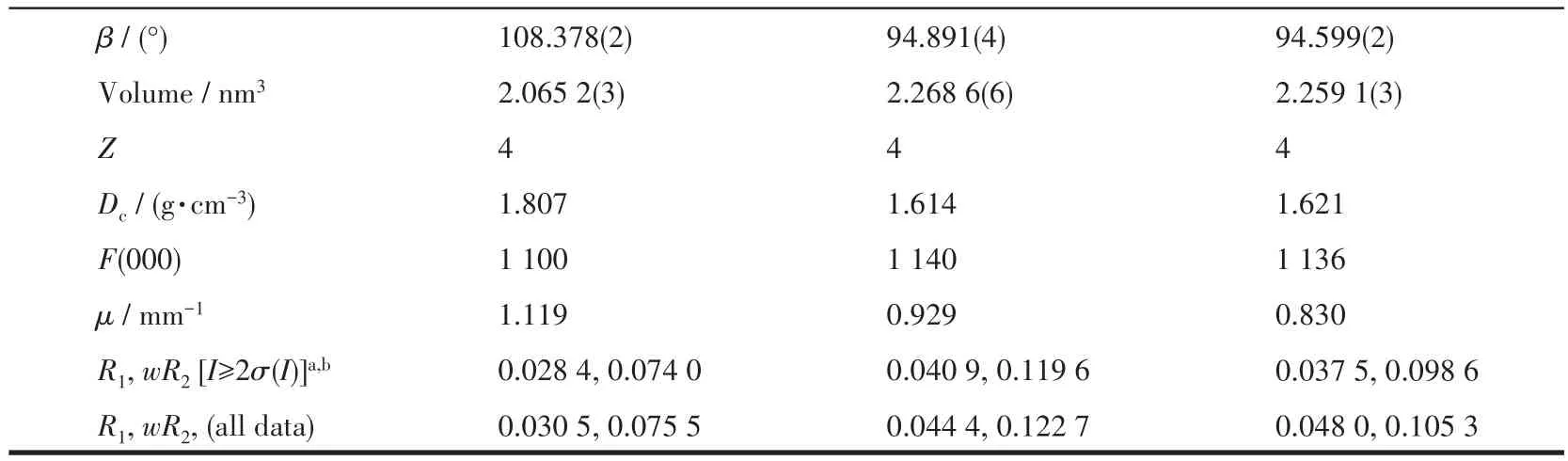
Continued Table 1
CCDC:1959821,1;2034447,2;2034524,3.
2 Results and discussion
2.1 Structure description
Crystals of complexes 1-3 were obtained through the self-assembly reactions of(H2Bpybc)Cl2,H3BTC,and Cd2+(1)/Ni2+(2)/Co2+(3),respectively.The purity of the synthesized samples was verified by the PXRD test(Fig.S1).The methylene group serving as the junction linking the pyridinium and 4-carboxyl phenyl units together imparts flexibility to Bpybc,which in turn leads to a zigzag conformation.Due to its flexible nature,Bpybc exhibits different conformations and coordination modes in the three complexes.Singlecrystal X-ray diffraction analysis shows that complex 1 crystallizes in the monoclinicP21/cspace group and has a 2D-layered structure.The asymmetric unit contains one Cd2+ion,half one Bpybc molecule,one HBTC2-ion,one coordinated water molecule,and some lattice waters(Fig.1a).Each Cd2+ion exhibits a pentagonal bipyramid geometry,coordinated by two O atoms from the Bpybc molecule,four O atoms from two different HBTC2-ions and one O atom from the water molecule.The Cd—O bond lengths range from 0.225 32(19)to 0.246 62(17)nm,which is consistent with that observed in previously reported Cd2+complexes[18].The two carboxylate groups of Bpybc and HBTC2-ligands coordinate with the Cd2+ion in a bidentate mode,leading to the formation of a wavy chain,which is linked to the other chain through another HBTC2-ion,forming a 2D layer along thea-axis andb-axis(Fig.2).The HBTC2-ion acts like an open arm pendant from either side of the chain into the macrocycle.Of the three carboxyl groups in each HBTC2-ion,only two carboxylate groups bridge Cd2+in the bidentate coordination mode,while another carboxyl group remains terminally unbridged.Intermolecularπ…πinteraction between the pyridinium ring of Bpybc and the benzene ring of HBTC2-is demonstrated(dCg-Cg=0.351 1 nm,Fig.S2),which can enhance coordination interactions between the layers.Moreover,there are O—H…O and C—H…O hydrogen bonds in the structure(Table S2).

Fig.1 X-ray crystal structures of 1(a)and 3(b)drawn with ellipsoids at a 30% probability level
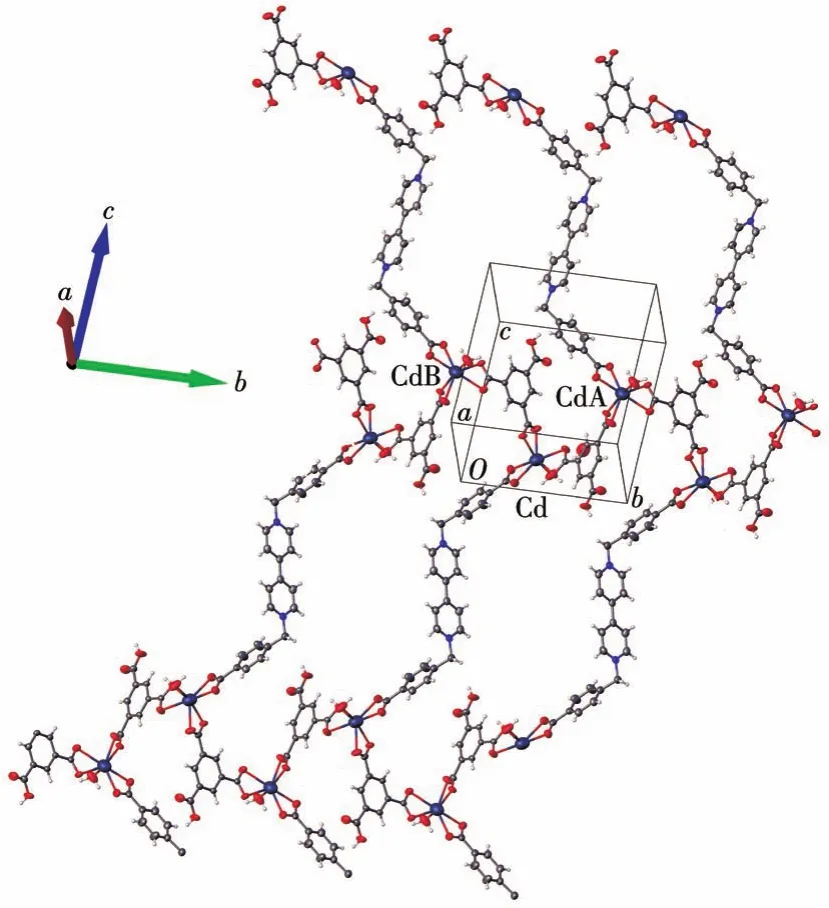
Fig.2 Two-dimensional infinite layer of 1
Single-crystal X-ray diffraction analysis reveals that complexes 2 and 3 are isomorphic,crystallizing in the monoclinic system with space groupP21/n,which contains one Ni2+/Co2+ion,half one Bpybc molecule,one HBTC2-ion,and four coordinated water molecules in an asymmetric unit(Fig.1b).In the crystal structure,each metal ion is coordinated by one carboxylate O atom from Bpybc,one O atom from HBTC2-and four water molecules in the distorted octahedral geometry,leading to an[MO6]octahedron.The M—O distances are in a range of 0.206 45(14)-0.214 14(14)nm for 2,and 0.206 23(13)-0.213 98(14)nm for 3.Note that only one O atom in the carboxylate of Bpybc is coordinated to Ni2+/Co2+ions,and the other is free.Unlike 1,neither the HBTC2-ligand in 2 nor 3 is bridged by another metal ion to create an extended structure(Fig.S3).
2.2 Chromic property
As functional organic ligands,viologen derivatives are known to possess interesting chromic properties owing to the electron-accepting ability and redox activity,and have received increasing attention in recent years[19].Complex 1 displayed a rapid visible color change from colorless to dark blue(Fig.3a)exposed to UV light(365 nm,175 W,Hg lamp)within 3 min at room temperature.The dark blue sample of 1(1P)was very stable in air and did not turn colorless after being placed in the dark for nearly a month.But after heating at 100℃for 5 h,the colored sample could be bleached to the initial state.This reversible color conversion could be repeated many times without noticeable color loss.UV-Vis and EPR tests have been used to study the photochromic process.As shown in Fig.4a,complex 1 exhibited strong absorption in the 200-430 nm region,which should be owing to the charge-transfer transition between the ligand and metal ions.Colorless 1 had no absorption in the 430-750 nm region,however,the absorption in the visible light region increased significantly after irradiation.In addition,new bands appeared at about 403,624,677,and 736 nm,which can be seen as characteristic absorptions of viologen radicals[20].The EPR study showed that no EPR signal was observed for the as-synthesized sample of 1,whereas after irradiation,a signal ofg=1.987 appeared(Fig.4d).Such EPR signal disappeared again after heating to colorless.PXRD study showed that the structure of 1 remained intact after irradiation(Fig.S1a).All these results indicate that the photochromism of 1 originates from the generation of Bpybc radicals[21].From the structural data,it can be found that the closest distance between the pyridinium N atom(acceptor:N1)and the carboxylate O atom(donor:O7)is 0.377 5 nm,and the O7…N1…C6 angle is 149.45°(Fig.S4a).Furthermore,the short contacts between hydrogen atoms and oxygen atoms(0.252 7 nm,C8—H…O7)may be a possible ET pathway[22].
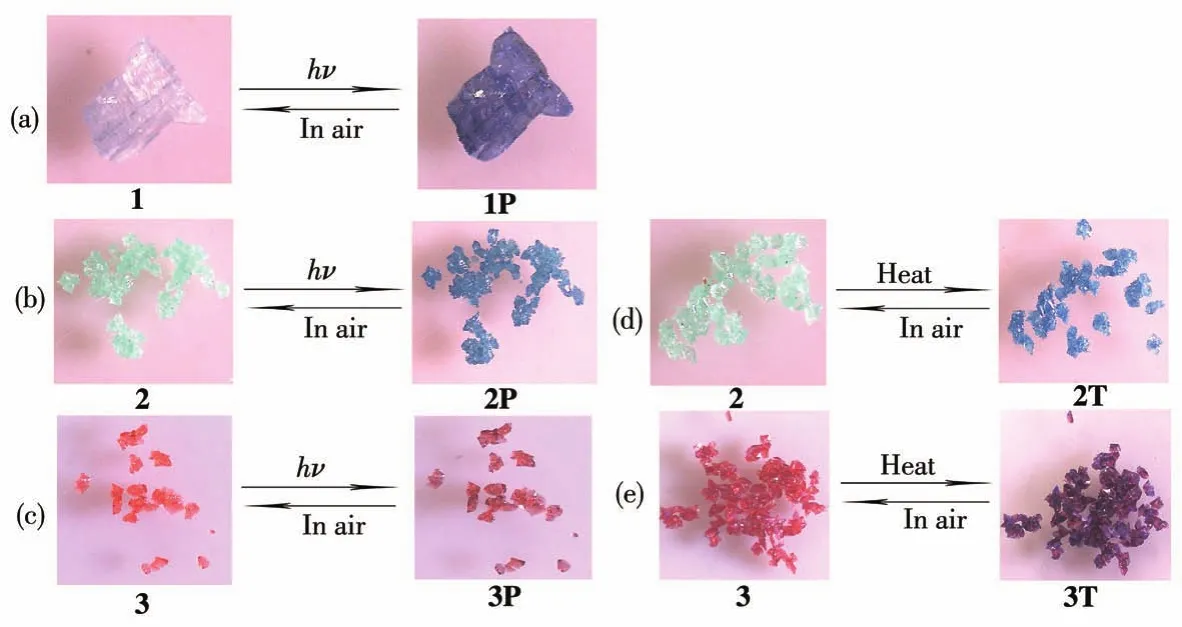
Fig.3 Photographs of chromic properties for complexes 1-3
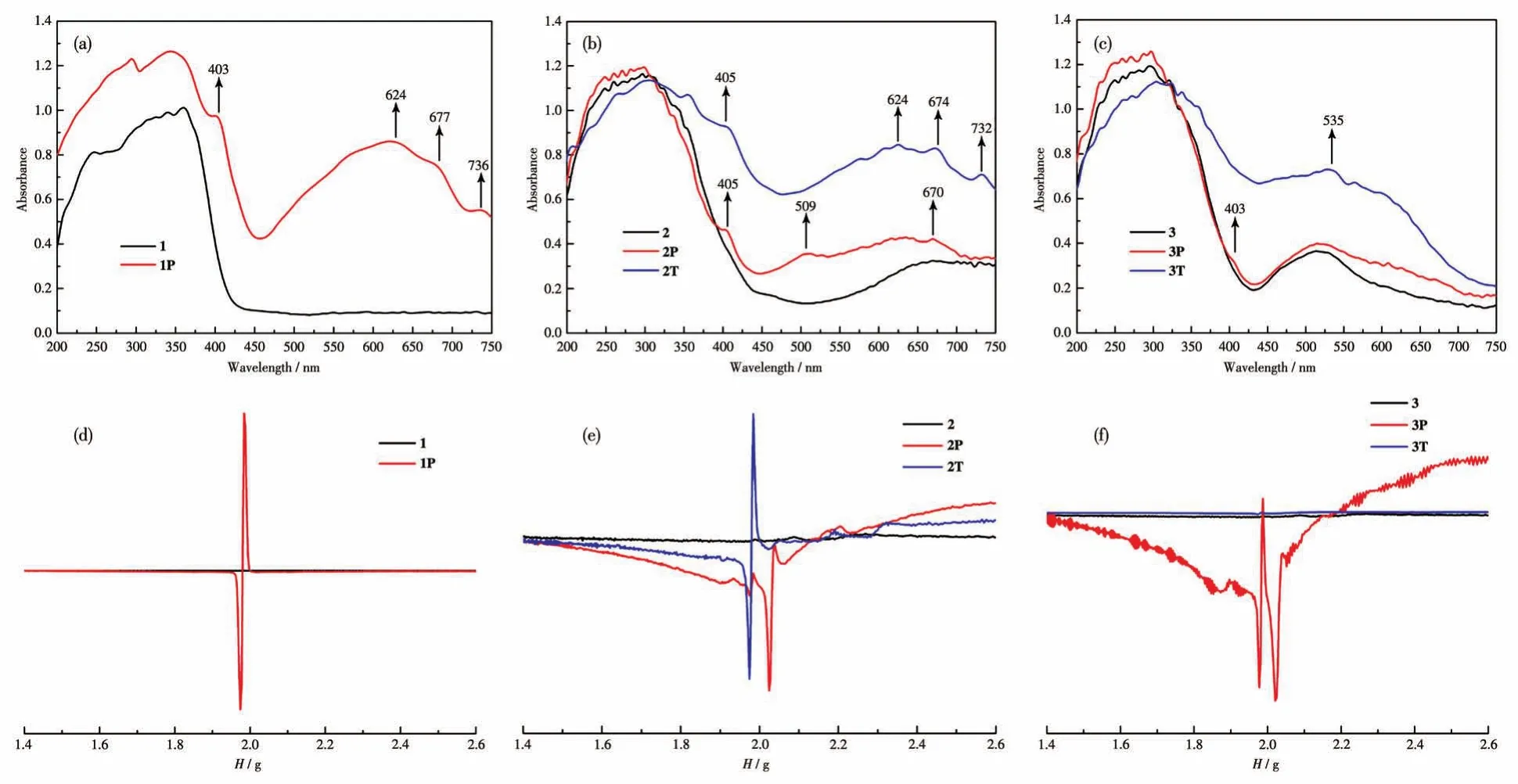
Fig.4 UV-Vis spectra of 1(a),2(b),and 3(c)before and after external stimuli;EPR spectra of 1(d),2(e),and 3(f)before and after external stimuli
As with 1,complex 2 can also respond to UV light.Upon UV irradiation for about 20 min at room temperature,the color change from light green to dark blue(Fig.3b)could be observed.The colored sample(2P)was stable in the dark for weeks,indicating that the photochromic transition of 2 cannot be easily reversed.As shown in Fig.4b,three new absorption peaks centered at 405,509,and 670 nm appeared after irradiation.Meanwhile,2P exhibited an EPR spectrum(Fig.4e)with axial anisotropy ingvalues(g1=1.987,g2=2.041).Similar to 1P,the resonance small signal of 1.987 should be owing to the generation of Bpybc radicals.While,another strong signal of 2.041 may be due to the Ni3+center,which is unusually detectable during the photochromic process[23].As reported,both the Ni2+ion and the carboxyl O atom can theoretically donate electrons to the pyridinium N atom,although the oxidation of Ni2+to Ni3+is difficult to occur under ordinary conditions[24].To gain insight,the XPS study of 2 was performed before and after irradiation.The core level spectra of N1s,O1s,and Ni2pchanged greatly after irradiation,while that of C1shardly varied(Fig.5a).The core level spectrum of N1scan be divided into two peaks at 401.48 and 398.98 eV before irradiation,cor-responding to the positively charged N atom and the N atom of pyridyl radicals.The weak peak of pyridyl N radicals may be due to the fact that complex 2 is sensitive to sunlight and generates a small number of radicals.The N1score level spectrum shifted to a lower binding energy position after irradiation,and the peak at 401.18 eV for the positively charged N atom became weaker.Meanwhile,a stronger signal appeared at 398.68 eV,corresponding to the generation of more pyridyl N radicals after irradiation(Fig.5b).The O1sspectrum shifted to the position from 530.68 to 530.98 eV with higher binding energy after irradiation(Fig.5c).At the same time,the Ni2pspectrum was not the same before and after irradiation,confirming that the oxidation state of Ni2+has changed(Fig.5d).These spectral changes show that both the Ni2+ion and the carboxyl O atom donate electrons to increase the binding energy,while the viologen moiety accepts electrons to reduce the binding energy of the pyridinium N atom.The above results show that we have reason to infer that the photochromism of 2 may originate from the generation of viologen radicals accompanied by redox reactions of metal ions.
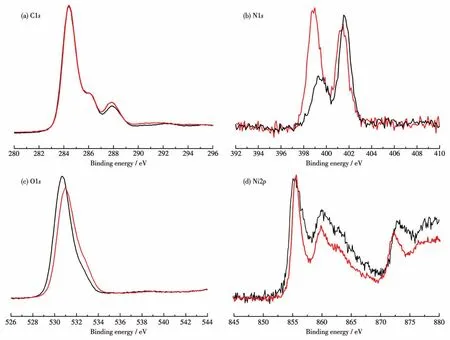
Fig.5 XPS core-level spectra of 2 before(black line)and after UV irradiation(2P,red line)
Strikingly,this chromic behavior could also happen when 2 was heated above 80℃for 30 min in air(2T,Fig.3d).In order to understand the chromic mechanism of 2 under heating conditions,relevant tests were carried out to study the thermal effect.TG indicated that the weight loss of 2 started at 103℃(Fig.S5).The PXRD patterns before and after heating were basically matched,which proves the retention of the structure(Fig.S1b).UV-Vis and EPR spectra after heating showed characteristic signals of Bpybc radicals similar to 1P(Fig.4b and 4e),indicating that the color change of 2 by heating also arises from the ET process,rather than structural transformation.To the best of our knowledge,thermochromism based on viologen derivatives is very rare,because high temperature will facilitate charge recombination[25].
Similar to 2,complex 3 is photochromic,resulting from the generation of viologen radicals and the oxidation process of Co2+,showing a color change from light red to dark red(3P,Fig.3c)upon UV irradiation within 15 min,which has also been proved by the UV-Vis and EPR tests(Fig.4c and 4f).Meanwhile,complex 3 underwent a reversible color change upon release and reabsorption of water molecules.After heating above 80℃in the air for 10 min,the edges of the crystals gradually changed from light red to purple.As the heating time was increased to 20 min,the crystals all turned purple(3T,Fig.3e).However,the absence of detectable EPR signals characterized by viologen radicals after heating indicates that this chromic process is independent of radical generation(Fig.4f).Furthermore,the UV-Vis diffuse reflectance spectrum after heating approximated the transition of Co2+ions in a trigonal-bipyramidal geometry(Fig.4c)[26].TG indicated that 3 underwent continuous weight loss starting at 65℃,associated with the removal of water molecules(Fig.S5).PXRD tests indicate that 3T is a new crystalline phase distinct from the original hydrate(Fig.S1c).We also heated 3 in water vapor at 80℃,and no color change was observed.Thus,this chromic behavior of 3 by heating can be attributed to the change of coordination environment of some Co2+ions because of dehydration.When in direct contact with liquid water or exposed to moist air,this phase easily turns light red.Chromic phenomena associated with dehydration and rehydration are very common for Co2+or Ni2+complexes,and the rearrangement of the coordination environment can affect theird-orbital configuration significantly,which then exhibits a pronounced color change[27-28].The mechanism of the observed phenomenon of 2 and 3 by heating is quite different,which involves the generation of radicals by ET for 2 and dehydration and rehydration for 3.Complexes 2 and 3 have the same structure but different metal ions,and the effect of metal ions on the construction of viologen complexes and chromic properties has been discussed in our previous study[14-16].
3 Conclusions
In our work,by utilizing the carboxybenzyl viologen ligand 1,1′-bis(4-carboxybenzyl)-4,4′-bipyridinium dichloride((H2Bpybc)Cl2)and auxiliary ligand 1,3,5-benzenetricarboxylic acid(H3BTC),and changing different metal ions,three new complexes{[Cd(Bpybc)0.5(HBTC)(H2O)]·0.6H2O}n(1),[Ni(Bpybc)0.5(HBTC)(H2O)4](2),and[Co(Bpybc)0.5(HBTC)(H2O)4](3)have been successfully synthesized.Single-crystal X-ray analysis reveals that complex 1 features a 2D layered structure,while complexes 2 and 3 show isolated structures.Complexes 1-3 all exhibited photochromic properties,which involve the generation of viologen radicals by ET for 1 and the generation of radicals accompanied by redox reactions of metal ions for 2 and 3.In addition,complex 2 exhibited thermochromic phenomena,which is associated with radical via electron transfer,and complex 3 showed a color change accompanying a reversible structural transition with the removal and reabsorption of water.Differences in metal ions have a large impact on the final structure and responding chromic properties.
Supporting information is available at http://www.wjhxxb.cn
