COVID-19 associated diabetes mellitus: A review
2022-09-16AjayGavkareNeetaNanawareAbhijitRayateSachinMumbreBasavrajNagoba
INTRODUCTION
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) was declared a global pandemic by the World Health Organization (WHO) in March 2020. It continues to spread worldwide with about 452201564 confirmed cases and 6029852 deaths to date[1].
The speed with which this deadly virus spreads leaves no place for doubt that, at some time, a significant proportion of the world’s population will be affected. Therefore, it is a matter of great concern to study the interaction of COVID-19 with other commonly occurring medical conditions to anticipate and find out how they will interact with each other and to decide a protocol for their management. Laboratory reports in almost all critically ill patients show severe hyperglycemia as a common finding and this is often considered a marker of disease severity[2]. A literature search for studies carried out during the pandemic shows that COVID-19 is associated with hyperglycemia in people with and without known diabetes mellitus. Hence, now there is sufficient evidence to support the fact that SARS-CoV-2 infection causes a diabetogenic state in COVID-19 patients[3,4]. In this minireview, an attempt has been made to understand how COVID-19 related diabetes develops, its pathogenesis, clinical presentation, outcome and management protocol of new-onset diabetes mellitus in COVID-19 patients.
DIABETOGENIC EFFECT OF SARS-CoV-2 INFECTION IN COVID-19
SARS-CoV-2 infection leading to a diabetogenic state in patients of COVID-19 is now a well-established fact. Different studies carried out in the earlier days of the pandemic support this fact and they report that many patients with SARS-CoV-2 infection were diagnosed with diabetes mellitus after COVID-19.It has been found that many patients presented with diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state and required higher units of insulin to normalize the blood sugar levels[4-6].
CAUSES/RISK FACTORS
The severity of hyperglycemic levels in confirmed cases of COVID-19 infection was found to be proportional to the severity of infection. This can be attributed to the involvement of one or more inter-related processes like stress response associated with severe illness, cytokine storm with elevated levels of inflammatory markers like interleukin (IL)-6, tumor necrosis factor (TNF)-α, C-reactive protein (CRP),lactate dehydrogenase and ferritin. Overdoses of steroids, pancreatic beta-cell damage/destruction resulting in a combined effect of insulin resistance and insufficiency disturbing glucose homeostasis have been reported as important risk factors. Apart from this, increasing age, high body mass index(BMI) and family history of diabetes are independent risk factors[7]. To make the situation worse, strict disciplinary actions taken to break the chain of infection (such as repeated rotatory lockdowns) could also have had an adverse impact such as limited access to clinical care, healthy diet and opportunities to exercise[8].
POSSIBLE POTENTIAL MECHANISMS
COVID-19 due to SARS-CoV-2 infection may manifest not only as new-onset diabetes but also causes worsening of pre-existing diabetes. Considering the evolving nature of the COVID-19 pandemic, it is not yet clearly understood whether SARS-CoV-2 infection causes new-onset diabetes by mechanisms similar to those established in the pathogenesis of type 1 or type 2 diabetes mellitus, or whether this itself is an atypical form of diabetes[9]. Moreover, it has also not been established whether COVID-19 patients remain at higher risk for developing new-onset diabetes or related complications following viral clearance and recovery. The literature reveals detailed discussions regarding the possible potential mechanisms for derangement of glucose metabolism leading to the development of hyperglycemia and new-onset diabetes in COVID-19 patients and these can be broadly attributed to the following factors(Figure 1).
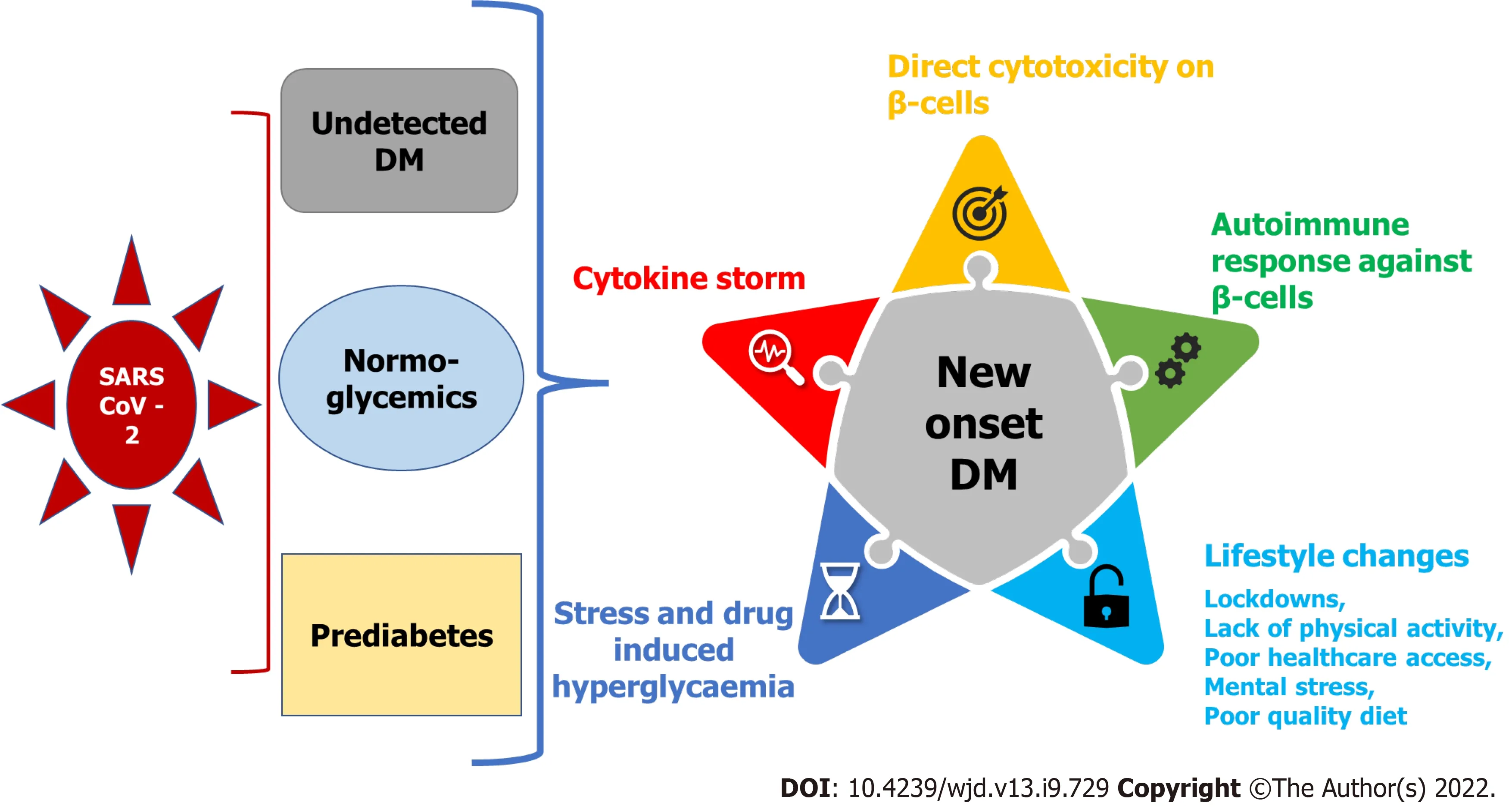
Cytopathic effect causing beta-cell damage
‘Madam,’ replied the prince, whose weakness would hardly allow him to speak, ‘do not think me so unnatural34 as to wish to deprive my father of his crown. As long as he lives I shall remain the most faithful of his subjects! And as to the princesses you speak of, I have seen none that I should care for as a wife, though I would always obey your wishes, whatever it might cost me.’
Autoimmune destruction of pancreatic beta cells
Apart from direct virus-induced cytotoxicity over insulin-secreting beta cells of the pancreas, another suggestion is that SARS-CoV-2 can trigger an autoimmune response against pancreatic beta-cell antigens, and it has emerged as one of the most prevalent hypotheses behind the etiopathogenesis of type 1 diabetes. According to this theory, the virus-mediated cytotoxicity toward beta cells leads to sequestration of antigens that in turn cause activation of autoreactive T lymphocytes. The resultant autoimmune response ultimately destroys the remainder of the beta-cell mass, leading to insulindependent type 1 diabetes in a few weeks to months after infection[12]. This theory cannot completely explain the pathogenesis of immediate onset of diabetes during the acute phase of COVID-19 infection;however, it may hold true for development of hyperglycemia in some patients and later development of diabetes within weeks to months post-COVID recovery. Further research about this would be helpful to reach a more meaningful conclusion.
Host response to COVID-19
As observed in any acute infectious condition, a profound and nonspecific activation of immune mechanisms also occurs in patients with severe COVID-19, escalating the release of counter-regulatory hormones and proinflammatory cytokines such as IL-6 and TNF-α in the form of cytokine storm. This rampant cytokine storm is known to induce insulin resistance and resultant hyperglycemia[13].
Drug-induced iatrogenic effect
The Fairy loved her with all her heart, for she was at once original and gentle, and she had nearly reached the age at which the gifts were generally bestowed4
Undetected pre-existing diabetes before infection with SARS-CoV-2
This sounded like a reasonable idea to all of us kids, so we kept on going with the stories. My mom knew the true story, though. Bobby s mom was a single parent, and she suspected that they just couldn t afford the Easter Bunny.
Acute illness and stress leading to hyperglycemia and new-onset diabetes
Any acute illness and associated stress are the two important common factors that may lead to hyperglycemia in many patients and these patients will form the category of new-onset diabetes mellitus. This was observed during the SARS-CoV-1 pandemic[17]. The cytokine storm due to acute infection by SARS-CoV-2 can cause elevated inflammatory markers like an increase in CRP, erythrocyte sedimentation rate and increased leukocyte count. Cellular stress during acute inflammation causes accelerated lipolysis, thereby increasing the levels of free fatty acids in the circulation, leading to relative insulin deficiency[18].
Long-term surveillance of COVID-19-associated newly diagnosed diabetes patients is necessary to control their risk factors and achieve adequate glycemic control. Patients with stress hyperglycemia during acute critical illness are at high risk of developing diabetes in the future. Meticulous tracking of such cases for early diagnosis, interventions, and long-term follow-up is necessary. Screening for diabetes in every COVID-19 patient would identify a significant number of cases and the cost-effectiveness of the screening would then need consideration. However, screening for diabetes is advisable at least for high-risk patients because if identified, appropriate management of these cases can be instituted. Also, COVID-19 patients with one or more comorbidities should undergo regular monitoring for cardiac and renal risk factors as well as micro/macrovascular complications.
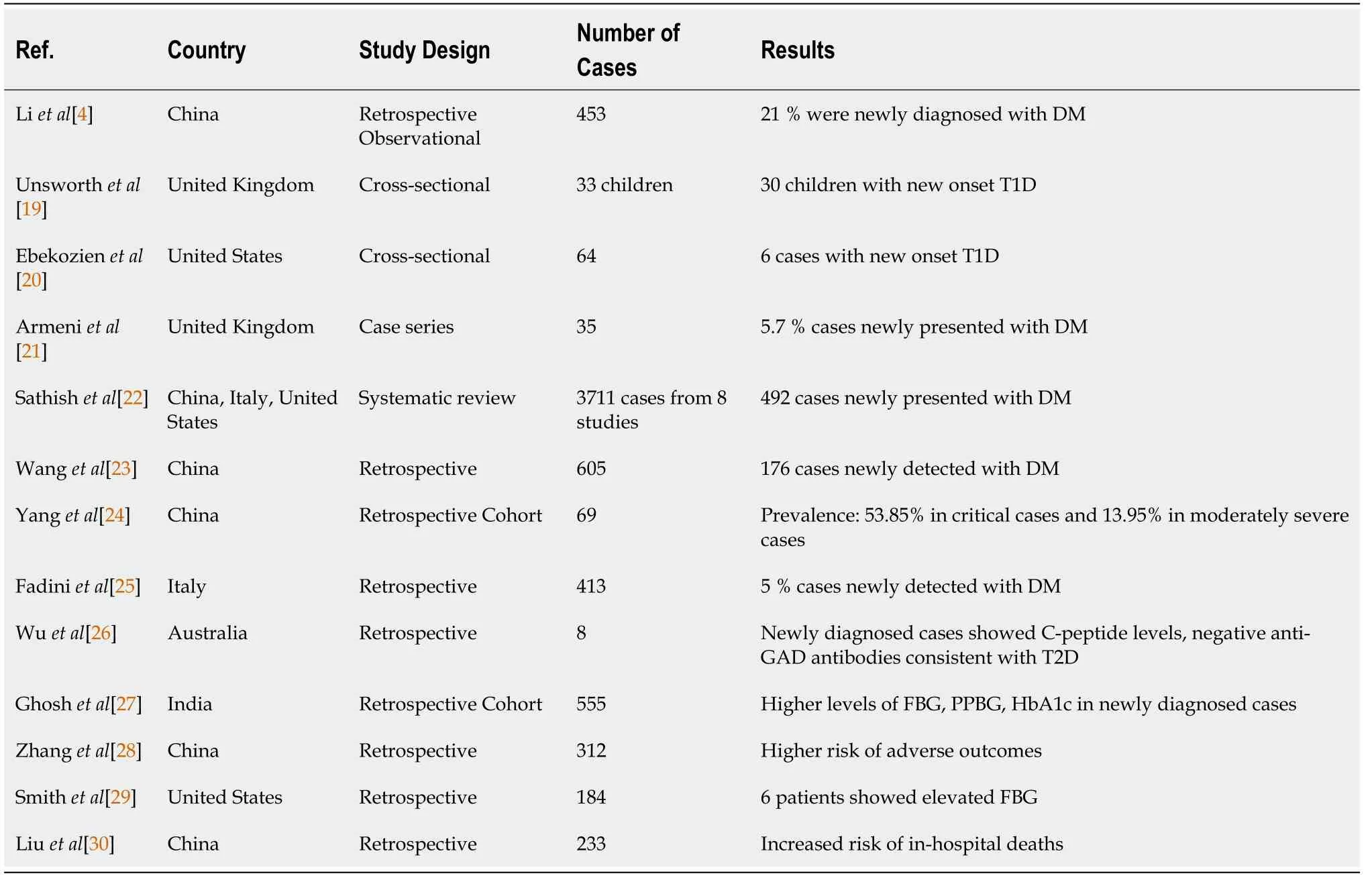
New-onset diabetes has been reported in most of the earlier studies from different parts of the globe(Table 1)[4,19-30].
The RECOVERY trial in ICU COVID-19 patients requiring respiratory support prompted WHO to reframe guidelines and recommend the use of corticosteroids to reduce the overall mortality and morbidity in such patients[14]. However, corticosteroids are a double-edged sword. On one side, they improve the clinical course of patients during the cytokine storm and thereby prevent death in patients with COVID-19 pneumonia. On the other side, they are also known to be highly diabetogenic drugs.Hyperglycemia is almost inevitable with the doses prescribed for this indication and some cases present with complications like DKA, especially in patients with previously undiagnosed diabetes or prediabetes[8].
CLINICAL OUTCOME AND ASSOCIATED MORBIDITIES
It has been observed that diabetes is the pre-existing condition in most of the patients with COVID-19 disease showing severe morbidity and mortality[31]. The diabetic patients in general were found to have a higher risk of developing diabetic nephropathy, ischemic heart disease, and pneumonia leading to multiorgan failure and acute respiratory distress syndrome (ARDS) as compared to nondiabetic individuals. In addition, diabetic individuals were found to be more prone to ICU admission[32,33]. In subjects with diabetes and COVID-19, the mortality rate ranges from 22% to 31% of all COVID-19 patients[34]. A UK-based study revealed that out of 23 804 deaths in hospitalized COVID-19 patients,32% had type 2 diabetes and 1.5% had type 1 diabetes mellitus[35].
Obesity, one of the independent risk factors for type 2 diabetes mellitus, is significantly associated with the severity of COVID-19. A cohort study of 2741 hospitalized patients found that among different factors, obesity was strongly associated with COVID-19 hospitalization and risk of critical illness[36]. A retrospective study from Kuwait consisting of 1158 hospitalized COVID-19 patients concluded that patients with morbid obesity needed more ICU admissions [odds ratio (OR), 5.18][37]. A study by Cai
[38] involving 383 hospitalized COVID-19 patients reported that COVID-19 manifestations were more severe in obese patients as compared to patients with normal BMI. They also found an increased OR of developing severe COVID-19 in overweight patients (OR, 1.84;
= 0.05), with the value of odds being higher in obese subjects (OR, 3.40;
= 0.007).
He is almost as light as the bubble, and he raises himself on his hind12 legs, and wants to be taken into the swing; but it does not stop, and the dog falls; then he barks and gets angry
The entry portal for SARS-CoV-2 is angiotensin-converting enzyme (ACE)-2 receptor. Along with respiratory epithelial cells, ACE-2 receptors are also present in the kidneys, gastrointestinal tract and pancreas. Following infection, SARS-CoV-2 replicates in human endocrine and exocrine secretory cells of the pancreas[10]. It has been postulated that this causes the destruction of insulin-secreting pancreatic beta cells, which leads to the development of new-onset diabetes in some patients with COVID-19. This phenomenon can be well correlated with that observed during SARS-CoV-1 infection; thus, giving due credit to this hypothesis[11].
Altered glucose homeostasis and insulin resistance resulting in acute hyperglycemia have been reported during infection in patients hospitalized with viral infections such as human herpes virus 8 and SARS-CoV as a part of normal antiviral responses. Such responses may further increase the risk of developing type 1 or type 2 diabetes mellitus[39]. During the SARS-CoV-1 outbreak in 2003, findingsfrom one study involving 39 patients without a history of diabetes mellitus, showed that 20 patients developed diabetes during hospitalization and two of these patients remained diabetic despite receiving 3 years of antidiabetic management during follow-up[11].
In a study by Li
[4], 94 out of 453 COVID-19 patients were diagnosed with new-onset diabetes.These newly diagnosed, post-COVID-19 diabetic patients required admission, intermittent mandatory ventilatory assistance, and demonstrated higher risk of all-cause mortality than those COVID-19 patients who were normoglycemic or had transient hyperglycemia. Also, these COVID-19 patients with pre-existing diabetes and new-onset diabetes demonstrated more severe complications including ARDS,acute renal failure, shock, or hypoalbuminemia as compared to those COVID-19 patients having normal or transiently raised blood sugar levels. Similarly, another multicenter retrospective study by Wang
[23], involving 605 COVID-19 patients found that 29% of patients with newly detected diabetes mellitus experienced a higher rate of in-hospital complications and all-cause mortality as compared to normoglycemic COVID-19 patients over a 28-d period. Finally, another study by Fadini
[25],comprising 413 subjects, reported a significant increase in ICU admissions and a higher percentage of death in patients with new-onset COVID-19-related diabetes compared to COVID-19 patients with preexisting diabetes or normal blood glucose levels. In a retrospective observational study from Wuhan by Zhang
[40], there was no significant increase in these parameters. Although many other studies have indicated a correlation between new-onset diabetes and COVID-19, experimental findings from several studies like Ibrahim
[41] and Drucker[42] have also reported an inconclusive relationship between the increase in type 1 diabetes mellitus during the COVID-19 pandemic. These observations can be attributed to a lack of strong supporting evidence. Therefore, there is a necessity for further research to elucidate the interconnected relationship between COVID-19-induced diabetes mellitus and associated complications.
MANAGEMENT OF COVID-19-ASSOCIATED DIABETES MELLITUS
Since the explicit mechanisms and epidemiological factors associated with the development of newonset diabetes following COVID-19 are unknown, it is difficult to frame treatment guidelines for such patients. However, in the light of increasing morbidity and mortality in people with newly diagnosed diabetes mellitus or those with hyperglycemia during admission, treatment protocols should prioritize the management of acute hyperglycemia. It is also indispensable to diagnose COVID-19-associated diabetes mellitus and manage its metabolic complications such as DKA in patients admitted to the hospital for better clinical outcomes. Insulin requirement is invariably higher in such patients when compared to that in patients with acute illness due to other reasons or non-COVID-19-related DKA[26,43,44]. The exact duration of hospital stay of patients with newly detected diabetes mellitus following SARS-CoV-2 infection cannot be defined. There is a paucity of data in the literature regarding the longterm follow-up of these patients. Patients with stress-induced hyperglycemia may revert to a normoglycemic state once they have recovered from the phase of acute illness. Our experience also shows that most patients who have developed new-onset diabetes following SARS-CoV-2 infection have been found to revert to normoglycemic state within 2–4 wk after recovery, especially patients aged < 60 years. These patients, therefore, may not be labeled as having full-blown diabetes requiring prolonged antidiabetic medication. However, these cases are at high risk for developing diabetes in the future;therefore, they require long-term follow-up to determine a further course of action.
At one moment it was so pitch dark that she could not see a single object, but a flash of lightning revealed the whole scene; she could see every one who had been on board excepting the prince; when the ship parted, she had seen him sink into the deep waves, and she was glad, for she thought he would now be with her; and then she remembered that human beings could not live in the water, so that when he got down to her father’s palace he would be quite dead
Considering associated comorbidities like obesity, hypertension, hypercholesterolemia, coronary artery disease, renal disease,
in COVID-19 patients, hypoglycemic agents that improve metabolic function without weight gain should be the preferred choice for long-term management in patients following acute SARS-CoV-2 infection and sustained symptoms (
, long COVID). Sodium–glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs) are the preferred novel therapeutic options that have a beneficial effect on factors like body weight, glycemic control, and cardiovascular and renal outcomes by reducing the duration of stay, overall morbidity and mortality from cardiac and noncardiac causes[46].
Therapeutic trials
DARE-19: This was a randomized, double-blind, placebo-controlled trial undertaken to study organprotective effects of dapagliflozin, an SGLT-2 inhibitor. The study was conducted in hospitalized COVID-19 patients with at least one cardiometabolic risk factor (
, hypertension, type 2 diabetes,coronary artery disease or chronic kidney disease). The trial excluded critically ill patients. The results showed that although the drug was well-tolerated by patients, it did not have an organ-protective effect.There was no significant improvement in clinical recovery within 30 d of starting the medication[47].
Recently, in a study report from India by Kuchay
[45], three COVID-19 patients presented with acute-onset diabetes mellitus with DKA and had favorable initial response to treatment with intravenous fluids and insulin. Later, these patients were managed with multiple doses of subcutaneous insulin, and after 4–6 wk, they were shifted from insulin to oral hypoglycemic agents. Glutamic acid decarboxylase antibodies were measured in two patients who had tested negative, suggesting a transient insulinopenia in these patients.
Ongoing trials: Various trials with dipeptidyl peptidase-4 inhibitors, pioglitazone, and the GLP-1RA semaglutide have been designed[48-53], but only a few are currently functional in the recruiting phase[52,53].
Death continued to stare at the emperor with his cold, hollow eyes, and the room was fearfully still. Suddenly there came through the open window the sound of sweet music. Outside, on the bough of a tree, sat the living nightingale. She had heard of the emperor’s illness, and was therefore come to sing to him of hope and trust. And as she sung, the shadows grew paler and paler; the blood in the emperor’s veins flowed more rapidly, and gave life to his weak limbs; and even Death himself listened, and said, “Go on, little nightingale, go on.”
CONCLUSION
The results of most of the earlier studies show that a significantly higher rate of new-onset diabetes in many COVID-19 patients is a frequently observed phenomenon. The resultant hyperglycemia is known to influence the clinical outcome and has been associated with considerable increase in morbidity and increased mortality in some cases. These issues increase the overall cost of treatment and the length of stay in hospital.
Hyperglycemia may return to normal glycemia in prediabetic or nondiabetic patients once they recover from acute illness and may not require antidiabetic medications. However, long-term follow-up is the key in such cases. Important prognostic factors include early diagnosis, associated other comorbidities, interventions, and longer surveillance of patients with stress hyperglycemia and/or newonset diabetes so that we can ensure that their risk factors are managed and good glycemic control is achieved.
Every evening after supper the Beast came to see her, and always before saying good-night asked her in his terrible voice: Beauty, will you marry me? And it seemed to Beauty, now she understood him better, that when she said, No, Beast, he went away quite sad
The latest report of Diabetes Atlas from the International Diabetes Federation states that almost 50% of the adult population may have undiagnosed diabetes and bear a lifetime risk of diabetes mellitus[15].This potential at-risk population is the reason for the hike in incidence of new-onset diabetes after COVID-19. Probable causes for this are recent weight gain, worsening of hyperglycemia due to changes in lifestyle such as lack of exercise and reduced physical activity due to lockdown, self-isolation, social distancing and poor diet as a result of lack of access to sufficient quality and quantity of fruits/vegetables during lockdown periods[16].
Studies published in recent times assessed the findings of hospitalized COVID-19 patients. There are no or limited data available from patients who were asymptomatic or had mild disease managed in community COVID care centers or in home isolation. So, there is likely to be a greater number of cases of newly detected diabetes in COVID-19 patients worldwide. Hence, a large population of patients needs to be followed up globally to have better understanding of this phenomenon, involving an epidemiological and interventional approach.
ACKNOWLEDGMENTS
Authors wish to thank Mr. Vinod Jogdand and Mr. Dipak Badne from Dept. of Medical Education for their assistance in preparation of manuscript.
FOOTNOTES
Gavkare AM, Nanaware N and Rayate AS contributed to the literature search, collection of the data and writing the paper; Mumbre S and Nagoba BS contributed to the idea behind the manuscript, writing the paper, modification of content and final approval of the draft.
The authors declare no conflicts of interest.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
India
Ajay M Gavkare 0000-0003-4711-5596; Neeta Nanaware 0000-0002-3176-4930; Abhijit S Rayate 0000-0002-6183-7029; Sachin Mumbre 0000-0002-9169-6001; Basavraj S Nagoba 0000-0001-5625-3777.
Chang KL
6. Little grey- haired man: An old man was . . . regarded as the personification of the age-old wisdom of humanity or the collective unconscious (Cirlot 243).The Jack Zipes edition uses the term dwarf20 here (Complete 256). [see below for more]Return to place in story.
Kerr C
Today we buried our 20-year-old son. He was killed instantly in a motorcycle accident on Friday night. How I wish I had known when I talked to him last that it would be the last time. If I had only known I would have said, Jim, I love you and I m so very proud of you.
Chang KL
1 WHO. WHO coronavirus disease (COVID-19) dashboard. [cited 14 March 2022]. Available from:https://covid19.who.int/
2 Jivanji CJ, Asrani VM, Windsor JA, Petrov MS. New-Onset Diabetes After Acute and Critical Illness: A Systematic Review.
2017; 92: 762-773 [PMID: 28302323 DOI: 10.1016/j.mayocp.2016.12.020]
3 Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, Klonoff DC. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States.
2020; 14: 813-821 [PMID:32389027 DOI: 10.1177/1932296820924469]
4 Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, Qiu K, Zhang J, Zeng T, Chen L, Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19.
2020; 22: 1897-1906 [PMID: 32469464 DOI: 10.1111/dom.14099]
5 Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus.
2020; 164: 108166 [PMID: 32339533 DOI: 10.1016/j.diabres.2020.108166]
6 Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, Yu X, Zhang S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19.
2020; 19: 58 [PMID: 32393351 DOI:10.1186/s12933-020-01035-2]
7 Farag AA, Hassanin HM, Soliman HH, Sallam A, Sediq AM, Abd Elbaser ES, Elbanna K. Newly Diagnosed Diabetes in Patients with COVID-19: Different Types and Short-Term Outcomes.
2021; 6 [PMID: 34449740 DOI: 10.3390/tropicalmed6030142]
8 Unnikrishnan R, Misra A. Diabetes and COVID19: a bidirectional relationship.
2021; 11: 21 [PMID:34168110 DOI: 10.1038/s41387-021-00163-2]
9 Metwally AA, Mehta P, Johnson BS, Nagarjuna A, Snyder MP. COVID-19-Induced New-Onset Diabetes: Trends and Technologies.
2021; 70: 2733-2744 [PMID: 34686519 DOI: 10.2337/dbi21-0029]
10 Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, Weil T, Koepke L, Bozzo CP, Read C, Fois G, Eiseler T, Gehrmann J, van Vuuren J, Wessbecher IM, Frick M, Costa IG, Breunig M, Grüner B, Peters L, Schuster M, Liebau S,Seufferlein T, Stenger S, Stenzinger A, MacDonald PE, Kirchhoff F, Sparrer KMJ, Walther P, Lickert H, Barth TFE,Wagner M, Münch J, Heller S, Kleger A. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas.
2021; 3: 149-165 [PMID: 33536639 DOI: 10.1038/s42255-021-00347-1]
11 Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes.
2010; 47: 193-199 [PMID: 19333547 DOI: 10.1007/s00592-009-0109-4]
12 Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19.
2020; 14: 2211-2217 [PMID: 33395782 DOI: 10.1016/j.dsx.2020.11.012]
13 Papachristou S, Stamatiou I, Stoian AP, Papanas N. New-Onset Diabetes in COVID-19: Time to Frame Its Fearful Symmetry.
2021; 12: 461-464 [PMID: 33367980 DOI: 10.1007/s13300-020-00988-7]
14 RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N,Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19.
2021; 384: 693-704 [PMID: 32678530 DOI:10.1056/NEJMoa2021436]
15 Diabetes now affects one in 10 adults worldwide. Nov 2, 2021 [cited 14 March 2022]. Available from:https://www.idf.org/news/240:diabetes-now-affects-one-in-10-adults-worldwide.html
16 Deschasaux-Tanguy M, Druesne-Pecollo N, Esseddik Y, de Edelenyi FS, Allès B, Andreeva VA, Baudry J, Charreire H,Deschamps V, Egnell M, Fezeu LK, Galan P, Julia C, Kesse-Guyot E, Latino-Martel P, Oppert JM, Péneau S, Verdot C,Hercberg S, Touvier M. Diet and physical activity during the coronavirus disease 2019 (COVID-19) lockdown (March-May 2020): results from the French NutriNet-Santé cohort study.
2021; 113: 924-938 [PMID: 33675635 DOI: 10.1093/ajcn/nqaa336]
17 Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, Sun GZ, Yang GR, Zhang XL, Wang L, Xu X, Xu XP, Chan JC.Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS.
2006; 23: 623-628 [PMID: 16759303 DOI: 10.1111/j.1464-5491.2006.01861.x]
18 Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.
2000; 355: 773-778 [PMID: 10711923 DOI: 10.1016/S0140-6736(99)08415-9]
19 Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, Kwong RMW, Kumar P, Logan KM. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the U.K.
2020; 43: e170-e171 [PMID: 32816997 DOI: 10.2337/dc20-1551]
20 Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 Diabetes and COVID-19: Preliminary Findings From a Multicenter Surveillance Study in the U.S.
2020; 43: e83-e85 [PMID: 32503837 DOI: 10.2337/dc20-1088]
21 Armeni E, Aziz U, Qamar S, Nasir S, Nethaji C, Negus R, Murch N, Beynon HC, Bouloux P, Rosenthal M, Khan S,Yousseif A, Menon R, Karra E. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series.
2020; 8: 660-663 [PMID: 32621809 DOI: 10.1016/S2213-8587(20)30221-7]
22 Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis.
2021; 23: 870-874 [PMID: 33245182 DOI:10.1111/dom.14269]
23 Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, Xu J, Wu F, Duan L, Yin Z, Luo H, Xiong N, Xu M, Zeng T, Jin Y.Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study.
2020; 63: 2102-2111 [PMID: 32647915 DOI: 10.1007/s00125-020-05209-1]
24 Yang J-K, Jin J-M, Liu S, Bai P, He W, Wu F, Liu X-F, Chai Z-L, Han D-M. New onset COVID-19–related diabetes: an indicator of mortality. 2020 Preprint. Available from: medRxiv: 2020.04.08.20058040 [DOI:10.1101/2020.04.08.20058040]
25 Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, Bonora BM, Selmin E, Arcidiacono G, Pinelli S,Farnia F, Falaguasta D, Russo L, Voltan G, Mazzocut S, Costantini G, Ghirardini F, Tresso S, Cattelan AM, Vianello A,Avogaro A, Vettor R. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration.
2020; 168: 108374 [PMID: 32805345 DOI:10.1016/j.diabres.2020.108374]
26 Wu L, Girgis CM, Cheung NW. COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients.
2020; 93: 390-393 [PMID: 32683745 DOI: 10.1111/cen.14288]
27 Ghosh A, Anjana RM, Shanthi Rani CS, Jeba Rani S, Gupta R, Jha A, Gupta V, Kuchay MS, Luthra A, Durrani S, Dutta K,Tyagi K, Unnikrishnan R, Srivastava BK, Ramu M, Sastry NG, Gupta PK, Umasankari G, Jayashri R, Mohan V, Misra A.Glycemic parameters in patients with new-onset diabetes during COVID-19 pandemic are more severe than in patients with new-onset diabetes before the pandemic: NOD COVID India Study.
2021; 15: 215-220 [PMID:33450530 DOI: 10.1016/j.dsx.2020.12.033]
28 Zhang J, Kong W, Xia P, Xu Y, Li L, Li Q, Yang L, Wei Q, Wang H, Li H, Zheng J, Sun H, Xia W, Liu G, Zhong X, Qiu K, Li Y, Wang Y, Song X, Liu H, Xiong S, Liu Y, Cui Z, Hu Y, Chen L, Pan A, Zeng T. Impaired Fasting Glucose and Diabetes Are Related to Higher Risks of Complications and Mortality Among Patients With Coronavirus Disease 2019.
2020; 11: 525 [PMID: 32754119 DOI: 10.3389/fendo.2020.00525]
29 Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, Dobesh DP, Brufsky A, Connor RI. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19.
2021; 93: 409-415 [PMID: 32589756 DOI: 10.1002/jmv.26227]
30 Liu Y, Lu R, Wang J, Cheng Q, Zhang R, Zhang S, Le Y, Wang H, Xiao W, Gao H, Zeng L, Hong T. Diabetes, even newly defined by HbA1c testing, is associated with an increased risk of in-hospital death in adults with COVID-19.
2021; 21: 56 [PMID: 33771154 DOI: 10.1186/s12902-021-00717-6]
31 Barbu MG, Thompson RJ, Thompson DC, Cretoiu D, Suciu N. The Impact of SARS-CoV-2 on the Most Common Comorbidities-A Retrospective Study on 814 COVID-19 Deaths in Romania.
2020; 7: 567199[PMID: 33015111 DOI: 10.3389/fmed.2020.567199]
32 Erener S. Diabetes, infection risk and COVID-19.
2020; 39: 101044 [PMID: 32585364 DOI:10.1016/j.molmet.2020.101044]
33 Selvin E, Juraschek SP. Diabetes Epidemiology in the COVID-19 Pandemic.
2020; 43: 1690-1694 [PMID:32540920 DOI: 10.2337/dc20-1295]
34 Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations.
2020; 14: 303-310 [PMID: 32298981 DOI: 10.1016/j.dsx.2020.04.004]
35 Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ,Young B, Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a wholepopulation study.
2020; 8: 813-822 [PMID: 32798472 DOI:10.1016/S2213-8587(20)30272-2]
36 Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI.Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study.
2020; 369: m1966 [PMID: 32444366 DOI: 10.1136/bmj.m1966]
37 Al-Sabah S, Al-Haddad M, Al-Youha S, Jamal M, Almazeedi S. COVID-19: Impact of obesity and diabetes on disease severity.
2020; 10: e12414 [PMID: 33079448 DOI: 10.1111/cob.12414]
38 Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, He Q, Wang Z, Liu Y, Liu L, Chen J, Xu L. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China.
2020; 43: 1392-1398 [PMID: 32409502 DOI:10.2337/dc20-0576]
39 Sobngwi E, Choukem SP, Agbalika F, Blondeau B, Fetita LS, Lebbe C, Thiam D, Cattan P, Larghero J, Foufelle F, Ferre P, Vexiau P, Calvo F, Gautier JF. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-saharan africans.
2008; 299: 2770-2776 [PMID: 18560004 DOI: 10.1001/jama.299.23.2770]
40 Zhang Y, Li H, Zhang J, Cao Y, Zhao X, Yu N, Gao Y, Ma J, Zhang H, Guo X, Liu X. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: A single-centre,retrospective, observational study in Wuhan.
2020; 22: 1443-1454 [PMID: 32406594 DOI:10.1111/dom.14086]
41 Ibrahim S, Monaco GSF, Sims EK. Not so sweet and simple: impacts of SARS-CoV-2 on the β cell.
2021; 13: 66-79 [PMID: 33970787 DOI: 10.1080/19382014.2021.1909970]
42 Drucker DJ. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning.
2021; 33:479-498 [PMID: 33529600 DOI: 10.1016/j.cmet.2021.01.016]
43 Coppelli A, Giannarelli R, Aragona M, Penno G, Falcone M, Tiseo G, Ghiadoni L, Barbieri G, Monzani F, Virdis A,Menichetti F, Del Prato S; Pisa COVID-19 Study Group. Hyperglycemia at Hospital Admission Is Associated With Severity of the Prognosis in Patients Hospitalized for COVID-19: The Pisa COVID-19 Study.
2020; 43:2345-2348 [PMID: 32788285 DOI: 10.2337/dc20-1380]
44 Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, Boehm B, Amiel S, Holt RI, Skyler JS,DeVries JH, Renard E, Eckel RH, Zimmet P, Alberti KG, Vidal J, Geloneze B, Chan JC, Ji L, Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19.
2020; 8: 546-550 [PMID: 32334646 DOI: 10.1016/S2213-8587(20)30152-2]
45 Kuchay MS, Reddy PK, Gagneja S, Mathew A, Mishra SK. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19.
2020; 14: 2039-2041 [PMID:33113470 DOI: 10.1016/j.dsx.2020.10.015]
46 Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus.
2019; 139: 2022-2031 [PMID: 30786725 DOI:10.1161/CIRCULATIONAHA.118.038868]
47 Kosiborod MN, Esterline R, Furtado RHM, Oscarsson J, Gasparyan SB, Koch GG, Martinez F, Mukhtar O, Verma S,Chopra V, Buenconsejo J, Langkilde AM, Ambery P, Tang F, Gosch K, Windsor SL, Akin EE, Soares RVP, Moia DDF,Aboudara M, Hoffmann Filho CR, Feitosa ADM, Fonseca A, Garla V, Gordon RA, Javaheri A, Jaeger CP, Leaes PE,Nassif M, Pursley M, Silveira FS, Barroso WKS, Lazcano Soto JR, Nigro Maia L, Berwanger O. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled,phase 3 trial.
2021; 9: 586-594 [PMID: 34302745 DOI: 10.1016/S2213-8587(21)00180-7]
48 Effects of DPP4 inhibition on COVID-19. [Accessed 2022 Feb 28]. In: ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine. Available from https://clinicaltrials.gov/ct2/show/NCT04341935 ClinicalTrials.gov identifier NCT04341935
49 Efficacy and safety of dipeptidyl peptidase-4 inhibitors in diabetic patients with established COVID-19. [Accessed 2022 Feb 28]. In: ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine. Available from https://clinicaltrials.gov/ct2/show/NCT04371978 ClinicalTrials.gov identifier NCT04371978
50 The effect of sitagliptin treatment in COVID-19 positive diabetic patients (SIDIACO). [Accessed 2022 Feb 28]. In:ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine. Available from https://clinicaltrials.gov/ct2/show/NCT04365517 ClinicalTrials.gov identifier NCT04365517
51 Metformin glycinate in patients with MS or DM2, hospitalized with COVID-19 and SARS secondary to SARS-CoV-2(DMMETCOV19). [Accessed 2022 Feb 28]. In: ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine.Available from https://clinicaltrials.gov/ct2/show/NCT04626089 ClinicalTrials.gov identifier NCT04626089
52 Effect of pioglitazone on T2DM patients with COVID-19 (PIOQ8). [Accessed 2022 Feb 28]. In: ClinicalTrials.gov.Bethesda (MD): U.S. National Library of Medicine. Available from https://clinicaltrials.gov/ct2/show/NCT04604223 ClinicalTrials.gov identifier NCT04604223
53 Semaglutide to reduce myocardial injury in patients with COVID-19 (SEMPATICO). [Accessed 2022 Feb 28]. In:ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine. Available from https://clinicaltrials.gov/ct2/show/NCT04615871 ClinicalTrials.gov identifier NCT04615871
杂志排行
World Journal of Diabetes的其它文章
- Different nutrient compositions in diet and taking hypoglycemic drugs can modulate gut microbial flora
- Mapping the global research landscape on insulin resistance:Visualization and bibliometric analysis
- Role of insulin in pancreatic microcirculatory oxygen profile and bioenergetics
- Relationship between age of pregnant women with gestational diabetes mellitus and mode of delivery and neonatal Apgar score
- Hyperglycemia and reduced adiposity of streptozotocin-induced diabetic mice are not alleviated by oral benzylamine supplementation
- Effectiveness and safety of COVID-19 vaccines in patients with diabetes as a factor for vaccine hesitancy
