Potential role of Limosilactobacillus fermentum as a probiotic with anti-diabetic properties: A review
2022-09-16DiegoCabralLacerdaPaulosarTrindadedaCostaPaulaBriellePontesLucasAlvesCarneirodosSantosJosPatrocnioRibeiroCruzNetoCristianeCosmoSilvaLuisVanessaPolyanadeSousaBritoJosLuizdeBritoAlves
INTRODUCTION
Diabetes mellitus (DM) is a chronic non-communicable disease that affects millions of people and has become one of the leading causes of death worldwide[1,2]. The Diabetes Atlas published by the International Diabetes Federation estimated that 537 million adults worldwide had type DM in 2021[3].Associated to this prevalence, clinical management of DM has elevated the costs of the health system,increasing by 316% in the last 15 years[3,4]. One of the etiological factors of this metabolic disorder includes long-term inappropriate diet such as regular consumption of sugary drinks, red meat, and low consumption of whole grains and fiber. In addition, smoking, physical inactivity, history of gestational diabetes or delivery of newborns > 4 kg weight, medications such as statins, thiazides, and betablockers, psychosocial stress, and depression have been described as risk factors for DM[5-7].
Clinical features and laboratory findings of DM include changes in body weight, increased blood glucose, insulin resistance, development of lipid metabolism disorder, polyuria, polydipsia, visual disturbances, ketoacidosis, and hyperosmolar non-ketoacidotic syndrome with risk of coma[8,9]. When uncontrolled, diabetes can induce grave complications, including death[10]. Insulin resistance in sensitive tissues such as liver, muscle, and adipose tissue and β-cell dysfunctions are the main factors involved in initiating and progressing the pathophysiology of type 2 DM[5,11]. Moreover, it has been reported that gut microbiota (GM) impairment plays a crucial role in developing DM[12].
DM patients show an altered intestinal microbiota resulting from an increase in opportunistic bacteria and Gram-negative toxin-producing bacteria that alter metabolism energetic[13]. Furthermore, the accumulation of gut-derived pro-inflammatory molecules, including lipopolysaccharide (LPS),peptidoglycans, and flagellin, appear to accelerate the inflammatory response in patients with DM[14].Deregulation of the GM, also called dysbiosis, promotes intestinal permeability and energy homeostasis changes, causing metabolic endotoxemia, inflammation, hyperglycemia, and hyperlipidemia[15,16].Dysbiosis impairs the integrity of the intestinal wall and allows the translocation of toxins from the intestinal lumen into the systemic circulation, promoting inflammation, autoimmunity, and oxidative stress that can lead to β-cell destruction or insulin resistance[17,18].
The findings involving the association between gut dysbiosis and DM reinforce the importance of gut-targeting approaches in the treatment of DM[19-21] Strategies capable of recovering the community of commensal GM and controlling DM have recently increased. Probiotic therapy has begun to be used to improve GM composition and management of DM[22,23]. Given this scenario, the identification of new potentially-probiotic strains with anti-diabetic properties is essential for the development of new probiotic products and testing in well-controlled trials.
Many knights went to battle and many knights were hurt as the dragon moved closer and closer to the castle. The King declared whosoever killed the dragon would be granted half his kingdom. Now knights came from across the sea. They were the most fierce, the bravest and the biggest knights anyone had ever seen. A thousand of them gathered to attack the dragon.
He never allowed the boy himself to go near the water. Beware! he said to him. If you touch the water a hand will appear, take hold of you, and pull you under.
Among
species, strains of
(
) has been reported to exert probiotic properties due to its ability to improve GM composition, reduce blood cholesterol,modulate the intestinal immune system, stimulate the release of immunoglobulin A, reduce intestinal inflammation, and increase the activity of antioxidant enzymes[24-27]. Although early studies have identified anti-diabetic properties in some
strains, an in-depth review focusing on
strains as a potential anti-diabetic has not been found in the available literature to the time of this writing[28,29].
The King couldn t refuse his only daughter. He rose from his throne and knighted the blacksmith. Then, for luck, the Princess unwound her long braid, pulled out a single hair and handed it to the littlest knight. He placed it in a pocket over his heart. May you have good fortune, my brave knight, she said.
This present literature review focuses on the emerging findings of experimental and clinical studies that have used
supplementation to prevent or treat complications of DM. To investigate the effectiveness of
more thoroughly, we focus on the type of strain, source of probiotics,dosage, duration of treatment, and the primary outcomes reported.
is a Gram-positive, rod- or coccoid-shaped, heterofermentative, and anaerobic or aerotolerant bacteria found in fermented cereals and other fermenting plant materials, dairy products,manure sewage and feces, and the human vagina[41]. The
genus is widely used as an intestinal modulator due to its safety and probiotic activity[40]. Among these bacterial groups,
is a well-studied species, mainly due to its action in improving metabolic function and oxidative stress, which may be considered for DM management[26,27,42].
PROBIOTIC THERAPY IN THE TREATMENT OF METABOLIC DISORDERS
Probiotics are live microorganisms that confer host health benefits when administrated adequately.Probiotics have significant importance in the industrial economy and are among the most consumed food supplements worldwide[30]. Experimental studies and clinical trials have documented that probiotics can modulate the GM, inducing beneficial effects and increasing overall wellness[28,31,32].Over the last few years, studies on probiotics have been growing sharply due to their beneficial health effects, which have been used as adjuvant therapy for metabolic disorders[33]. A number of preclinical and clinical studies have investigated the effectiveness of probiotics by evaluating the intestinal microbiota after probiotics use, showing promising results in treating metabolic diseases[31].
Recently, an Indian research group analyzed the activity of the probiotic
MCC2759 and MCC2760 on intestinal markers of inflammation using a high-fat diet model associated with the STZinduced diabetic model[48]. Both
strains were administered in a concentration of 1 × 10
CFU/mL for 4 wk. The main findings of the study revealed that diabetic female rats treated with
MCC2759 and MCC2760 reduced blood glucose levels, increased insulin levels, and improved the lipid profile[48]. Coupled with biochemical changes,
administration downregulated TNF-α mRNA and up-regulated mRNA IL-10 in the intestine, liver, mesenteric adipose tissue, and muscle, suggesting that the anti-diabetic effect promoted by
MCC2759 and MCC2760 can be associated with a decrease in inflammatory markers[48]. In addition,
MCC2759 and MCC2760 administration modulated other gene expressions, such as reduced expression of Toll-like receptor 4, enhanced expression of tight junction protein ZO-1, endocannabinoid receptor CB2 and GLP1, glucose transporter type 4 in mesenteric adipose tissue and muscle tissue. The results demonstrated that
MCC2759 and MCC2760 might be a potential probiotic in treating type 2 DM.
Impairment in commensal homeostasis of GM and intestinal functional capacity, called gut dysbiosis,is associated with the development of metabolic diseases such as colitis, obesity, liver, obesity, and DM[34]. Thus, a probiotic may be able to relieve GM dysbiosis, through various mechanisms including improvement in the composition and diversity of the GM, induction of immunomodulation, protection against physiological stress, and pathogen suppression[35]. Probiotics also promote health benefits to the host through other mechanisms of action, such as the production of organic acids, including lactic acid and short-chain fatty acids (SCFA) (mainly acetate, propionate, and butyrate)[36,37]. Another mechanism reported is the capacity of probiotics to protect the integrity of the intestinal wall by stimulating mucin production and upregulating tight-junction claudin, occludin, and zonulin protein expression[37]. Furthermore, probiotics are also responsible for producing small molecules with systemic effects essential for maintaining vital functions, such as cortisol, serotonin, gammaaminobutyric acid (GABA), tryptophan, histamine derivatives, satiety hormones, and conjugated linoleic acid[37].
Coupled with the mechanisms mentioned above, some experimental and clinical evidence has demonstrated that probiotics have anti-inflammatory and antioxidant properties[27,37,38]. This antioxidant capacity results from signaling pathways that produce antioxidant enzymes and molecules,reducing serum and tissues levels of oxidative stress[38,39]. Concerning their anti-inflammatory properties, probiotics have been reported to reduce inflammatory markers, including LPS, tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, as well as to promote an increase of anti-inflammatory markers, such as IL- 10.
Later that evening, I began pulling weeds from around my lopsided azalea bush. As my mind wandered back to the love Jonathan showed the little girl, a biblical verse came to me: ...these three remain: faith, hope and love. But the greatest of these is love. While my son had put love into practice, I had only felt anger.
LIMOSILACTOBACILLUS FERMENTUM, LEAKY GUT AND DIABETES MELLITUS
Most diabetes treatments, particularly drug therapies, use agents that act directly on signaling pathways to regulate glucose. Because of this, it is pertinent to explore therapies that adjunctively attenuate deregulation of GM, such as probiotics[44]. Among the main harmful effects in GM induced by DM, gram-negative bacteria in the colon increase the concentration of LPS in the lumen. LPS causes high production of free radicals, increasing intestinal permeability and generating a systemic chronic inflammatory process. This pro-inflammatory state is a critical mechanism in the genesis of chronic diseases, such as DM[38,40]. Additionally, GM imbalances observed in DM patients are characterized by changes in the composition of SCFAs, including increasing acetate levels and decreasing butyrate production. As a consequence, there may be acetate excess and reduction of butyrate, caused by dysbiosis, and impaired blood glucose homeostasis[45].
12 Then he pulled the skin off the bear13 and said, This shall be thy cloak, and thy bed also, for thereon shalt thou sleep, and in no other bed shalt thou lie, and because of this apparel shalt thou be called Bearskin
DM is one of the main metabolic diseases related to leaky gut, oxidative stress, and chronic inflammation. GM impairment has been described in the pathogenesis of DM and metabolic syndrome. Due to the high mortality rate of patients with of DM and this direct relationship with intestinal health, the number of studies involving probiotic therapy has increased in recent years.
has been proven to alleviate metabolic disorder-related symptoms, including improvement in glucose and insulin levels, control of the lipid profile, to decrease in pro-inflammatory cytokines and to increase antioxidant capacity[27,41,43]. However, these protective responses need to be further investigated in clinical studies to elucidate the responsiveness of
therapy in DM patients.
Probiotics have shown satisfactory results as an adjunct treatment in DM[40,42]. Both single strain,combined with other foods, and multiple strain probiotics can be used as supplements. Among more effective probiotic strains, the therapeutic potential of
has been investigated for adjuvant management of DM[40,48,55].
On the other hand,
manipulation could attenuate GM imbalance, which may decrease DM complications. Considering the inversely proportional relationship between butyrate and acetate levels and the effects of excess acetate on the worsening of DM, keeping these fatty acids in balance becomes an important way to assist glycemic control[45]. Increased butyrate production by
regulates acetate production, preventing increased hepatic gluconeogenesis and insulin resistance.Additionally, the increase in butyrate production resulting from
supplementation may repair enterocyte tight junctions and improve intestinal permeability[46]. Experimental evidence has revealed that increasing levels of SCFA, especially acetate and succinate, decreases cellular damage of enterocytes, leading to a reduction in inflammation state, oxidative stress, and leaky gut in DM-induced rodents[28].
Another antidiabetic property of
is to maintain normal levels of the intestinal hormone GLP-1[47]. GLP-1 has been shown to stimulate proliferation and prevent apoptosis of pancreatic beta cells, upregulating insulin synthesis and promoting a reasonable glycemic control[29,36]. In the liver,GLP1 decreases gluconeogenesis and stimulates glycolysis, contributing to reducing glycemic levels in individuals with DM. The main consequence of reducing these peptides is the exacerbation of hunger,the search for palatable food, and the preference for hypercaloric foods, which can be a predisposing factor for developing obesity and insulin resistance[30]. Leaky gut also generates chronic low-grade inflammation in organs such as the liver, skeletal muscle, and adipose tissue, causing metabolic changes such as hyperglycemia and dyslipidemias.
also promotes benefits in these organs because it stimulates the synthesis of the fasting-induced adipose factor, a protein that regulates the function of the LPL enzyme and prevents hepatic steatosis and dyslipidemia, common in diabetic subjects[48].Therefore, it is suggested that
may improve intestinal permeability, normalize GLP-1 Levels, and reduce DM complications.
Another important action of
is to reduce oxidative stress and glycation. Studies indicate that pathophysiological findings of DM, including macular degeneration, vascular endothelial injury,hepatic fibrosis, renal failure, are related to the glycation process. This process occurs when circulating glucose binds to proteins, inactivating them and increasing inflammatory cytokines such as interferongamma (IFN-γ), IL-6, and IL-4. The main biochemical marker for glycation is glycated hemoglobin(HB1ac), but this process can occur with any protein, including antioxidant enzymes. When glycation events occur more expressively, oxidative stress is even higher due to the increase in reactive oxygen species (ROS) and inactivation of the enzymatic antioxidant systems, such as superoxide dismutase(SOD) and glutathione peroxidase[40,49].
The authors are grateful to the Fundação de Apoio à Pesquisa do Estado da Paraíba (FAPESQ, Brazil)and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarships awarded to Lacerda DC, Trindade da Costa PC, Pontes PB, Carneiro dos Santos LA, Cruz Neto JPR,Silva Luis CC. Additionally, the authors give thanks for the research productivity fellowship granted by the Brazilian National Council for Scientifc and Technological Development (CNPq) to de Brito Alves,JL.
To complete the evaluation of the therapeutic potential of
MF423, the authors investigated the role of this probiotic in the modulation of GM. Diabetic rats treated either to high dose of defatted rice fermented by
or pioglizatone showed GM composition similar to the control group, compared to untreated diabetic animals[31]. The relative abundances of
(20%) and
(40%) were increased in both mentioned groups compared to a diabetic group without treatment[31]. A decreased abundance of
can be found in diabetic patients compared to their non-diabetic counterparts[53]. These two major phyla may play an essential role in hyperglycemia,hyperlipidemia, and inflammation. Moreover, probiotic treatment increased the relative abundance of SCFA-producing bacteria in diabetic mice, including
_group, and
. Interestingly, a decrease in the genus
was significant in diabetic mice, while treatment with defatted rice bran fermented by
MF423 increased its abundance, similar to control mice[31].
is known for its probiotic role in food consumption, which could modify abnormalities in intestinal microbes and retard hyperglycemia. In conclusion, defatted rice bran fermentation by
MF423 Lessened damage to the structure and function of GM induced by type 2 DM.
ANTI-DIABETIC PROPERTIES OF DIFFERENT STRAINS OF LIMOSILACTOBACILLUS FERMENTUM
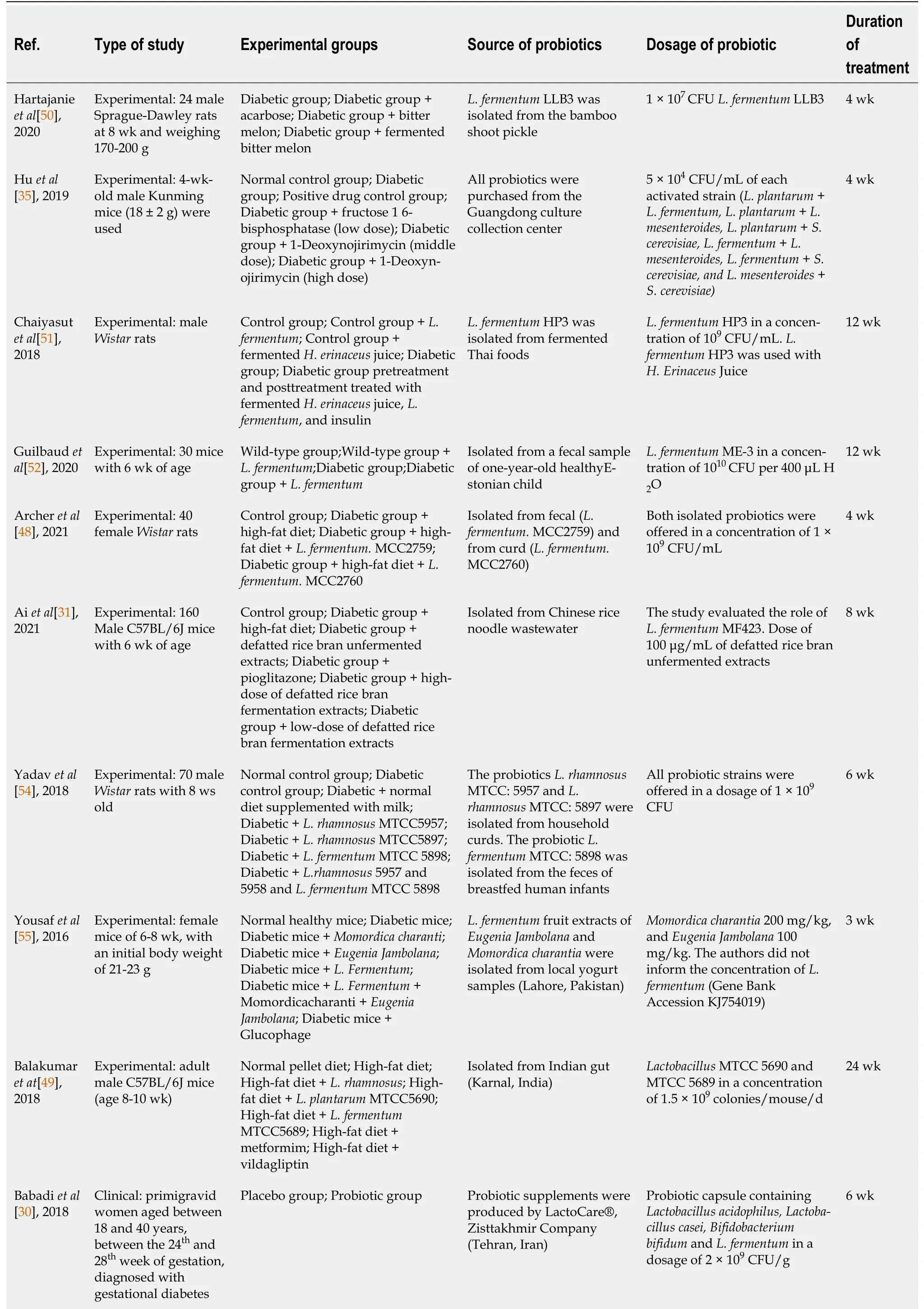
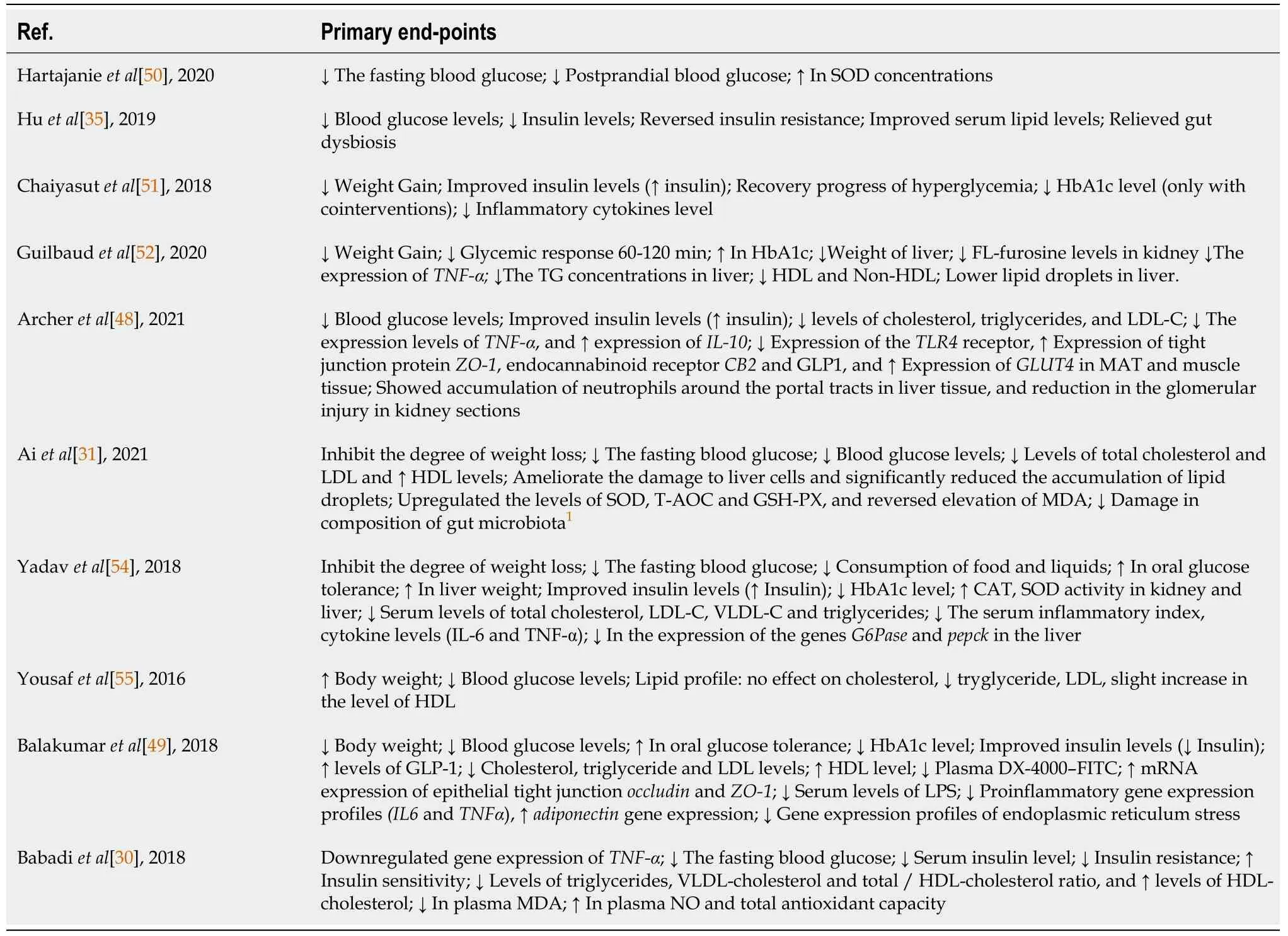
We investigated studies that analyzed the role of
administered singly or combined with other therapies to alleviate DM complications. Among ten of the studies included, nine evaluated antidiabetic properties in experimental studies using rats or mice. Only one clinical study assessed the antidiabetic potential of probiotic intervention in women with gestational DM. Since the majority of beneficial effects following administration of
come from animal studies, this present review investigated emerging findings of their potential role in DM management. The characteristics of the studies and the primary outcomes are summarized in Table 1 and Table 2, respectively.

L. fermentum LLB3
An experimental study revealed that treatment with
LLB3 isolated from the bamboo shoot pickle and offered in fermented bitter melon (
), in a concentration of 1 × 10
CFU during 4 wk, reduced fasting glucose and postprandial blood glucose levels and increased SOD enzyme activity in rats subjected to type 2 DM induced by streptozotocin (STZ)[50]. This suggests that
LLB3 might be considered an adjuvant therapy to attenuate type 2 DM-related symptoms[50].
L. fermentum HP3
Administration of a fermented
juice containing 10
CFU/mL of
HP3 for 12 wk reduced weight gain, increased insulin level, and reduced hyperglycemia in diabetic mice induced by STZ[51]. In addition, treated mice showed lower levels of inflammatory cytokines, including IL-6, IL-17, and IFN-γ[51], suggesting that fermented
juice can be used as nutritional manipulation in the treatment of type 2 DM.
L. fermentum ME-3
A previous preclinical study investigated the anti-diabetic effect of
ME-3 in genetically diabetic mice[52].
ME-3 was administered in mice at 6 wk of age, in a concentration of 10
CFU, for 12 wk[52]. The treatment with
ME-3 reduced body weight, inhibited expression of TNF-α, but did not improve glycemic control[52]. In addition, supplementation with
ME-3 reduced the formation of glycation products, including FL-furosine levels in the kidney. However, the researchers found an increase in HbA1c, another marker of early glycation. The authors noted that while HbA1c reflects early glycation mainly in red blood cells, FL-furosine provides information on the extent of early glycation in fluids, tissues, and organs and offers a broader view of the early glycation status of the whole organism[52]. In summary,
ME-3 has the therapeutic potential to reduce the formation of some glycation products in kidneys and attenuate some typical type 2 DMrelated symptoms.
L. fermentum MCC2759 and MCC2760
There were splendid dresses fit for a queen, with all the ornaments49 that were to be worn with them; and when Beauty opened the cupboards she was quite dazzled by the gorgeous jewels that lay in heaps upon every shelf
At the concert, I sat with Aaron and his girlfriend in the third row, stuffing cotton in my ears to block out the loud, ear-splitting amplified4 music of the first performer. I stood when the kids stood, clapped when they clapped, and never let anyone know how nervous I was to feel the floor vibrate beneath my feet. Aaron and his friends were amused at my enthusiasm.
L. fermentum MF423
MF423 is a strain isolated from Chinese rice noodle wastewater[31]. The authors analyzed adverse effects triggered by an experimental model of type 2 DM induced by STZ and tested the effectiveness of different therapies, including supplementation with unfermented extracts of defatted rice bran, high and low doses of defatted rice bran fermented by
MF423, and drug intervention(pioglitazone)[31]. Mice receiving a high dose (1 g/kg) of defatted rice bran fermentation extracts containing
MF423 for 8 wk evidenced weight loss and reduced fasting blood glucose, lipid accumulation, and liver cells damage[31]. Moreover, probiotic groups intensified antioxidant activity in diabetic mice through up-regulation levels of SOD, total antioxidant capacity (T-AOC), and reversed elevation of malondialdehyde (MDA) in the liver[31]. It is important to mention that no effects were found in animals treated only by unfermented extracts of rice bran, highlighting the antioxidant activity of
MF423.
To evaluate the effectiveness of
the following sections refer to the findings on the antidiabetic properties of different strains of
investigated in preclinical and clinical studies.
L. fermentum MTCC: 5898
Probiotic fermented milk prepared using different probiotic strains, including
MTCC: 5957,
MTCC: 5897, and
MTCC: 5898, were evaluated in an experimental study[54].Probiotic strains were offered independently or in combination for treating STZ induced type 1 DM in male
rats. All probiotic strains were provided in a dosage of 1 × 10
CFU for 6 wk. The study demonstrated that the diabetic rats who received fermented milk containing
MTCC: 5898 had less weight loss, improved glucose metabolism by reducing fasting blood glucose, HbA1c associated with increased insulin level, reduced diabetic dyslipidemia, and attenuated inflammation status through reduction of IL-6 and TNF-α[54]. In addition, supplementation with
MTCC:5898 showed antioxidant properties by increasing catalase (CAT) and SOD activities in the kidney and liver[54]. Moreover, administration of probiotics reduced mRNA expression of phosphoenolpyruvate carboxykinase and Glucose 6-phosphatase genes that code the key enzymes of the gluconeogenesis pathway[54]. Compared to other lactobacilli strains, rats receiving
MTCC: 5898 displayed the most effective responses including oral glucose tolerance, serum insulin, serum, liver CAT, serum triglycerides, VLDL[54]. Therefore, it is suggested that daily consumption of probiotic fermented milk,especially
MTCC: 5898, may be effective in attenuating complications of type 1 DM.
L. fermentum MTCC 5690 and MTCC 5689
MTCC 5690 and MTCC 5689 were isolated from the Indian gut and used to treat high-fat diet-induced type 2 DM mice[49]. The present study compared the anti-diabetic effect of
MTCC 5690 and MTCC 5689 to other probiotics (
,
MTCC5690) and drug intervention (metformin, vildagliptin).
MTCC 5690 and MTCC 5689 were administered in a concentration of 1.5 × 10
colonies/mouse/day for 24 wk[49]. Both probiotics and drugs groups reduced body weight, improved oral glucose tolerance, and reduced fasting glucose and Hb1Ac levels in diabetic mice[49]. Concerning insulin levels, probiotic groups contributed to normalizing levels of this hormone, which approximated the levels observed in the control group[49]. In addition, a significant reduction of insulin levels was found in the vildagliptin group compared with other groups, which may be considered a possible adverse mild effect of this drug. Furthermore, all treatment groups improved lipid profile by reduction of levels of cholesterol, triglycerides, LDL,associated with an increase in HDL levels.
Additionally, the study evaluated the gut integrity after 24 wk of treatment. Probiotic treatment and drug therapy were able to reduce damage in gut integrity, which may contribute to normalizing gut permeability[49]. The authors quantified mRNA expression of epithelial tight junction occludin and ZO-1 and LPS levels. All the probiotic and anti-diabetic drugs increased gene expression of the intestinal tight junction occludin and ZO-1[49]. To evaluate endotoxemia state and intestinal barrier integrity,probiotic treatments decreased LPS levels and pro-inflammatory cytokines IL-6 and TNF-α in mice subjected to type 2 DM[49]. In addition, the authors verified the effectiveness of treatments in attenuating endoplasmic reticulum stress of skeletal muscle of diabetic mice. Results showed that both probiotic and drug therapies reduced endoplasmic reticulum stress markers[49]. The findings suggested that
MTCC 5690 and MTCC 5689 act like anti-diabetic drugs, highlighting the therapeutic potential of these probiotic strains in alleviating type 2 DM complications.
Non mentioned strain of L. fermentum
The role of
fruit extracts of
and
, isolated from yogurt samples (Pakistan) on STZ induced DM mice, was previously investigated[55].
and the extracts were administered individually as well as in combination with DM-induced mice for 3 wk.Results were compared with mice that received drug intervention (Glucophage). Administration of probiotics and natural extracts improved body weight, and reduced blood glucose levels and both results were similar with the Glucophage group[55]. Concerning lipid profile,
and natural extracts improved almost all lipid profile parameters, including reduced triglycerides, LDL, and increased HDL serum levels[55]. The study demonstrated that Glucophage treatment might affect some parameters, such as increased total cholesterol, triglycerides, and LDL concentration. These findings showed that
and natural extracts have hypoglycemic and hypolipidemic activity, which may reduce DM complications.
“I think she’s perfect,” said Mother. “Baby Jesus probably didn’t have much hair when he was born either, and I bet he’d like to be represented by a doll who’s had so many hugs.”
Another experimental study demonstrated that mixed probiotics containing
could reverse insulin resistance, reduce blood glucose levels, and improve lipid profile in STZ induced DM in old male Kunming mice after 4 wk of treatment[35]. In addition, the authors showed a significant impact of the supplementation of
on the relief of gut dysbiosis, lowering the damage in the composition of GM[35]. However, the authors did not specify which strain of
was administered, limiting the understanding of these effects.
Regarding clinical data, we found only one randomized, double-blind, placebo-controlled trial that evaluated the effectiveness of
in attenuating complications of DM[30]. This study was carried out to assess the effects of probiotic supplementation on genetic and metabolic profiles in patients with gestational DM, aged 18-40 years (at weeks 24-28 of gestation)[30]. Participants were randomly divided into two groups: a control group and a probiotic group, made up of women who received a probiotic capsule containing
(2 × 10
CFU/g each) for 6 wk[30]. However, the authors did not inform the specific strain of
used, limiting the interpretation of results. The probiotic group showed lower levels of fasting blood glucose serum insulin, reduced insulin resistance, and significantly increased insulin sensitivity compared with the control group[30]. In addition, probiotic supplementation decreased triglycerides, VLDL, and increased HDL levels compared with the control group. Additionally,probiotic administration reduced plasma MDA, and an elevation in plasma nitric oxide and T-AOC was found compared with the control group. Therefore, the probiotic treatment showed great therapeutic potential in alleviating complications found in women with gestational DM. Future clinical studies are needed to investigate further the specific strains of
to elucidate which strains are more effective in attenuated DM.
CONCLUSION
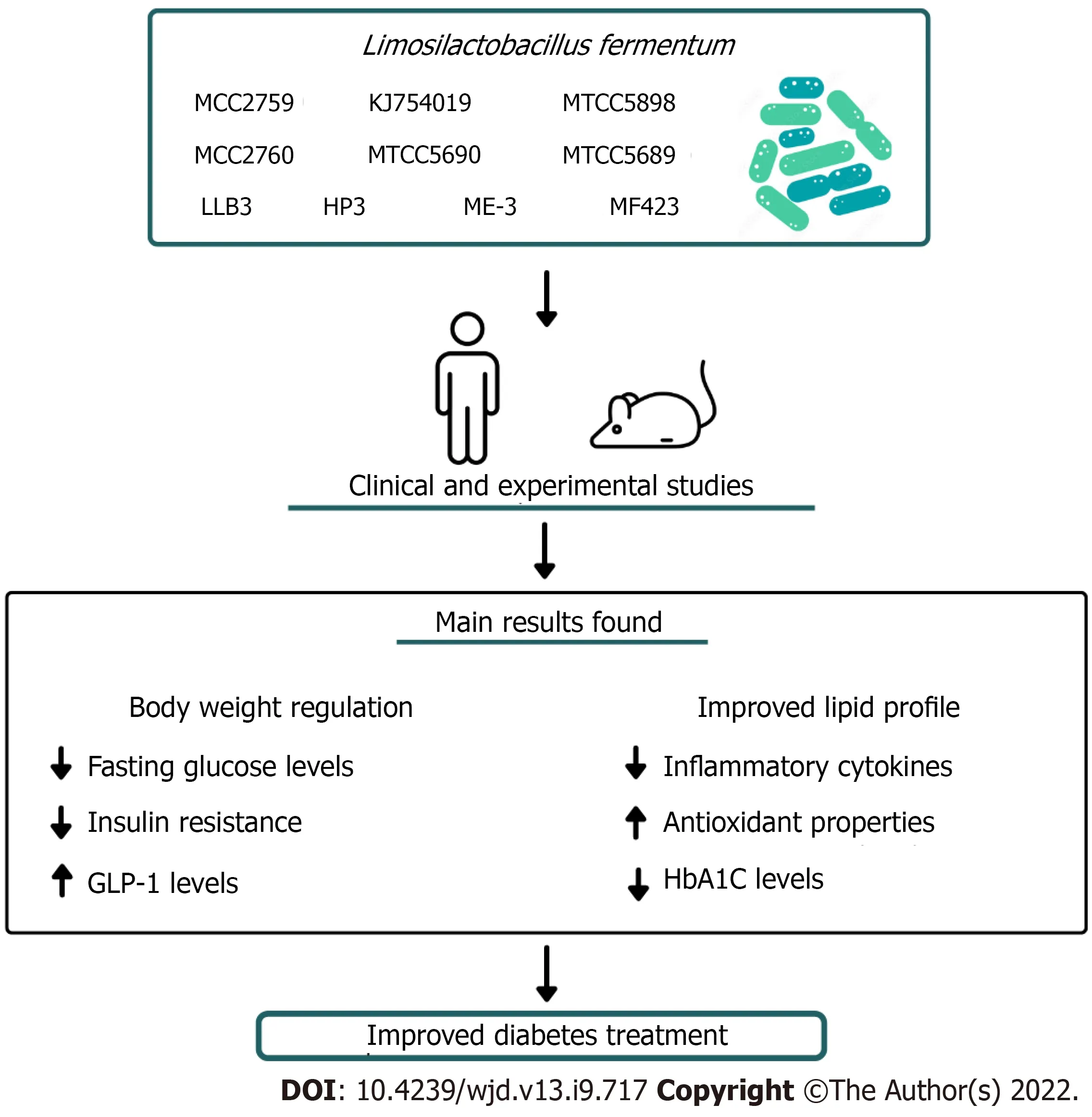
This literature review showed that
is a promising strain for the management of DM(Figure 1). Evidence from experimental and clinical study verified that
supplementation contributed to normalizing body weight, reduced blood glucose and fasting blood glucose levels,reduced insulin resistance, and improved lipid profile. Coupled with these biochemical changes,
therapy showed anti-oxidant and anti-inflammatory properties, which contributed to alleviating related symptoms of DM. However, the heterogeneities of studies, including variations in dosage, and duration of treatment, limit the elucidation of the most effective way to use
as adjuvant therapy of DM. Moreover, it is relevant to explore the effectiveness of co-intervention with
associated with bioactive compounds with antioxidant and anti-inflammatory properties,such as quercetin and resveratrol [15]. We also highlight that most of the available data came from preclinical studies, hence, therapeutic potential of different strains of
in minimizing complications of DM needs to be further investigated in randomized, double-blind, placebo-controlled trials to confirm these findings in human studies.
ACKNOWLEDGEMENTS
Conversely, the administration of
decreased the glycation events and oxidative stress through the increasing production of ferulic acid (FA). This potent antioxidant metabolite can significantly reduce ROS formation and prevent glycation events. This mechanism is related to decreasing inflammatory markers, Hb1ac, and serum glucose. High levels of FA are also related to lower cardiometabolic risk in diabetic individuals[40,44].
FOOTNOTES
de Brito Alves JL contributed to the conceptualization; Lacerda DC, Trindade da Costa PC,Pontes PB, Carneiro dos Santos LA, Cruz Neto JP, Silva Luis CC, de Sousa Brito VP, de Brito Alves JL wrote,reviewed and edited the manuscript.
The authors declare no competing interests.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Terrified, the couple jumped up and scrambled6 toward the other end of the car. The laborer aimed a kick at the retreating old woman but missed as she scuttled7 to safety. This so enraged8 the drunk that he grabbed the metal pole in the center of the car and tried to wrench9 it out of its stanchion(). I could see that one of his hands was cut and bleeding. The train lurched ahead, the passengers frozen with fear. I stood up.
It s just a small, white envelope stuck among the branches of our Christmas tree. No name, no identification, no inscription(,). It has peeked1 through the branches of our tree for the past 10 years or so.
Brazil
José Luiz de Brito Alves 0000-0003-4696-3809.
Chang KL
A
Despair filled her heart anew, but a dream led her a third time to the old woman s house. She went there, and the wise woman gave her a golden spinning wheel, comforted her, and said, Everything is not yet fulfilled. Wait until the full moon comes, then take the spinning wheel, sit on the bank, and spin the spool23 full. When you have done this place the spinning wheel at the water s edge, and you will see what will happen.
Chang KL
1 Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends.
2020; 10: 107-111 [PMID: 32175717 DOI:10.2991/jegh.k.191028.001]
2 Tinajero MG, Malik VS. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective.
2021; 50: 337-355 [PMID: 34399949 DOI: 10.1016/j.ecl.2021.05.013]
3 Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045.
2022; 183: 109119 [PMID: 34879977 DOI: 10.1016/j.diabres.2021.109119]
4 Jin Q, Ma RCW. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies.
2021; 10 [PMID: 34831057 DOI: 10.3390/cells10112832]
5 Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications.
2018; 14: 88-98 [PMID: 29219149 DOI: 10.1038/nrendo.2017.151]
6 Taylor R. Type 2 diabetes: etiology and reversibility.
2013; 36: 1047-1055 [PMID: 23520370 DOI:10.2337/dc12-1805]
7 Lee E. Diabetes: Screening, Diagnosis, and Prevention of Type 2 Diabetes.
2021; 504: 16-21 [PMID:33970587]
8 Roden M. [Diabetes mellitus: Definition, classification and diagnosis].
2012; 124 Suppl 2: 1-3[PMID: 23250457 DOI: 10.1007/s00508-012-0269-z]
9 Cloete L. Diabetes mellitus: an overview of the types, symptoms, complications and management.
2022; 37:61-66 [PMID: 34708622 DOI: 10.7748/ns.2021.e11709]
10 Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease.
2019;21: 21 [PMID: 30828746 DOI: 10.1007/s11886-019-1107-y]
11 Ceriello A, Prattichizzo F. Variability of risk factors and diabetes complications.
2021; 20: 101[PMID: 33962641 DOI: 10.1186/s12933-021-01289-4]
12 Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome.
2014; 20: 16079-16094 [PMID: 25473159 DOI: 10.3748/wjg.v20.i43.16079]
13 Chakaroun RM, Massier L, Kovacs P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders?
2020; 12 [PMID: 32295104 DOI: 10.3390/nu12041082]
14 Woldeamlak B, Yirdaw K, Biadgo B. Role of Gut Microbiota in Type 2 Diabetes Mellitus and Its Complications: Novel Insights and Potential Intervention Strategies.
2019; 74: 314-320 [PMID: 31870137 DOI:10.4166/kjg.2019.74.6.314]
15 Sampaio KB, do Nascimento YM, Tavares JF, Cavalcanti MT, de Brito Alves JL, Garcia EF, de Souza EL. Development and
evaluation of novel nutraceutical formulations composed of Limosilactobacillus fermentum, quercetin and/or resveratrol.
2021; 342: 128264 [PMID: 33041168 DOI: 10.1016/j.foodchem.2020.128264]
16 Slingerland AE, Schwabkey Z, Wiesnoski DH, Jenq RR. Clinical Evidence for the Microbiome in Inflammatory Diseases.
2017; 8: 400 [PMID: 28446909 DOI: 10.3389/fimmu.2017.00400]
17 Arora T, Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives.
2016; 280: 339-349 [PMID: 27071815 DOI: 10.1111/joim.12508]
18 Sohail MU, Althani A, Anwar H, Rizzi R, Marei HE. Role of the Gastrointestinal Tract Microbiome in the Pathophysiology of Diabetes Mellitus.
2017; 2017: 9631435 [PMID: 29082264 DOI:10.1155/2017/9631435]
19 Cabello-Olmo M, Araña M, Urtasun R, Encio IJ, Barajas M. Role of Postbiotics in Diabetes Mellitus: Current Knowledge and Future Perspectives.
2021; 10 [PMID: 34359462 DOI: 10.3390/foods10071590]
20 Bastos RMC, Rangel ÉB. Gut microbiota-derived metabolites are novel targets for improving insulin resistance.
2022; 13: 65-69 [PMID: 35070060 DOI: 10.4239/wjd.v13.i1.65]
21 Xia F, Wen LP, Ge BC, Li YX, Li FP, Zhou BJ. Gut microbiota as a target for prevention and treatment of type 2 diabetes:Mechanisms and dietary natural products.
2021; 12: 1146-1163 [PMID: 34512884 DOI:10.4239/wjd.v12.i8.1146]
22 Xi Y, Xu PF. Diabetes and gut microbiota.
2021; 12: 1693-1703 [PMID: 34754371 DOI:10.4239/wjd.v12.i10.1693]
23 Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Stanton C. Gut microbiota, obesity and diabetes.
92: 286-300 [PMID: 26912499 DOI: 10.1136/postgradmedj-2015-133285]
24 Liang T, Wu L, Xi Y, Li Y, Xie X, Fan C, Yang L, Yang S, Chen X, Zhang J, Wu Q. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis.
2021; 61: 1670-1688 [PMID: 32436397 DOI: 10.1080/10408398.2020.1764488]
25 Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis.
2021; 12: 722-734 [PMID: 33126241 DOI:10.1093/advances/nmaa133]
26 Jang YJ, Kim WK, Han DH, Lee K, Ko G.
species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota.
2019; 10: 696-711 [PMID: 30939976 DOI: 10.1080/19490976.2019.1589281]
27 Yadav R, Khan SH, Mada SB, Meena S, Kapila R, Kapila S. Consumption of Probiotic Lactobacillus fermentum MTCC:5898-Fermented Milk Attenuates Dyslipidemia, Oxidative Stress, and Inflammation in Male Rats Fed on Cholesterol-Enriched Diet.
2019; 11: 509-518 [PMID: 29754388 DOI: 10.1007/s12602-018-9429-4]
28 de Luna Freire MO, do Nascimento LCP, de Oliveira KÁR, de Oliveira AM, Dos Santos Lima M, Napoleão TH, da Costa Silva JH, Lagranha CJ, de Souza EL, de Brito Alves JL. Limosilactobacillus fermentum Strains with Claimed Probiotic Properties Exert Anti-oxidant and Anti-inflammatory Properties and Prevent Cardiometabolic Disorder in Female Rats Fed a High-Fat Diet.
2021 [PMID: 34817804 DOI: 10.1007/s12602-021-09878-1]
29 Kim JE, Lee JY, Kang CH.
MG4295 Improves Hyperglycemia in High-Fat Diet-Induced Mice.
2022; 11 [PMID: 35053962 DOI: 10.3390/foods11020231]
30 Babadi M, Khorshidi A, Aghadavood E, Samimi M, Kavossian E, Bahmani F, Mafi A, Shafabakhsh R, Satari M, Asemi Z.The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus:a Randomized, Double-Blind, Placebo-Controlled Trial.
2019; 11: 1227-1235 [PMID:30535534 DOI: 10.1007/s12602-018-9490-z]
31 Ai X, Wu C, Yin T, Zhur O, Liu C, Yan X, Yi C, Liu D, Xiao L, Li W, Xie B, He H. Antidiabetic Function of
MF423-Fermented Rice Bran and Its Effect on Gut Microbiota Structure in Type 2 Diabetic Mice.
2021; 12: 682290 [PMID: 34248898 DOI: 10.3389/fmicb.2021.682290]
32 Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012.
2015; 1-16 [PMID: 25671660]
33 Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, Kim HB, Lee JH. Role of Probiotics in Human Gut Microbiome-Associated Diseases.
2019; 29: 1335-1340 [PMID: 31434172 DOI:10.4014/jmb.1906.06064]
34 Chang CJ, Lin TL, Tsai YL, Wu TR, Lai WF, Lu CC, Lai HC. Next generation probiotics in disease amelioration.
2019; 27: 615-622 [PMID: 31324278 DOI: 10.1016/j.jfda.2018.12.011]
3535 Hu TG, Wen P, Shen WZ, Liu F, Li Q, Li EN, Liao ST, Wu H, Zou YX. Effect of 1-Deoxynojirimycin Isolated from Mulberry Leaves on Glucose Metabolism and Gut Microbiota in a Streptozotocin-Induced Diabetic Mouse Model.
2019; 82: 2189-2200 [PMID: 31393724 DOI: 10.1021/acs.jnatprod.9b00205]
36 Aziz G, Zaidi A, Tariq M. Compositional Quality and Possible Gastrointestinal Performance of Marketed Probiotic Supplements.
2022; 14: 288-312 [PMID: 35199309 DOI: 10.1007/s12602-022-09931-7]
37 Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease:from biology to the clinic.
2019; 16: 605-616 [PMID: 31296969 DOI:10.1038/s41575-019-0173-3]
38 Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, Xu XY, Li HB. Effects and Mechanisms of Probiotics,Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review.
2021; 13 [PMID: 34579087 DOI: 10.3390/nu13093211]
39 Mañé J, Lorén V, Pedrosa E, Ojanguren I, Xaus J, Cabré E, Domènech E, Gassull MA. Lactobacillus fermentum CECT 5716 prevents and reverts intestinal damage on TNBS-induced colitis in mice.
2009; 15: 1155-1163[PMID: 19266568 DOI: 10.1002/ibd.20908]
40 Feng T, Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review.
2020; 12: 1801944 [PMID: 32795116 DOI: 10.1080/19490976.2020.1801944]
41 Dowarah R, Verma AK, Agarwal N, Singh P, Singh BR. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets.
2018; 13:e0192978 [PMID: 29518093 DOI: 10.1371/journal.pone.0192978]
42 Sun Z, Sun X, Li J, Li Z, Hu Q, Li L, Hao X, Song M, Li C. Using probiotics for type 2 diabetes mellitus intervention:Advances, questions, and potential.
2020; 60: 670-683 [PMID: 30632770 DOI:10.1080/10408398.2018.1547268]
43 Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O'Toole PW, Pot B, Vandamme P, Walter J,Watanabe K, Wuyts S, Felis GE, Gänzle MG, Lebeer S. A taxonomic note on the genus
: Description of 23 novel genera, emended description of the genus
Beijerinck 1901, and union of
and
.
2020; 70: 2782-2858 [PMID: 32293557 DOI: 10.1099/ijsem.0.004107]
44 Paulino do Nascimento LC, Lacerda DC, Ferreira DJS, de Souza EL, de Brito Alves JL. Limosilactobacillus fermentum,Current Evidence on the Antioxidant Properties and Opportunities to be Exploited as a Probiotic Microorganism.
2022 [PMID: 35467236 DOI: 10.1007/s12602-022-09943-3]
45 de Luna Freire MO, do Nascimento LCP, de Oliveira KÁR, de Oliveira AM, Napoleão TH, Lima MDS, Lagranha CJ, de Souza EL, de Brito Alves JL. Effects of a Mixed
Formulation with Claimed Probiotic Properties on Cardiometabolic Variables, Biomarkers of Inflammation and Oxidative Stress in Male Rats Fed a High-Fat Diet.
2021; 10 [PMID: 34574313 DOI: 10.3390/foods10092202]
46 Cavalcante RGS, de Albuquerque TMR, de Luna Freire MO, Ferreira GAH, Carneiro Dos Santos LA, Magnani M, Cruz JC, Braga VA, de Souza EL, de Brito Alves JL. The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats.
2019; 29: 1408-1417 [PMID: 31640890 DOI:10.1016/j.numecd.2019.08.003]
47 Adeshirlarijaney A, Gewirtz AT. Considering gut microbiota in treatment of type 2 diabetes mellitus.
2020;11: 253-264 [PMID: 32005089 DOI: 10.1080/19490976.2020.1717719]
48 Archer AC, Muthukumar SP, Halami PM. Lactobacillus fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats.
2021;13: 1068-1080 [PMID: 33575913 DOI: 10.1007/s12602-021-09744-0]
49 Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R, Raghavan S, Soundarajan A, Grover S, Batish VK, Mohan V, Balasubramanyam M. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice.
2018; 57: 279-295 [PMID: 27757592 DOI:10.1007/s00394-016-1317-7]
50 Hartajanie L, Fatimah-Muis S, Heri-Nugroho Hs K, Riwanto I, Sulchan M. Probiotics Fermented Bitter Melon Juice as Promising Complementary Agent for Diabetes Type 2: Study on Animal Model.
2020; 2020: 6369873[PMID: 32190386 DOI: 10.1155/2020/6369873]
51 Chaiyasut C, Woraharn S, Sivamaruthi BS, Lailerd N, Kesika P, Peerajan S. Lactobacillus fermentum HP3-Mediated Fermented Hericium erinaceus Juice as a Health Promoting Food Supplement to Manage Diabetes Mellitus.
2018; 23: 2515690X18765699 [PMID: 29619846 DOI: 10.1177/2515690X18765699]
52 Guilbaud A, Howsam M, Niquet-Léridon C, Delguste F, Fremont M, Lestavel S, Maboudou P, Garat A, Schraen S,Onraed B, Foligné B, Boulanger É, Tessier FJ. The Effect of Lactobacillus fermentum ME-3 Treatment on Glycation and Diabetes Complications.
2020; 64: e1901018 [PMID: 31991062 DOI: 10.1002/mnfr.201901018]
53 Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults.
2010; 5:e9085 [PMID: 20140211 DOI: 10.1371/journal.pone.0009085]
54 Yadav R, Dey DK, Vij R, Meena S, Kapila R, Kapila S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats.
2018; 125: 454-462 [PMID: 30316007 DOI: 10.1016/j.micpath.2018.10.015]
55 Yousaf S, Hussain A, Rehman S, Aslam MS, Abbas Z. Hypoglycemic and hypolipidemic effects of Lactobacillus fermentum, fruit extracts of Syzygium cumini and Momordica charantia on diabetes induced mice.
2016;29: 1535-1540 [PMID: 27731809]
杂志排行
World Journal of Diabetes的其它文章
- Different nutrient compositions in diet and taking hypoglycemic drugs can modulate gut microbial flora
- Mapping the global research landscape on insulin resistance:Visualization and bibliometric analysis
- Role of insulin in pancreatic microcirculatory oxygen profile and bioenergetics
- Relationship between age of pregnant women with gestational diabetes mellitus and mode of delivery and neonatal Apgar score
- Hyperglycemia and reduced adiposity of streptozotocin-induced diabetic mice are not alleviated by oral benzylamine supplementation
- Effectiveness and safety of COVID-19 vaccines in patients with diabetes as a factor for vaccine hesitancy
