Evolving spectrum of diabetic wound: Mechanistic insights and therapeutic targets
2022-09-16RajaChakrabortyPobitraBorahParthaPratimDuttaSaikatSen
INTRODUCTION
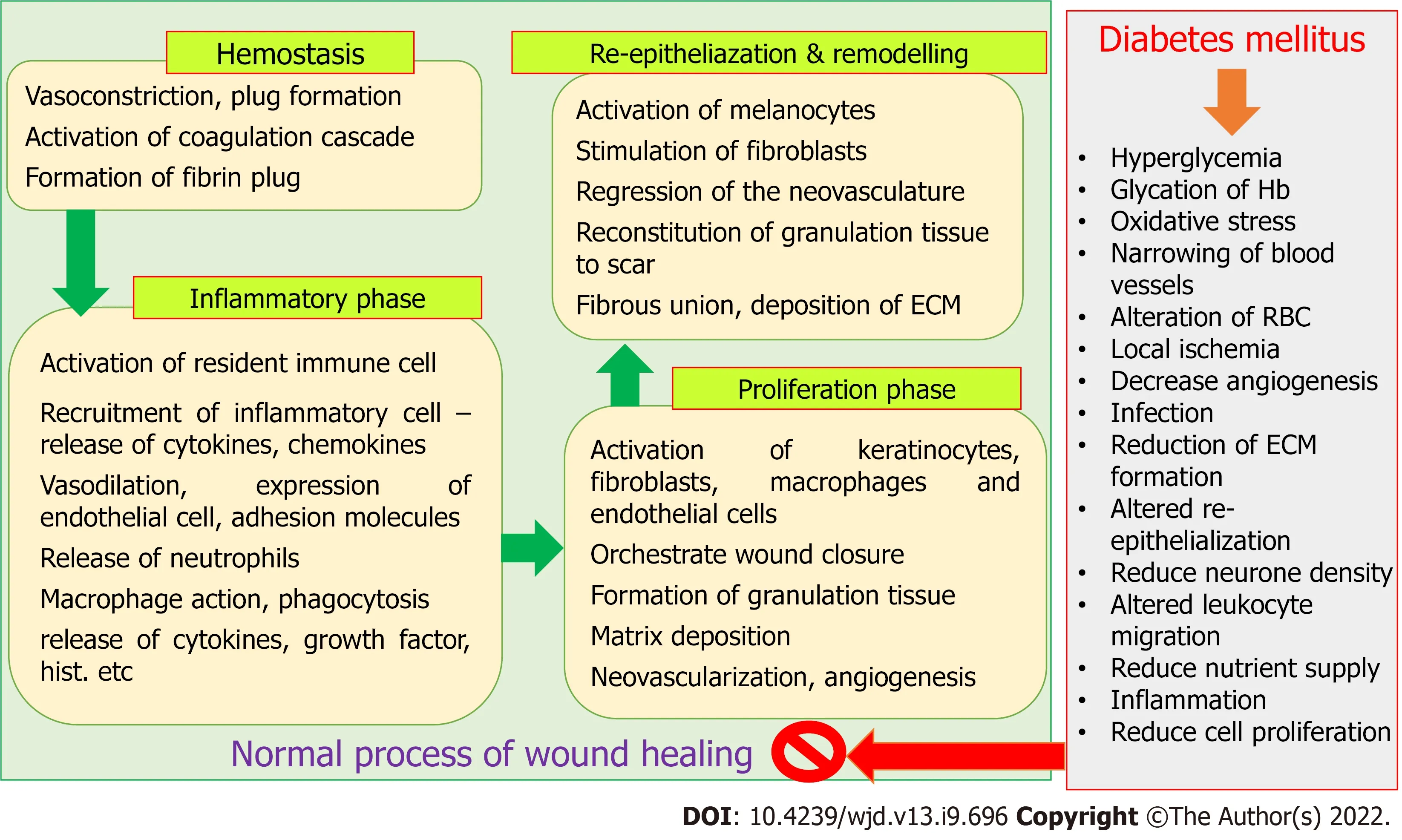
The process of wound healing is complex and requires spatial as well as temporal synchronization between different types of cells with specific functions. Hemostasis, inflammatory phase, proliferative phase, re-epithelialization, and remodelling phase are the four major phases of wound healing process,which result in the restoration of functional integrity of tissues[1-3]. Alteration in the microenvironment due to diabetes mellitus (DM) results in a change in the level of oxygen, chemokines, synthesis of growth factors, extracellular matrix, oxidative stress that in turn alter normal cellular recruitment and activation, and induce impaired or delayed wound healing[1,3]. Ageing, genetic disorders, obesity, and metabolic disorders including DM, are responsible for the abnormal wound healing process, that enhances the risk of developing chronic wound[1,3,4]. It was estimated that chronic wounds directly affect the quality of life of about 2.5% population of the United States, and the medicare cost for management of all wounds and the related situation was projected between $28.1 to $96.8 billion[5].Management of wounds in the diabetic individual is a major clinical and social concern. The hyperglycaemic environment in diabetic people causes impaired and delayed wound healing[4],making the situation more perilous as the number of diabetic people is increasing day by day. It was also found that treatment and management of diabetic ulcers and surgical wounds were the most expensive[5]. The IDF Diabetes Atlas estimated that the prevalence of DM in 2021 was 10.5% (536.6 million people in the 20-79 year age group), which will increase to 12.2% (783.2 million in the 20-79 year age group) by 2045. Global health expenditures related to the management of DM and its complications are expected to reach $966 billion in 2021[6]. It was also predicted that almost half of the adult population (44.7%; 239.7 million of 20-79 years old) were unaware of their diabetic condition. People may develop micro and macrovascular complications during an asymptomatic diabetic state[7].Impaired or delayed wound healing affects about 25% of diabetic people. A study suggested that 1 in 3 to 1 in 5 diabetic individuals are at risk of chronic non-healing wounds, including diabetic foot (with a very high recurrence rate) in their lifetime[8]. Zhang
[9] estimated that the global prevalence of diabetic foot is 6.3%, which usually affects type 2 diabetic people, older people, and people with a longer duration of DM.
Both the extrinsic and intrinsic factors are responsible for delay in the wound healing process in diabetic patients. Repeated trauma or mechanical stress in the diabetic foot can lead to neuropathy and ischemic situation. Glucose-rich environment results in increased generation of advanced glycation endproducts (AGEs) and elevated levels of inflammatory cytokines [
, interleukin (IL)-1β and tumor necrosis factor α (TNF-α)] for a persistent period that hinders the normal process of wound healing[10,11]. In turn, hyperglycemia reduces collagen synthesis, growth factor production, macrophage function,angiogenic response, migration and proliferation of fibroblast and keratinocyte, epidermal nerve count,and the balance between extracellular matrix (ECM) component accumulation and matrix metalloproteinase (MMP) induced remodelling [4,12]. The normal wound healing process and effect of the hyperglycemic condition are depicted in Figure 1.
Reuben couldn t ask his father for the money. Everything Mark Earle made through fishing in Bay Roberts, Newfoundland, Canada. Reuben s mother, Dora, stretched like elastic2() to feed and clothe their five children.
Despite the presence of protocols to standardize care in the diabetic wounds, as well as numerous advancements in scientific research and in clinical fronts, DM remains a problematic situation for wound healing. This paper is an attempt to highlight the mechanistic insights, plausible therapeutic targets, and pharmacotherapeutic approaches, particularly the role of phytochemicals in the management of diabetic wounds, in light of recent shreds of evidence.
MECHANISTIC INSIGHTS OF DIABETIC WOUND
Wound healing, being an evolutionary conservation process, restores impaired epithelial barriers through a cascade of events including inflammatory responses, proliferation, cellular migration,angiogenesis, and remodeling of tissues[13]. DM interferes with the normal healing process to provoke non-healing wound, and leads to complications including walking difficulty and infections like septicemia, abscess, cellulitis, osteomyelitis, and gangrene[4]. Acute diabetic wounds with an impaired healing process of unknown aetiology are the first signs of chronic diabetic wounds. Hyperglycemia,hypoxia, chronic inflammation, neuropathy, circulatory dysfunctions, alteration in neuropeptide signalling, and infections impede the diabetic wound healing process. Importantly, due to the heterogeneous nature of the diabetic wound, there exist no clear implications from the pathogenic vantage.The following subsections discuss the potential factors underlying the pathogenesis of diabetic wound.
But in front of the tan-yard, close to the entrance, stood a little girl clothed in rags, very pretty to look at, with curly hair, and eyes so blue and clear that it was a pleasure to look into them
Molecular implications
Impaired wound healing associated with diabetes remains inconclusive. However, the alterations in cellular factors and biochemical mediators have been believed to be involved in the development and progression of diabetic wound (Figure 2). Factors like hyperglycemia and oxidative stress in diabetic patients result in dysregulated macrophage polarization through modulation of epigenetic codes to delay the process of wound healing[14,15]. Hyperglycemia is implicated in impaired wound closure in diabetic foot ulcers (DFUs), with reduced skin cell function and the formation of atherosclerosis and neuropathy as possible contributors[8]. The development of atherosclerosis leads to alteration in the physiology of endothelial cells along with deprivation of nutrients in the wound site, critically affecting the healing process. Patients with type 1 DM are more prone to macrovascular diseases, especially affecting femoral and metatarsal arteries[16,17]. DM associated early microvascular deficiencies include decreased capillary size, basement membrane thickening, and arteriolar hyalinosis. Thickening of the membrane disrupts the physiological exchanges and causes altered leucocyte migration, and thereby increasing the risk of microbial infections[18,19]. Hyperglycemia disrupts protein translation as well as the migration and proliferation of fibroblasts and keratinocytes involved in the process of re-epithelialization[20-22]. For instance, altered expression of proteins like cytoskeletal keratin proteins(K2/K6/K10) associated with keratinocyte differentiation, and LM-3A32, a laminin-5 α3 chain precursor protein that regulates epithelial cell binding to the basement membrane, was reported in subjects with DFUs[23]. As LM-3A32 is required for the survival and differentiation of keratinocytes, reduction in this protein affects the re-epithelialization process[24]. Interestingly, the expression of mRNA and microRNA was found to be non-significant in diabetic and non-diabetic foot skin fibroblasts[25].However, fibroblasts from DFUs were reported to exhibit altered morphology, growth factor unresponsiveness, ECM deposition, and reduced proliferation and migration[26-29]. Impaired vasculogenesis and angiogenesis due to deregulation of the growth factors and receptors leads to impaired wound healing. Dysfunctional endothelial progenitor cells (EPC) or reduction in their numbers and transition to proinflammatory EPC phenotypes have been implicated in DM[30,31]. Notably, EPC dysfunction and altered recruitment are contributed by hyperglycemia, chronic inflammation, oxidative stress, and activation of NADPH oxidase associated with diabetes pathogenesis[32]. Prolonged IL-1β production and reduced expression of peroxisome proliferator-activated receptor-γ are also involved in the impairment of wound healing in DM[33]. Higher expression of MMPs including MMP-1, 2, 8, 9, 14,and 26, and lower expression of their tissue inhibitors were also reported in diabetes[34,35]. In patients with DFUs, it was reported that there is an increase in MMPs and a decrease in tissue inhibitor of metalloproteinase (TIMP)-2, which supports the fact that in a proteolytic environment, diabetic wound fails to heal due to reduction in ECM formation[36]. An increase in MMPs also contributes to matrix degradation, delayed cell migration, and inhibition of collagen deposition[36]. Oxidative stress resulting due to decrease action of enzymes like superoxide dismutase and glutathione peroxidase augment diabetic wound. Overproduction of reactive oxygen species (ROS) from hexosamine, polyol, and AGE pathways affects the later stages of diabetic wound healing, particularly by damaging the peripheral nerves. Consequently, the detrimental effect on the structure, supply, and metabolism of peripheral nerves (neuropathy) increases the risk of DFU development[37]. Hyperglycemia not only contributes to impaired healing but also makes skin prone to injury. Decrease in nuclear factor erythroid factor 2-related factor 2 (Nrf2) in diabetic patients increases the oxidized proteins and ROS generation[38,39]. In Nrf2 knockout mice, delayed wound healing was reported when compared to a Nrf2
mice, possibly due to oxidative DNA damage, elevated MMP-9 expression, and lower level of transforming growth factor-beta 1 (TGF-β1) expression[40]. Hyperglycemia also leads to an increase in neutrophil extracellular traps release, which has been implicated in delayed wound healing in both murine and human models[13,41].
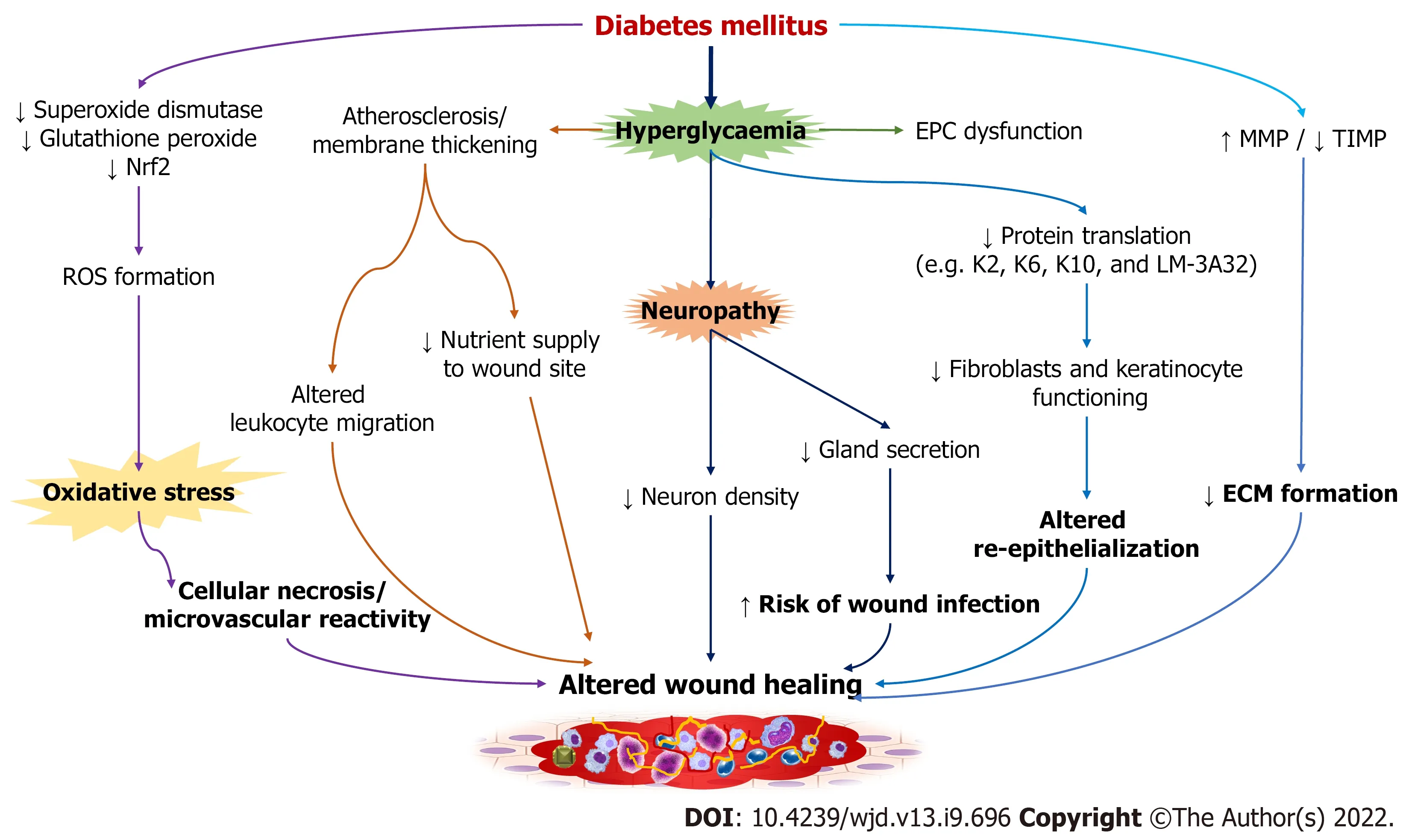
The formation of a diabetic wound is also linked to several forms of neuropathy, including sensory,motor, and autonomic. Sensory deficits, for example, cause a loss of protective symptoms, whereas motor neuropathy causes anatomical deformities and ischemic death in the plantar region of the foot[42], and autonomic neuropathy reduces sweat secretion from the glands, making skin dry and increasing the risk of infection and pruritus[43]. Furthermore, neuropathy causes a decrease in neuron density, causing impairment in wound healing. Primarily, diabetic neuropathy occurs in nerves that are dependent on nerve growth factor[8]. Impaired microvascular processes along with autonomic neuropathy and denervation of sympathetic nerves disrupt the blood flow. Cellular necrosis and microvascular reactivity are also triggered by Poly (ADP-ribose) polymerase enzyme
oxidative DNA damage[16,44]. In diabetic neuropathy, impairment of C-fibre dependent neurovascular responses leads to abnormality in the release of histamine, substance P, and calcitonin related peptide, thus exhibiting altered vasodilatation in case of stress like pressure or trauma[44]. However, the direct link between neuropathic abnormalities and glucose control is yet to be proven.
Epigenetic mechanisms
Despite the fact that epigenetic pathways have a role in a variety of diabetic complications, evidence of epigenetics in diabetic wound or impaired wound healing is still emerging. The role of microRNA in diabetic wound was first highlighted by a study that revealed up-regulation of miR-503 in plasma obtained from DFUs and in human umbilical vein endothelial cells (HUVEC).
experiment suggested the detrimental effect of forced miR-503 expression on function of HUVEC cells resulting due to impaired migration, proliferation, and formation of blood vessels[45]. Blockage of miR-503 expression showed improvement in angiogenesis in diabetic animals with limb ischemia[45,46]. Another study demonstrated that diabetic mice had a distinct microRNA signature, with differential expression of fourteen microRNAs[47]. Of these, expression of miR-146b was found to be up-regulated by 30 fold.Though miR-21 was up-regulated in diabetic skin, it was reduced in diabetic wound healing. This study suggested the necessity of miR-21 expression for fibroblast migration and miR-21 knock-down results in altered cellular migration. Similarly, another study showed the stabilisation of hypoxia-inducible factor α leads to miR210 expression, which silences the expression of E2F3, an important element of wound healing[46]. This implies the fact that epigenetic changes in miR-210 lead to impaired wound re-epithelialization and reduced proliferation. The involvement of DNA methylation in impaired diabetic wound healing is being studied in many experiments. A study reported inhibition of DNA methyltransferase 1(DNMT1), an enzyme that transfers a methyl group to the cytosine ring to produce 5-methylcytosine associated with transcriptional repression, suppresses inflammatory signals in bone marrow derived macrophages and also promotes M2-like macrophage formation[48]. This finding was supported by DNMT1 knockdown in db/db mice showing improvement in wound healing[49]. In contrast to that,demethylation of MMP-9 promoter in keratinocytes was reported to be involved in the induction of diabetic wound[50,51]. Apart from the role of DNA methylation in wound healing, it is also associated with metabolic memory, insulin resistance, and other diabetic complications[52,53]. Unlike DNA methylation, histone methylation does not always lead to transcriptional repression. Instead, it silences or promotes transcription based on the target residue and methyl groups. Methylation of histone H3K4 is regulated by SET domain containing protein family, particularly MLL1 that promotes inflammatory gene expression in nuclear factor kappa B-dependent manner[54-56]. A study reported the role of mixed-lineage leukemia-1 (MLL1) is to catalyze H3K4me3 deposition in macrophages during the process of wound healing[57]. Delayed wound healing and reduced pro-inflammatory cytokine generation were reported in a myeloid-specific MLL1 deletion in mice. Monocytes isolated from patients with type-2 DM demonstrated higher MLL1 expression, indicating dynamic regulation of MLL1 expression during diabetic wound healing. In diet-induced obesity model of diabetes, increased expression of histone demethylase (
, lysine-specific demethylase 6B or JMJD3) that targets H3K27me3 was seen in wound macrophages[58,59]. Epigenetic regulation of IL-6 expression in neutrophils was believed to be impacted by toll-like receptor (TLR) activation associated with increased H3K27ac,H3K4me3, and acetylated histone H4[60]. Re-epithelialization is also promoted by JMJD3 expression,which induces keratinocyte migration to the wound site[61]. Similar report suggests upregulation of JMJD3 expression on the wound site is necessary for early onset of the wound healing process, which is absent in diabetic wound[62]. However, it will be too oversimplified interpolation as most of these findings are limited to normal wounds; thus, further studies are warranted. Other histone modifications including histone acetylation or deacetylation, histone phosphorylation, histone-arginine demethylation or methylation, and ATP-dependent chromatin remodelling are also being studied for their potential role in diabetic wound healing[62].
Whereupon he began, with a voice like a screech-owl s, to chant the words of his opera, only this time happily not at such a length, and without the frog accompaniment
Microbiological perspectives
Growth factors are biologically active polypeptides that play an important role in the onset and maintenance of wound healing[97]. In diabetics, any change,
, down-regulation of growth factor receptors and rapid degradation of growth factors, causes wound healing to be delayed. Factors such as VEGF, IGF, TGF-β, KGF24, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), TNF-α, and IL-6 are significantly reduced in diabetes patients, and several of them have been demonstrated to significantly enhance wound healing in many studies.Growth factors that cause a molecular alteration in the wound micro-environment may help patients with non-responsive wounds. PDGF is a major serum mitogen that promotes fibroblast proliferation,matrix formation, and connective tissue maturation[98]. They attract fibroblasts and inflammatory cells and aid in the production of glycosaminoglycans, proteoglycans, and collagen. During the healing process, it is a critical mediator in fibroblast migration and proliferation, the formation of granulation tissue proteins and provisional extracellular matrix, and angiogenesis[99]. The expression of PDGF and its receptors is reduced in diabetic wounds, and many clinical studies employing PDGF have shown improved healing time[100,101]. It is indicated for the treatment of infections that have spread to deeper subcutaneous tissues or beyond areas with a sufficient blood supply[102]. Another growth factor, bFGF,has a stimulatory effect on fibroblast growth and differentiation, as well as the proliferation of vascular smooth muscle cells, endothelial cells, ECM metabolism, growth, and movement of mesodermally derived cells, all of which speed up the formation of granulation tissue and promote wound healing[103]. Angiogenesis, cell proliferation, migration, differentiation, neo-vascularization, re-epithelialization, and collagen disposition were all stimulated by the clinical application of bFGF, all of which contribute to wound healing[104]. It promotes mesodermal cell chemotaxis and extracellular matrix growth and expedites both acute and chronic wound healing, which gives a scar-free cure[105]. VEGF is a potent angiogenic cytokine that has a substantial impact on healing and promotes rate-limiting processes in vasculogenesis and angiogenesis[106]. Low VEGF levels cause impaired wound healing and aberrant VEGF receptor patterns in diabetics. Decreased VEGF mRNA levels, increased VEGF receptor (VEGFR)-1 Levels, and decreased VEGFR-2 Level are some of the key causes of wound nonhealing[107]. In diabetic wounds, VEGF leads to an increase in capillary density, which enhances blood perfusion and metabolism in the wounded tissue[108]. VEGF causes an increase in capillary density,which improves blood perfusion and metabolism in the wounded tissue. This leads to the facilitation of the supply of oxygen and nutrients to assist the growth and function of reparative cells. It is the primary regulator of wound revascularization and permeability and participates in the formation of granulation tissue. On binding with the EGF receptors, EGF causes an increase in epidermal cell, cell motility,cellular migration, mesenchymal regeneration, angiogenesis and cell proliferation[109]. Application of EGF into the wound site results in a greater pharmacodynamic response in terms of granulation tissue growth and wound closure. IGF-1 promotes wound healing by assisting in cell granulation and reepithelisation, promoting endothelial cell chemotaxis and keratinocyte and fibroblast proliferation,while lower levels of both IGF-1 and TGF-β in wound tissue cause wound healing to be delayed[110,111]. TGF-β attracts and stimulates inflammatory cells such as neutrophils, macrophages, lymphocytes,keratinocytes, and fibroblasts, as well as the synthesis of growth factors, which speed up vascularisation, angiogenesis, and ECM synthesis while slowing down ECM degradation[112]. Therapeutic agents or strategy, which can ameliorate this positively, are useful in diabetic wound condition. Several drugs and phytoconstituents have shown their positive effect in diabetic wounds targeting these biomolecules. The use of platelet-rich plasma (PRP), EGF, PDGF, and FGF has shown promising effects in the treatment of diabetic wound in a better way.
to be the most common colonizers in DFUs[64]. Stratification of DFUs as per infection severity revealed higher bacterial diversity in severely infected DFUs, while
and
were found to be the most abundant in mild-to-moderate DFUs[65]. It was similar to a previous finding that found diverse microbiota (with higher incidence of anaerobic and Proteobacteria infection) in deep chronic ulcers, while
was found to be abundant in acute and superficial ulcers[66]. However,contrary to these reports, another study reported
spp. to be the primary culture detected in the microbiome in diabetic foot osteomyelitis[67]. A higher prevalence of
colonization in DFUs and intact diabetic skin lead to systemic infection and osteomyelitis[63]. Indeed, in a Nigerian observational multi-center study, it was reported that the presence of osteomyelitis is an important predictor of wound healing in hospitalized patients with DFUs[68]. The expression of proteolytic factors by
and
are believed to be the disruptor of skin barrier. For example, SpeB released by
leads to cleavage of desmoglein 1 and 3 that causes epidermal barrier damage[69]. The alkaline environment of DFUs contributes to the formation of bacterial biofilm, leading to complex host–microbiome interaction[70]. The formation of bacterial biofilm along with alkaline pH affects drug action and is responsible for antibiotic resistance[71]. One should appreciate various other factors influencing this intricate microbiome network and its potential correlation with clinical significance. Keeping all this evidence in sight, more longitudinal studies are anticipated for an adequate understanding of the probable relevance of the microbiome to clinical outcomes.
PLAUSIBLE DRUG TARGETS
They were to eat peaches, as planned, after her nap, and now she sat across from the man who would have been a total stranger except that he was in fact her father. They had been together again (although she couldn t quite remember when they had been together before) for almost a hundred years now, or was it only since day before yesterday? Anyhow, they were together again, and he was kind of funny. First, he had the biggest mustache she had ever seen on anybody, although to her it was not a mustache at all; it was a lot of red and brown hair under his nose and around the ends of his mouth. Second, he wore a blue-and-white striped jersey1() instead of a shirt and tie, and no coat. His arms were covered with the same hair, only it was a little lighter2 and thinner. He wore blue slacks3 , but no shoes and socks, He was barefoot, and so was she, of course.
There were many pictures of my mother in those books, given to me by my father before he died an old man just a few years ago. I had barely known the woman on those pages and only imagined that she must have loved me. She, too, had died, when I was very little, leaving me to miss the presence of a relationship that seemed so innate18, so necessary.
As a result of elevated blood sugar levels in diabetic conditions, damage to nerve fiber occurs, leading to diabetic neuropathy, which can be sensory, motor or autonomic[72]. Sensory neuropathy can result in one of two outcomes: a painful foot or a foot that is devoid of sensation[12]. Motor neuropathy is accompanied by muscle weakening, atrophy, and paresis. The inability of an intrinsic muscle to keep the foot in its normal state resulting because of weakening of inter-osseous muscles in the foot, which contributes to foot deformity. When the foot deforms, the pressure distribution throughout the foot changes, and aberrant pressure develops at various locations on the foot[73]. Keratosis and callus development occur as a result of repeated pressure, which leads to damage to callused areas and induces ulcer formation beneath the callus that further causes cracks on the foot[74-77]. Malfunctioning of the sympathetic nerves supplying the sweat glands in the foot reduces the sweat and moisture in the feet, which leads to the development of cracks[78]. Inflammation, necrosis, and ulceration result when an unnoticed injury is combined[16,79]. Currently, duloxetine, anticonvulsant pregabalin and opioid tapentadol are being prescribed for diabetic peripheral neuropathy. Besides these, a substance like αlipoic acid has shown effectiveness in delaying or reversing peripheral diabetic neuropathy through its multiple antioxidant properties[80]. Neuropeptides such as substance-P and neuropeptide-Y are also been found to be effective in diabetic neuropathy and associated wound[81,82].
Peripheral vascular dysfunction is another major cause of diabetic wound in a majority of diabetic patients. The wound healing process in the diabetic condition is hampered by the altered physiological response due to glycation of hemoglobin, alteration of the red blood cell membrane and narrowing of blood vessels which cause the decreased supply of nutrients and oxygen to tissues[12]. The development of atherosclerotic plaque in diabetic patients also leads to the development of non-healing wound. Therefore, pharmacotherapeutic agents like antioxidant phytochemicals that can avert oxidative stress and formation of AGEs could be useful in treating diabetic wound.
Bacterial infection is one of the most common causes of wounds, and diabetic individuals are more vulnerable to it because of delayed wound healing and immunosuppression[83,84]. The biofilms created by microorganisms protect them from antimicrobial agents and the immune system while also interfering with the healing process, which is one of the most prevalent reasons for amputation of lower limbs in diabetic wounds[14]. Therefore, empiric therapy should include broad-spectrum antibiotics. In recent times, drug resistance is a bigger problem and several drugs are in use in the treatment of DFU.
Neutrophils, monocytes, macrophages, keratinocytes, fibroblasts, T cells, B cells, mast cells, and endothelial cells are all involved in wound healing and are responsible for the formation and modulation of pro-inflammatory cytokines and growth factors such IL-1, TNF-α, IL-6, vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and TGF-β. Hyperglycemia and oxidative stress lead to dis-regulation of these cells, resulting in delayed wound healing[85-87].Increased amounts of pro-inflammatory cytokines cause an inflammatory cascade to be disrupted,resulting in hyper-inflammation and insulin resistance. These also lead to reduced angiogenesis and microvascular issues, impaired macrophage and neutrophil function, impaired keratinocyte and fibroblast migration and proliferation, and impaired growth factor generation[15,88-90]. Many of these cells play a vital role in the immune response, which is also important for wound healing. Various chemokines whose expression can regulate the function of immune cells have the potential to enhance wound healing. Mast cells with close coordination with macrophages, endothelial cells, and fibroblasts,play a key role in matrix remodeling and disrupt the balance of pro- and anti-angiogenic molecules in wound tissues, affecting angiogenesis and vascular regression in the proliferative and remodeling phases, respectively[91-93]. As a result, mast cell degranulation inhibitors such as disodium cromoglycate, quercetin, and luteolin may be promising options for improving diabetic wound healing[92]. Heat shock proteins (HSPs) aid wound healing by attracting dermal fibroblasts, stimulating cell proliferation and keratinocyte differentiation, reducing oxidative stress, ameliorating actin microfilaments, aiding endothelial cell migration, and enhancing pro-collagen synthesis and protein homeostasis[94,95]. Reduced levels of HSPs and their downstream components TLR4 and p38-MAPK(mitogen-activated protein kinases) in diabetic patients are responsible for the slowed healing process[96]. Therefore, targeting this as a therapeutic target could be useful in diabetic wound conditions.
At last his confidants searched his heart and lifted the veil from the face of his love, and then set the matter before his father, King Saman-lal-posh
Certainly, pathogenic infections are not directly associated with the pathophysiology of diabetic wounds but are critical from the vantage of impaired wound healing, hospitalization, morbidity, and amputation. However, the role in the initiation of diabetic wound in case of trauma remains unclear.The rapid spreading of infections and high microbial burden exhibit detrimental effect on the wound healing process. Injury to the superficial skin layer allows polymicrobial contamination and colonization, affecting diabetic wound. Particularly, infections like cellulitis, osteomyelitis, and abscesses are of major concern[16]. The advent of high-throughput sequencing technologies like microarray, 16S rRNA sequencing, and whole-genome sequencing have enabled the expansion of diabetic wound microbiome. Diabetic wound has demonstrated higher colonization of
and
[63]. Another study reported
,
, and
Infection caused by microbes is a major problem in DFU. Antimicrobial therapy targeting Gram -ve,Gram +ve, anaerobic bacteria, and certain fungi, contributing to the pathophysiology of DFU, is also a key approach. Povidone iodine solution (10%), chlorhexidine, acetic acid (5%), and silver compounds can be used to treat the mild wound. Antibiotics like amoxicillin + clavulanate, ampicillin, dicloxacilline,cephalosporin (
, cephalexin, cefoxitin, ceftriaxone, and ceftazidime), quinolones (
, ciprofloxacin,levofloxacin), metronidazole, and clindamycin used to cure moderate DFU[126]. Drugs like piperacillin/tazobactam + clindamycin, meropenem, imipenem, ertapenem, ampicillin + sulbactam are used to cure severe infections caused by different Gram +ve and Gram -ve bacteria, anaerobes, MRSA,
, while vancomycin, linezolid, and daptomycin can cure MRSA[11]. Cefepime, ceftazidime,meropenem, and piperacillin/tazobactam are recommended to use against
Metronidazole and clindamycin are used to manage infection caused by anaerobes, while osteomyelitis can be managed with quinolones and linezolid. Further, the effectiveness of different drugs like penicillin,cephalosporin (ceftazidime, ceftriaxone, and cefuroxime), dicloxacillin, vancomycin aztreonam,cefalotin, clindamycin, cefoxitin, gentamicin, imipenem, piperacillin/tazobactam, imipenem,metronidazole, amikacin, levofloxacin, and cefalotin against microbes contributing in DFU infection also established[126]. Dressings with hydrogels, acrylics, hydrofibers, films, hydrocolloids, calcium alginates, polyurethane foam and ciprofloxacin-loaded calcium alginate wafer are also used to control the infection[126,127].
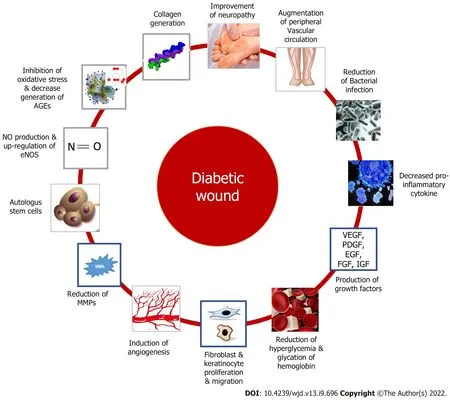
Autologous stem cells capable of self-renewal and multi-lineage differentiation have been used in diabetic wound. Clinically, bone marrow-derived mononuclear cells and mesenchymal stem cells are the most successful stem cell therapies[116,117]. Besides these, several other targets to promote diabetic wound healing include stimulation of nitric oxide production and up-regulation of endothelial NO synthase and nitric oxide (NO) expression[118-120], a decrease of AGE receptors[121], collagen generation and epitheliazation
[122-124]
Figure 3 represents plausible drug targets for diabetic wound.
The prince accepted gladly, and they went back to the palace, where the bald-headed king s daughter, who was still more beautiful than the other princess, welcomed them at the door and led the way into a large hall and to a table covered with silver dishes
Then all too soon the garden was harvested, the vegetables canned and stored, and the school reopened. Soon the leaves fell and the winds blew cold and gusty12 from the bay. Reuben wandered the streets, diligently13 searching for his hessian() treasures.
MANAGING DFU: PHARMACOTHERAPY
Hyperglycemic environments worsen the wound situation and delay the wound healing process, thus controlling blood sugar levels is important for the wound healing process. Several antidiabetic medications [
, insulin, metformin, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors,thiazolidinediones] found effective in controlling not only blood sugar levels but also promoting the healing of a wound. Metformin was found to be effective in wound healing, which may act by improving epidermis and deposition of collagen, and increasing TGF-β production that may link with angiogenesis process, inflammatory reaction, re-epithelization, and remodeling process. DPP-4 inhibitors may act by increasing the generation of SDF1α, which is crucial for wound repair process[11,125]. Drugs like simvastatin (enhance VEGF and NO content at wound site) and phenytoin (enhance VEGF and FGF in wound area) have also shown some positive effects in wound healing[11,125].
MMPs are a class of endopeptidase that play a key part in wound debridement, as well as angiogenesis, epithelialization, and extracellular matrix remodeling[113]. Matrix proteins such as collagens, basement membrane collagens, proteoglycans, elastin, and fibronectin are digested by the MMPs. TIMPs form a complex with MMPs, limiting interaction with the active site. A balance between MMPs and TIMPs is required for wound healing, which is impaired in diabetes patients[114]. Increased protease activity caused by high MMP levels in diabetic wounds causes tissue damage and slows down normal repair processes[115]. This is due to altered MMP expression and decreased TIMP expression in diabetic conditions, which results in high levels of pro-inflammatory and pro-fibrotic cytokines due to increased inflammatory cell activation and invasion, and indirectly affects MMPs through the formation of advanced glycation products which leads to the loss of growth factors, receptors, and matrix proteins essential for wound healing[111,114]. Drugs, therapy that is useful in diabetic wounds are found to act by enhancing collagen synthesis, decreasing inflammatory cytokines, AGEs
Further, identifying the therapeutic agents that can inhibit MMPs,
, MMP-1, MMP-8, and MMP-9 could be important in the management of diabetic wounds.
Debridement (elimination of bacterial biofilm and necrotic tissue from wound site), offloading(complete/partial removal of pressure), PRP, EGF, PDGF, FGF, oxygen therapy, negative pressure wound therapy (NPWT), low-level laser therapy (LLLT), extracorporeal shock wave therapy (ESWT),bio-engineered skin substitutes/ soft tissue substitutes (amniotic membrane, autologous stem cell therapy, bi-layered bio-engineered skin substitute, human fibroblast-derived dermis, porcine small intestine submucosa), and maggot therapy are also used successfully to cure DFU[127-129].
In clinical practice, control of blood glucose level, periodical foot screening, patient education, use of therapeutic footware by susceptible people, prophylactic arterial revascularization are important in prevention of DFU[130]. Off-loading in different grades of DFUs (grade 1B, 1C), and diagnosis of diabetic foot osteomyelitis are recommended in grade 1B, 1C, 2C DFU using different screening methods by Society of Vascular Surgery[130]. Surgical debridement to remove necrotic and devitalized tissue, use of proper dressing to create and maintain moist environment, reducing plantar pressure and shear stress (off-loading), vascular assessment in patient with peripheral arterial disease are the vital part of wound care in diabetic people[130,131]. Microbial infection is a major concern in DFU, and in such cases, use of antimicrobial agents is advised conserving extent of infection[131]. A number of adjuvant therapy as discussed in previous section are also used for effective wound healing. Further,adequate glycemic control is important also in acceleratd healing of DFU’s[130,131].
RECENT APPROACHES IN CLINICAL TRIALS
PRP is an important treatment approach investigated in DFU. A systematic review with metaanalyses of 10 studies reported that PRP promotes chronic diabetic wound healing (RR = 1.32; 95%CI:1.11-1.57,
= 15%) by reducing the volume and time of wound healing[160]. PRP may act
promoting the proliferation of wound cells, upregulation of cyclin A and cyclin-dependent kinase 4 proteins,modification of macrophage phenotype, reduction of TNF-α, enhancement of TGF-β and VEGF,increased secretion of fibroblast of collagen type I and III[161].
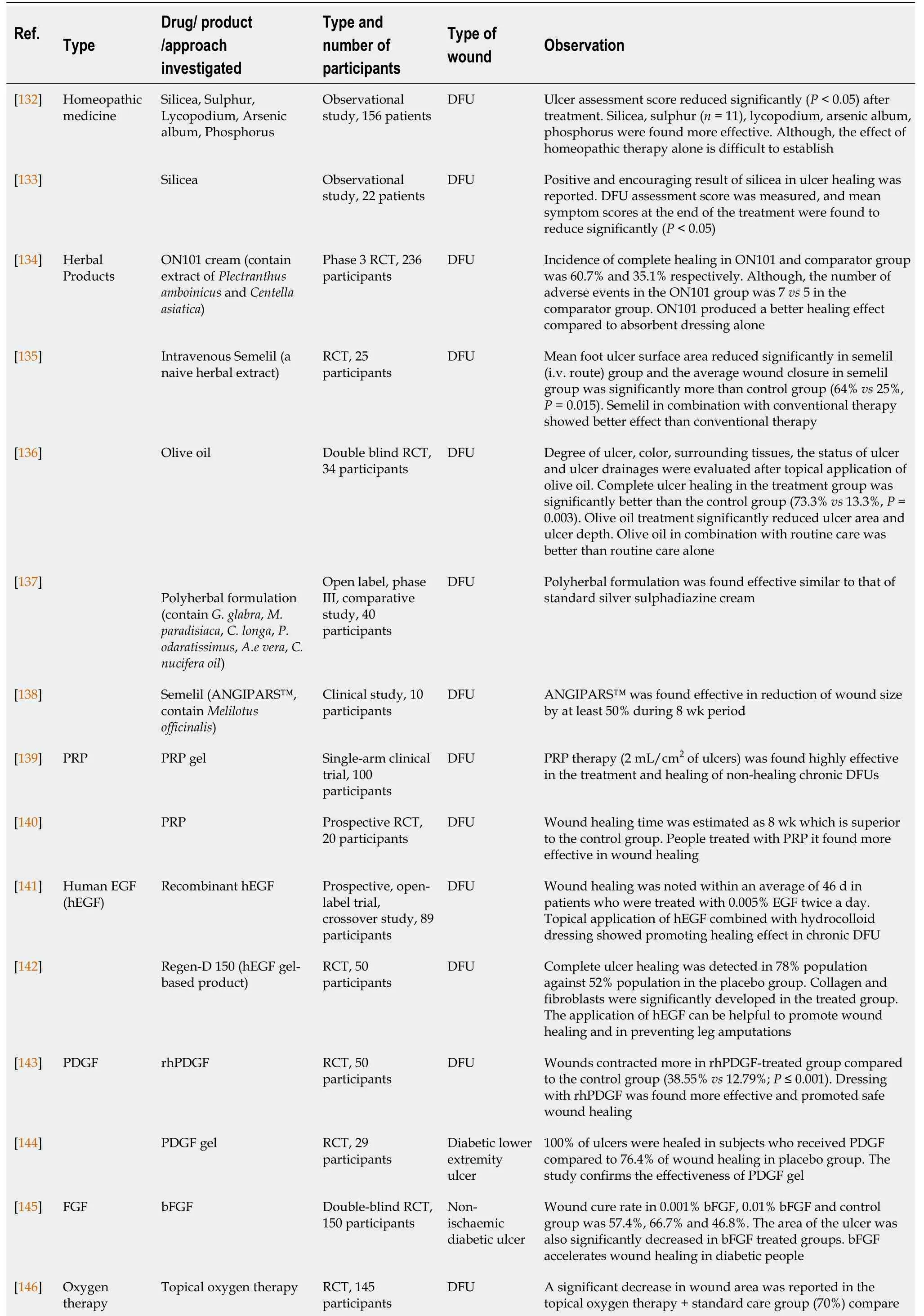
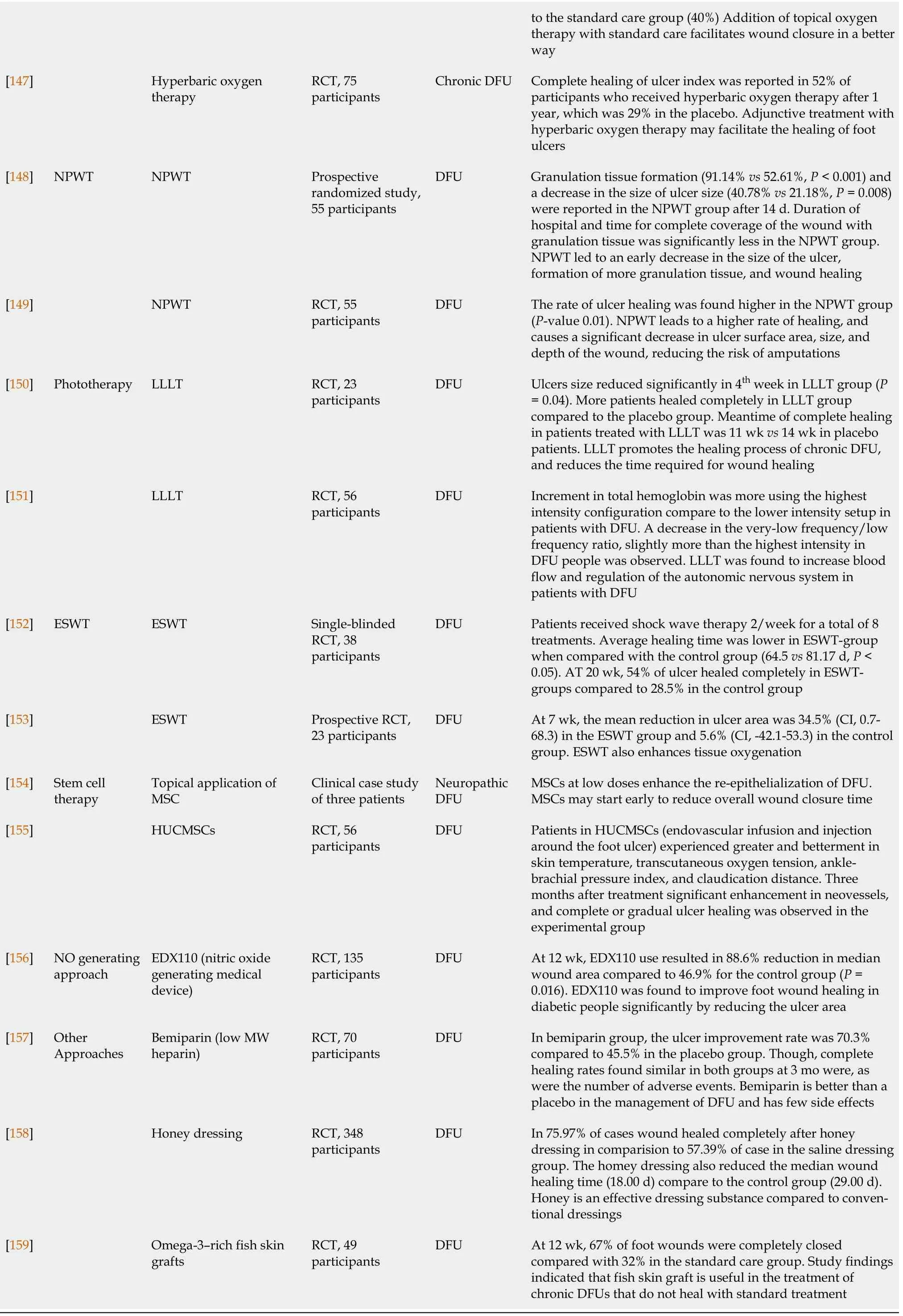
Several treatments /medicines have been successfully investigated clinically for their beneficial effects on the diabetic wound, especially in DFUs which was tabulated in Table 1[132-159].
A systematic review of 26 randomized controlled trials (RCTs) examined the usefulness of different recombinant proteins and growth factors in the treatment of DFU. EGF can be used as intralesional injection, topical cream, or gel. This meta-analysis reported that EGF significantly improves wound healing and the study failed to find a significant effect of EGF in reducing the risk of amputation[162].Decreasing the level of circulating C-reactive protein, decreasing NFκB1, TNF-α, and IL-1a expression,and increasing the wound expression of platelet-derived growth factors (PDGF)-B, cyclin-dependent kinase 4, P21, TP53, angiopoietin 1, collagen 1A1, MMP-2, and TIMP-2 after the treatment with hEGF may be linked to its beneficial effect[161]. Recombinant human PDGF (rhPDGF) also exhibits their beneficial effect in several clinical studies[143,144], but few clinical studies also failed to find statistically significant effects of rhPDGF compared to the control group[163,164] when used to cure DFU. The effectiveness of PDGF may be related to the promotion of fibroblast and leukocyte migration, and synthesis of extracellular matrix[161]. FGF, a family of cell signaling proteins, is found to stimulate angiogenesis, induce fibroblasts proliferation, and promote wound healing. About 23 subtypes of FGF from 7 subfamilies were identified[165]. Some clinical studies have reported the beneficial effect of FGF in diabetic wound[145], some studies failed to report the advantageous effect of FGF over the placebo group[166], while clinical studies reported the beneficial effect of combined use of human EGF and acidic FGF on wound healing in the later stage[167]. Very few clinical studies evaluated the effectiveness of granulocyte-colony-stimulating factor, topical telbermin, epoetin-β, talactoferrin, TGF-β2 in the diabetic wound. But the results are inconsistent[162].
LLLT was found to increase blood flow and regulation of the autonomic nervous system in patients suffering from DFU[151]. Reduce wound inflammation, enhance fibroblasts and angiogenesis[161],which may play an important role in diabetic wound healing. A metanalysis of 13 RCTs that included 413 patients concluded that LLLT significantly enhanced complete healing rate (RR = 2.10, 95%CI: 1.56-2.83,
< 0.00001), decreased wound ulcer area, and reduce mean healing time of wound healing in patients suffering from DFU[171].
It was evaluated that NPWT results in macro- and micro-deformation that stimulate different wound healing cascade like promotion of tissue granulation, epithelialization, proliferation of vessel, neoangiogenesis, pro-angiogenic condition, removal of surplus extracellular fluid, anti-inflammatory effect,increase expression of VEGF, FGF2, modification of circulating micro-RNAs, alteration of DNA methylation of genes linked with wound repair[161,168]. Liu
[169] in their study (meta-analysis of 11 RCTs) concluded that NPWT is a safe, cost-effective and effective strategy in the treatment of DFU,while Liu also evaluated 11 RCTs and found that compared with wound dressings NPWT may enhance the proportion of wounds healed, decrease the time of postoperative foot wound healing[170].
Oxygen therapy is found to improve cell metabolism and energy, decrease proinflammatory cytokines and ROS, and promote the synthesis of matrix and wound repair[161]. Few clinical studies confirmed the beneficial effect of hyperbaric as well as topic oxygen therapy in promoting diabetic wound[146,147]. A systematic review and meta-analysis of 20 RCTs and 1263 trials analysed that hyperbaric oxygen therapy confer benefits in DFU treatment by increasing the healing of ulcers (relative risk, 1.901; 95%CI: 1.484-2.435,
< 0.0001), reducing healing time, and also decrease the risk of major amputation[148].
In recent studies, neuropathy, peripheral vascular disease, and impaired wound healing have all been identified as key contributors to diabetic wounds and have all become critical targets in improving wound healing in diabetic patients. But despite of this well-established knowledge, comparatively fewer treatment options are being implemented in routine practice. The expression of cellular components of participating cells, as well as cytokines, growth factors, and other molecular factors required for coordinating the normal healing process, is impaired in diabetic wounds, and as a result, they are unable to progress in synchrony and are primarily checked in the inflammatory phase. Therapeutic strategy targeting such cellular and molecular pathway could be useful for effective management of diabetic wound.
Huang
[172] performed a meta-analysis of 8 RCTs and concluded that ESWT treatment reduced wound surface area in greater proportion, enhances re-epithelialization and can reduce treatment inefficiency. ESWT is useful as an adjuvant strategy in the management of DFUs, which can improve the complete wound cure rate and reduce the healing period of DFUs. ESWT may enhance the angiogenesis process, decrease macrophage number, and enhance the production of macrophage of growth factors from macrophages that help in the healing of wound[161].
In light of current evidence, it can be suggested that stem cell-based therapy (delivery through both local and systemic route is effective to heal DFU and considered a promising regenerative medicine, and mechanisms of stem cell therapy include improved angiogenesis, decrease inflammation, ameliorating neuroischemia, improved collagen deposition,
[173].
Wound healing in diabetic people can be promoted by providing endogenous or exogenous NO.Products (
, patches/ matrices) that release NO are used to treat diabetic wounds by different mechanisms like enhancement of angiogenic activity, endothelial cell proliferation, conferring antimicrobial substances, and promoting cell migration to the injured site[174]. Only a few clinical studies have reported the beneficial effects of NO-releasing devices, and several products are in the clinical trial.
Homeopathic medicines (like silicea, sulphur, lycopodium, and arsenic) were also investigated in the clinical trial and concluded that homeopathic medicine may be useful in the management of diabetic wounds[132,133]. Several other products, like honey, fish skin grafts,
were also clinically investigated for their beneficial effects against DFU[158,159]. Further, several herbal products were also successfully investigated in the treatment of diabetic wound[134,135,136-138].
MEDICINAL PLANTS AND PHYTOCONSTITUENTS IN DIABETIC WOUND
Medicinal plants and phytoconstituents always represent an important, effective and alternative treatment strategy to cure diseases. Phytochemicals have showed their effectiveness in different diabetes complications. Anti-inflammatory mechanism of phytochemicals is considered important in the management of diabetes wound. Epigallocatechin gallate in pre-clinical investigation was found to reduce reduced levels of IL-1β, TNF-α and IL-6, producing inhibition of Notch signaling and accumulation of macrophage at a wound site. Kaempferol, is an important dietary flavonoid found to exert different pharmacological activities, including antioxidant, anti-inflammatory and cardioprotective activity. An ointment containing Kaempferol was found effective in diabetic excisional and non-diabetic incisional wounds in experimental animals[175]. Flavonoids an important class of phytoconstituents,exerted anti-inflammatory and antioxidant effect, and also enhances angiogenesis and re-epithelialization. Preclinical trials found the effectiveness of isoliquiritin, isoflavonoid, naringenin,dihydromyricetin, dihydroquercetin, quercetin, hesperidin, kaempferol, proanthocyanidins, icariin,puerarin, rutin, genistein, luteolin, rutoside, silymarin, daidzein, genistein, and epigallocatechin gallate to cure wound[174]. Flavonoids positively regulate MMP-2, MMP-8, MMP-9, MMP-13, Ras/Raf/MEK/ERK, PI3K/Akt, and NO pathways. Phytochemicals are found to reduce oxidative stress,expression/release of proinflammatory/inflammatory cytokines,
, TNF-α, IL-1β, IL-6, NF-κB and upregulate IL-10 and antioxidant enzymes. Flavonoids also act on macrophages, fibroblasts and endothelial cells by facilitating expression/release of TGF-β1, VEGF, angiopoietin, tyrosine kinase with immunoglobulin and epidermal growth factor homology domains, and small mothers against decapentaplegic 2 and 3[176]. Oguntibeju[177] in his paper highlighted different medicinal plants like
,
,
,
,
,
,
,
,
,
, and
that has been investigated in the treatment of diabetic wound. Benefits of the plants may link to different mechanisms like an increase in fibroblast cell, fibroplasia, increase in collagen formation, enhancement of tissue regeneration, angiogenesis, antimicrobial, anti-inflammatory and antioxidant effect. A recent clinical study established the effectiveness and safety of nano-hydrogel embedded with quercetin and oleic acid when used in the management of lower limb skin wound in diabetic patients. The formulation effectively treated the wound and reduce the wound healing time compared to the control group[178].Infections caused by different microbes like
,
β-hemolytic
,
,
,
,
,
. and anaerobes are posing a serious situation in diabetic people[126]. Medicinal plants and phytochemicals with antimicrobial activity may also play an important role in the management of diabetic wound.Several plants have shown their potential against the microbial strain responsible for infection in the diabetic wound[126]. Formulation designed with
,
,
,
,
,
,
, and
has shown their potential in the treatment of diabetic wound[126]. Isoflavones isolated from plant sources were also found to be effective against DFU bacteria[179] Phytofabricated silver nanoparticles (
reduced silver nanoparticles) at 20 μg/mL were found highly effective against multi antibiotic-resistant DFU isolates like
,
,
. Identified phytochemicals of
include rutin,quercetin, kaempferol, gallic acid and ellagic acid[180]. The use of phytoextracts/active compounds may be considered as an important strategy for addressing the wound problem associated with DM in a better way.
CONCLUSION
Diabetes adversely acts on the phases of normal wound healing phases, i.e., hemostasis, inflammatory phase, proliferative phase, re-epithelialization and remodeling phase, and poses a big burden on the quality of life of a diabetic individual. Hyperglycemia can trigger oxidative stress, increase inflammatory cytokines, interrupt angiogenesis, decrease the functioning of fibroblast and keratinocyte,induce neuropathy associated events, increase MMPs, and reduce TIMPs that is responsible for impaired states of wound healing. An understanding sequence of the molecular and cellular cascade,epigenetic mechanisms, microbial perspective, complexity and plasticity of impaired wound healing in diabetic conditions is required for targeted research focusing on treatment of diabetic wound. Pharmacotherapy/strategy involving angiogenesis stimulation, growth factors, cytokines modulators, MMP inhibitors, ECM stimulators, anti-inflammatory drugs, antidiabetic agents, antimicrobial drugs,debridement, offloading, PRP, oxygen therapy, NPWT, LLLT, ESWT, stem cells, bio-engineered substitutes, and various natural-based products have shown their benefit. There has been a lack of quality-based evidence of efficacy of different adjuvant therapies tested through different clinical trials,thus more structured and quality studies are required. Indeed, the utilisation of medicinal plants/products in diabetic wound care holds prodigious potential in the future, and the development of innovative pharmaceutical formulations for advanced wound care is equally critical. Effective diabetic wound management necessitates a combination of techniques, including medication and nonpharmacological intervention. Hence, the treatment strategy of the future can only succeed if research concentrated on plausible drug targets after comprehending the inherent pathological complexities,evaluating non-pharmacological approaches through well-designed clinical trials, and targeting natural sources for new drug development.
The whole court applauded her with hands and tails; and for a moment her heart felt quite gay, for she knew she had the loveliest voice25 of any on earth or in the sea
FOOTNOTES
Chakraborty R, Borah P, Sen S, Dutta PP performed data accusation and writing; Borah P,Dutta PP prepared the figures; Chakraborty R provided the input in writing the paper; Sen S designed the outline and coordinated the writing of the paper.
There is no conflict of interest associated with any of the authors contributed their efforts in this manuscript.
I tried to be invisible within a sea of faces. I wanted to run away. Disappear. Most of all I wanted no one to guess whose kid I was. I was betrayed by my last name, which began with the letter A, so I was the first graduate on the first row; Being a red-head gave me even more exposure, and the baccalaureate() speaker, who had never met me, decided6 to use me as his audio-visual aid.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
India
Raja Chakraborty 0000-0002-2097-9178; Pobitra Borah 0000-0003-1047-4552; Partha Pratim Dutta 0000-0003-0907-3030; Saikat Sen 0000-0002-5279-1532.
Halloa! you Turkish nurse, said he, what is that great castle there close to the town? The one with the windows so high up? The sultan s daughter lives there, she replied
Chang KL
A
Chang KL
1 Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev 2019; 99: 665-706 [PMID: 30475656 DOI: 10.1152/physrev.00067.2017]
2 Shah A, Amini-Nik S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int J Mol Sci 2017; 18[PMID: 28509885 DOI: 10.3390/ijms18051068]
3 Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol 2020; 10:200223 [PMID: 32993416 DOI: 10.1098/rsob.200223]
4 Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother 2019; 112: 108615 [PMID: 30784919 DOI:10.1016/j.biopha.2019.108615]
5 Sen CK. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv Wound Care (New Rochelle)2021; 10: 281-292 [PMID: 33733885 DOI: 10.1089/wound.2021.0026]
6 Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045.Diabetes Res Clin Pract 2022; 183: 109119 [PMID: 34879977 DOI: 10.1016/j.diabres.2021.109119]
7 Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, Sun H, Boyko EJ, Magliano DJ. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract 2022; 183: 109118[PMID: 34883189 DOI: 10.1016/j.diabres.2021.109118]
8 Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic Wound-Healing Science. Medicina (Kaunas)2021; 57 [PMID: 34684109 DOI: 10.3390/medicina57101072]
9 Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis
. Ann Med 2017; 49: 106-116 [PMID: 27585063 DOI: 10.1080/07853890.2016.1231932]
10 Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int 2013; 2013: 385641 [PMID: 23653894 DOI: 10.1155/2013/385641]
11 Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals (Basel) 2020; 13 [PMID: 32244718 DOI: 10.3390/ph13040060]
12 Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117: 1219-1222[PMID: 17476353 DOI: 10.1172/JCI32169]
13 Pastar I, Ojeh N, Glinos GD, Stojadinovic O, Tomic-Canic M. Physiology and Pathophysiology of Wound Healing in Diabetes. In: The Diabetic Foot. 2018: 109-130 [DOI: 10.1007/978-3-319-89869-8_7]
14 Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 2007; 170: 1178-1191 [PMID: 17392158 DOI: 10.2353/ajpath.2007.060018]
15 Okizaki S, Ito Y, Hosono K, Oba K, Ohkubo H, Amano H, Shichiri M, Majima M. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice.Biomed Pharmacother 2015; 70: 317-325 [PMID: 25677561 DOI: 10.1016/j.biopha.2014.10.020]
16 Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005; 366: 1736-1743 [PMID: 16291068 DOI:10.1016/S0140-6736(05)67700-8]
17 LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med 1984; 311: 1615-1619 [PMID: 6390204 DOI: 10.1056/NEJM198412203112506]
18 Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des 2005; 11: 2301-2309 [PMID: 16022669 DOI:10.2174/1381612054367328]
19 Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int J Low Extrem Wounds 2005; 4: 154-159 [PMID: 16100096 DOI: 10.1177/1534734605280130]
20 Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous Manifestations of Diabetes Mellitus: A Review. Am J Clin Dermatol 2017; 18: 541-553 [PMID: 28374407 DOI: 10.1007/s40257-017-0275-z]
21 Andrade TAM, Masson-Meyers DS, Caetano GF, Terra VA, Ovidio PP, Jordão-Júnior AA, Frade MAC. Skin changes in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun 2017; 490: 1154-1161 [PMID: 28668393 DOI:10.1016/j.bbrc.2017.06.166]
22 Kim JH, Yoon NY, Kim DH, Jung M, Jun M, Park HY, Chung CH, Lee K, Kim S, Park CS, Liu KH, Choi EH. Impaired permeability and antimicrobial barriers in type 2 diabetes skin are linked to increased serum levels of advanced glycation end-product. Exp Dermatol 2018; 27: 815-823 [PMID: 29151267 DOI: 10.1111/exd.13466]
23 Blakytny R, Jude EB. Altered molecular mechanisms of diabetic foot ulcers. Int J Low Extrem Wounds 2009; 8: 95-104[PMID: 19443898 DOI: 10.1177/1534734609337151]
24 Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells.
1999; 145: 1309-1323 [PMID: 10366601 DOI:10.1083/jcb.145.6.1309]
25 Ramirez HA, Liang L, Pastar I, Rosa AM, Stojadinovic O, Zwick TG, Kirsner RS, Maione AG, Garlick JA, Tomic-Canic M. Comparative Genomic, MicroRNA, and Tissue Analyses Reveal Subtle Differences between Non-Diabetic and Diabetic Foot Skin.
2015; 10: e0137133 [PMID: 26318001 DOI: 10.1371/journal.pone.0137133]
26 Maione AG, Smith A, Kashpur O, Yanez V, Knight E, Mooney DJ, Veves A, Tomic-Canic M, Garlick JA. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair.
2016; 24: 630-643 [PMID: 27102877 DOI: 10.1111/wrr.12437]
27 Liang L, Stone RC, Stojadinovic O, Ramirez H, Pastar I, Maione AG, Smith A, Yanez V, Veves A, Kirsner RS, Garlick JA, Tomic-Canic M. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions.
2016; 24: 943-953 [PMID:27607190 DOI: 10.1111/wrr.12470]
28 Maione AG, Brudno Y, Stojadinovic O, Park LK, Smith A, Tellechea A, Leal EC, Kearney CJ, Veves A, Tomic-Canic M, Mooney DJ, Garlick JA. Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic
features of chronic wounds.
2015; 21: 499-508 [PMID: 25343343 DOI: 10.1089/ten.TEC.2014.0414]
29 Berlanga-Acosta J, Mendoza-Mari Y, Martínez MD, Valdés-Perez C, Ojalvo AG, Armstrong DG. Expression of cell proliferation cycle negative regulators in fibroblasts of an ischemic diabetic foot ulcer. A clinical case report.
2013; 10: 232-236 [PMID: 23194110 DOI: 10.1111/j.1742-481X.2012.12000.x]
30 Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells.
2007; 56: 960-967 [PMID: 17395742 DOI: 10.2337/db06-1254]
31 Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing.
2008; 10: 1869-1882 [PMID: 18627349 DOI: 10.1089/ars.2008.2121]
32 Yu CG, Zhang N, Yuan SS, Ma Y, Yang LY, Feng YM, Zhao D. Endothelial Progenitor Cells in Diabetic Microvascular Complications: Friends or Foes?
2016; 2016: 1803989 [PMID: 27313624 DOI: 10.1155/2016/1803989]
33 Mirza RE, Fang MM, Novak ML, Urao N, Sui A, Ennis WJ, Koh TJ. Macrophage PPARγ and impaired wound healing in type 2 diabetes.
2015; 236: 433-444 [PMID: 25875529 DOI: 10.1002/path.4548]
34 Krisp C, Jacobsen F, McKay MJ, Molloy MP, Steinstraesser L, Wolters DA. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients.
2013; 13: 2670-2681 [PMID: 23798543 DOI: 10.1002/pmic.201200502]
35 Liu Y, Min D, Bolton T, Nubé V, Twigg SM, Yue DK, McLennan SV. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers.
2009; 32: 117-119 [PMID: 18835949 DOI:10.2337/dc08-0763]
36 Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients.
2002; 45: 1011-1016 [PMID:12136400 DOI: 10.1007/s00125-002-0868-8]
37 Obrosova IG. Update on the pathogenesis of diabetic neuropathy.
2003; 3: 439-445 [PMID: 14611738 DOI: 10.1007/s11892-003-0005-1]
38 Wang C, Li C, Peng H, Ye Z, Zhang J, Liu X, Lou T. Activation of the Nrf2-ARE pathway attenuates hyperglycemiamediated injuries in mouse podocytes.
2014; 34: 891-902 [PMID: 25200066 DOI:10.1159/000366307]
39 Lee YJ, Kwon SB, An JM, Kim CH, Lee SH, Choi CY, Nam DH, Park JW, Nam HS, Lee MW, Cho MK. Increased protein oxidation and decreased expression of nuclear factor E2-related factor 2 protein in skin tissue of patients with diabetes.
2015; 40: 192-200 [PMID: 25557240 DOI: 10.1111/ced.12487]
40 Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, Zhou S, Wong PK, Wondrak GT, Zheng H, Zhang DD. An Essential Role of NRF2 in Diabetic Wound Healing.
2016; 65: 780-793 [PMID: 26718502 DOI:10.2337/db15-0564]
41 Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A, Ciciliot S, Mammano F, Ciubotaru CD,Brocco E, Marescotti MC, Cappellari R, Arrigoni G, Millioni R, Vigili de Kreutzenberg S, Albiero M, Avogaro A.NETosis Delays Diabetic Wound Healing in Mice and Humans.
2016; 65: 1061-1071 [PMID: 26740598 DOI:10.2337/db15-0863]
42 Deng L, Du C, Song P, Chen T, Rui S, Armstrong DG, Deng W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing.
2021; 2021: 8852759 [PMID: 33628388 DOI: 10.1155/2021/8852759]
43 Yamaoka H, Sasaki H, Yamasaki H, Ogawa K, Ohta T, Furuta H, Nishi M, Nanjo K. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy.
2010; 33: 150-155 [PMID: 20040674 DOI:10.2337/dc09-0632]
44 Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress.
2002; 3: 275-283 [PMID:12164482 DOI: 10.2174/1389201023378265]
45 Caporali A, Meloni M, Völlenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH,Agami R, le Sage C, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia.
2011; 123: 282-291 [PMID: 21220732 DOI: 10.1161/CIRCULATIONAHA.110.952325]
46 Rafehi H, El-Osta A, Karagiannis TC. Epigenetic mechanisms in the pathogenesis of diabetic foot ulcers.
2012; 26: 554-561 [PMID: 22739801 DOI: 10.1016/j.jdiacomp.2012.05.015]
47 Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing:promotive role of miR-21 in fibroblast migration.
2012; 9: 355-361 [PMID: 22067035 DOI:10.1111/j.1742-481X.2011.00890.x]
48 Wang X, Cao Q, Yu L, Shi H, Xue B. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity.
2016; 1: e87748 [PMID: 27882346 DOI: 10.1172/jci.insight.87748]
49 Yan J, Tie G, Wang S, Tutto A, DeMarco N, Khair L, Fazzio TG, Messina LM. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages.
2018; 9:33 [PMID: 29295997 DOI: 10.1038/s41467-017-02425-z]
50 Ling L, Ren M, Yang C, Lao G, Chen L, Luo H, Feng Z, Yan L. Role of site-specific DNA demethylation in TNFαinduced MMP9 expression in keratinocytes.
2013; 50: 279-290 [PMID: 23417766 DOI:10.1530/JME-12-0172]
51 Zhang J, Yang C, Wang C, Liu D, Lao G, Liang Y, Sun K, Luo H, Tan Q, Ren M, Yan L. AGE-induced keratinocyte MMP-9 expression is linked to TET2-mediated CpG demethylation.
2016; 24: 489-500 [PMID:26913994 DOI: 10.1111/wrr.12426]
52 Dhawan S, Tschen SI, Zeng C, Guo T, Hebrok M, Matveyenko A, Bhushan A. DNA methylation directs functional maturation of pancreatic β cells.
2015; 125: 2851-2860 [PMID: 26098213 DOI: 10.1172/JCI79956]
53 Chen Z, Miao F, Paterson AD, Lachin JM, Zhang L, Schones DE, Wu X, Wang J, Tompkins JD, Genuth S, Braffett BH,Riggs AD; DCCT/EDIC Research Group, Natarajan R. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort.
2016;113: E3002-E3011 [PMID: 27162351 DOI: 10.1073/pnas.1603712113]
54 Robert I, Aussems M, Keutgens A, Zhang X, Hennuy B, Viatour P, Vanstraelen G, Merville MP, Chapelle JP, de Leval L, Lambert F, Dejardin E, Gothot A, Chariot A. Matrix Metalloproteinase-9 gene induction by a truncated oncogenic NFkappaB2 protein involves the recruitment of MLL1 and MLL2 H3K4 histone methyltransferase complexes.
2009; 28: 1626-1638 [PMID: 19219072 DOI: 10.1038/onc.2009.6]
55 Carson WF 4th, Cavassani KA, Soares EM, Hirai S, Kittan NA, Schaller MA, Scola MM, Joshi A, Matsukawa A,Aronoff DM, Johnson CN, Dou Y, Gallagher KA, Kunkel SL. The STAT4/MLL1 Epigenetic Axis Regulates the Antimicrobial Functions of Murine Macrophages.
2017; 199: 1865-1874 [PMID: 28733487 DOI:10.4049/jimmunol.1601272]
56 Wang X, Zhu K, Li S, Liao Y, Du R, Zhang X, Shu HB, Guo AY, Li L, Wu M. MLL1, a H3K4 methyltransferase,regulates the TNFα-stimulated activation of genes downstream of NF-κB.
2012; 125: 4058-4066 [PMID:22623725 DOI: 10.1242/jcs.103531]
57 Kimball AS, Joshi A, Carson WF 4th, Boniakowski AE, Schaller M, Allen R, Bermick J, Davis FM, Henke PK, Burant CF, Kunkel SL, Gallagher KA. The Histone Methyltransferase MLL1 Directs Macrophage-Mediated Inflammation in Wound Healing and Is Altered in a Murine Model of Obesity and Type 2 Diabetes.
2017; 66: 2459-2471[PMID: 28663191 DOI: 10.2337/db17-0194]
58 Gallagher KA, Joshi A, Carson WF, Schaller M, Allen R, Mukerjee S, Kittan N, Feldman EL, Henke PK, Hogaboam C,Burant CF, Kunkel SL. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes.
2015; 64: 1420-1430 [PMID: 25368099 DOI: 10.2337/db14-0872]
59 De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 Lysine-27 demethylase Jmjd3 Links inflammation to inhibition of polycomb-mediated gene silencing.
2007; 130: 1083-1094 [PMID: 17825402 DOI: 10.1016/j.cell.2007.08.019]
60 Zimmermann M, Aguilera FB, Castellucci M, Rossato M, Costa S, Lunardi C, Ostuni R, Girolomoni G, Natoli G,Bazzoni F, Tamassia N, Cassatella MA. Chromatin remodelling and autocrine TNFα are required for optimal interleukin-6 expression in activated human neutrophils.
2015; 6: 6061 [PMID: 25616107 DOI: 10.1038/ncomms7061]
61 Na J, Shin JY, Jeong H, Lee JY, Kim BJ, Kim WS, Yune TY, Ju BG. JMJD3 and NF-κB-dependent activation of Notch1 gene is required for keratinocyte migration during skin wound healing.
2017; 7: 6494 [PMID: 28747631 DOI:10.1038/s41598-017-06750-7]
62 den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing.
2019; 204: 39-50 [PMID: 30392877 DOI: 10.1016/j.trsl.2018.10.001]
63 Thimmappaiah Jagadeesh A, Prakash PY, Karthik Rao N, Ramya V. Culture characterization of the skin microbiome in Type 2 diabetes mellitus: A focus on the role of innate immunity.
2017; 134: 1-7 [PMID:28951341 DOI: 10.1016/j.diabres.2017.09.007]
64 Dörr S, Holland-Letz AK, Weisser G, Chatzitomaris A, Lobmann R. Bacterial Diversity, Antibiotic Resistance, and the Risk of Lower Limb Amputation in Younger and Older Individuals With Diabetic Foot Infection.
2021; 1534734621992290 [PMID: 33745353 DOI: 10.1177/1534734621992290]
65 Radzieta M, Sadeghpour-Heravi F, Peters TJ, Hu H, Vickery K, Jeffries T, Dickson HG, Schwarzer S, Jensen SO,Malone M. A multiomics approach to identify host-microbe alterations associated with infection severity in diabetic foot infections: a pilot study.
2021; 7: 29 [PMID: 33753735 DOI: 10.1038/s41522-021-00202-x]
66 Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors.
2013; 62: 923-930 [PMID: 23139351 DOI: 10.2337/db12-0771]
67 van Asten SA, La Fontaine J, Peters EJ, Bhavan K, Kim PJ, Lavery LA. The microbiome of diabetic foot osteomyelitis.
2016; 35: 293-298 [PMID: 26670675 DOI: 10.1007/s10096-015-2544-1]
68 Ezeani IU, Ugwu ET, Adeleye FO, Gezawa ID, Okpe IO, Enamino MI. Determinants of wound healing in patients hospitalized for diabetic foot ulcer: results from the MEDFUN study.
2020; 54: 207-216 [PMID: 32857716 DOI: 10.2478/enr-2020-0023]
69 Sumitomo T, Mori Y, Nakamura Y, Honda-Ogawa M, Nakagawa S, Yamaguchi M, Matsue H, Terao Y, Nakata M,Kawabata S. Streptococcal Cysteine Protease-Mediated Cleavage of Desmogleins Is Involved in the Pathogenesis of Cutaneous Infection.
2018; 8: 10 [PMID: 29416987 DOI: 10.3389/fcimb.2018.00010]
70 Greener B, Hughes AA, Bannister NP, Douglass J. Proteases and pH in chronic wounds.
2005; 14: 59-61[PMID: 15739652 DOI: 10.12968/jowc.2005.14.2.26739]
71 McArdle CD, Lagan KM, McDowell DA. Effects of pH on the Antibiotic Resistance of Bacteria Recovered from Diabetic Foot Ulcer Fluid An In Vitro Study.
2018; 108: 6-11 [PMID: 29547034 DOI:10 .7547/16-033]
72 Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification.
1998; 57:1325-1332, 1337 [PMID: 9531915]
73 Bowering CK. Diabetic foot ulcers. Pathophysiology, assessment, and therapy.
2001; 47: 1007-1016[PMID: 11398715]
74 Frykberg RG. Diabetic foot ulcers: pathogenesis and management.
2002; 66: 1655-1662 [PMID:12449264]
75 Lavery LA, Peters EJ, Williams JR, Murdoch DP, Hudson A, Lavery DC; International Working Group on the Diabetic Foot. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot.
2008; 31: 154-156 [PMID: 17934155 DOI:10.2337/dc07-1302]
76 Schaper NC, Nabuurs-Franssen MH. The diabetic foot: pathogenesis and clinical evaluation.
2002; 2:221-228 [PMID: 16222613 DOI: 10.1055/s-2002-32045]
77 Searles JM Jr, Colen LB. Foot reconstruction in diabetes mellitus and peripheral vascular insufficiency.
1991; 18: 467-483 [PMID: 1889157]
78 Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers.
2004; 351: 48-55[PMID: 15229307 DOI: 10.1056/NEJMcp032966]
79 Chand G, Mishra AK, Kumar S, Agarwal A. Diabetic foot.
2012; 2: 144-150
80 Vallianou N, Evangelopoulos A, Koutalas P. Alpha-lipoic Acid and diabetic neuropathy.
2009; 6: 230-236 [PMID: 20043035 DOI: 10.1900/RDS.2009.6.230]
81 Benrath J, Zimmermann M, Gillardon F. Substance P and nitric oxide mediate would healing of ultraviolet photodamaged rat skin: evidence for an effect of nitric oxide on keratinocyte proliferation.
1995; 200: 17-20 [PMID: 8584255 DOI: 10.1016/0304-3940(95)12062-9]
82 Ghersi G, Chen W, Lee EW, Zukowska Z. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding.
2001; 22: 453-458 [PMID: 11287101 DOI:10.1016/s0196-9781(01)00340-0]
83 Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control.
2007; 23: 3-13 [PMID: 16960917 DOI: 10.1002/dmrr.682]
84 Smith K, Collier A, Townsend EM, O'Donnell LE, Bal AM, Butcher J, Mackay WG, Ramage G, Williams C. One step closer to understanding the role of bacteria in diabetic foot ulcers: characterising the microbiome of ulcers.
2016; 16: 54 [PMID: 27005417 DOI: 10.1186/s12866-016-0665-z]
85 Basu Mallik S, Jayashree BS, Shenoy RR. Epigenetic modulation of macrophage polarization- perspectives in diabetic wounds.
2018; 32: 524-530 [PMID: 29530315 DOI: 10.1016/j.jdiacomp.2018.01.015]
86 Kwon KT, Armstrong DG. Microbiology and Antimicrobial Therapy for Diabetic Foot Infections.
2018; 50: 11-20 [PMID: 29637748 DOI: 10.3947/ic.2018.50.1.11]
87 Brownlee M. The pathobiology of diabetic complications: a unifying mechanism.
2005; 54: 1615-1625 [PMID:15919781 DOI: 10.2337/diabetes.54.6.1615]
88 Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS.Diabetic foot ulcers: Part I. Pathophysiology and prevention.
2014; 70: 1.e1-18; quiz 19 [PMID:24355275 DOI: 10.1016/j.jaad.2013.06.055]
89 Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S,Tousoulis D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives.
2019; 14:50-59 [PMID: 31131037 DOI: 10.15420/ecr.2018.33.1]
90 Loot MA, Kenter SB, Au FL, van Galen WJ, Middelkoop E, Bos JD, Mekkes JR. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls.
2002; 81: 153-160 [PMID: 11998867 DOI: 10.1078/0171-9335-00228]
91 Nishikori Y, Shiota N, Okunishi H. The role of mast cells in cutaneous wound healing in streptozotocin-induced diabetic mice.
2014; 306: 823-835 [PMID: 25218083 DOI: 10.1007/s00403-014-1496-0]
92 Tellechea A, Leal EC, Kafanas A, Auster ME, Kuchibhotla S, Ostrovsky Y, Tecilazich F, Baltzis D, Zheng Y, Carvalho E, Zabolotny JM, Weng Z, Petra A, Patel A, Panagiotidou S, Pradhan-Nabzdyk L, Theoharides TC, Veves A. Mast Cells Regulate Wound Healing in Diabetes.
2016; 65: 2006-2019 [PMID: 27207516 DOI: 10.2337/db15-0340]
93 Bevan D, Gherardi E, Fan TP, Edwards D, Warn R. Diverse and potent activities of HGF/SF in skin wound repair.
2004; 203: 831-838 [PMID: 15221943 DOI: 10.1002/path.1578]
94 Singh K, Agrawal NK, Gupta SK, Mohan G, Chaturvedi S, Singh K. Decreased expression of heat shock proteins may lead to compromised wound healing in type 2 diabetes mellitus patients.
2015; 29: 578-588[PMID: 25746357 DOI: 10.1016/j.jdiacomp.2015.01.007]
95 Luong M, Zhang Y, Chamberlain T, Zhou T, Wright JF, Dower K, Hall JP. Stimulation of TLR4 by recombinant HSP70 requires structural integrity of the HSP70 protein itself.
2012; 9: 11 [PMID: 22448747 DOI:10.1186/1476-9255-9-11]
96 Park KH, Han SH, Hong JP, Han SK, Lee DH, Kim BS, Ahn JH, Lee JW. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A phase III multicenter, double-blind, randomized, placebo-controlled trial.
2018; 142: 335-344 [PMID: 29902542 DOI: 10.1016/j.diabres.2018.06.002]
97 Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing.
2008; 16: 585-601 [PMID: 19128254 DOI: 10.1111/j.1524-475X.2008.00410.x]
98 Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse.
1990; 136: 1235-1246 [PMID: 2356856]
99 Doxey DL, Ng MC, Dill RE, Iacopino AM. Platelet-derived growth factor levels in wounds of diabetic rats.
1995; 57: 1111-1123 [PMID: 7658918 DOI: 10.1016/0024-3205(95)02056-o]
100 Li H, Fu X, Zhang L, Huang Q, Wu Z, Sun T. Research of PDGF-BB gel on the wound healing of diabetic rats and its pharmacodynamics.
2008; 145: 41-48 [PMID: 18082770 DOI: 10.1016/j.jss.2007.02.044]
101 Lobmann R, Zemlin C, Motzkau M, Reschke K, Lehnert H. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing.
2006; 20: 329-335 [PMID:16949521 DOI: 10.1016/j.jdiacomp.2005.08.007]
102 Curran MP, Plosker GL. Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers.
2002; 16: 439-455 [PMID: 12463767 DOI:10.2165/00063030-200216060-00005]
103 Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice.
1990; 172: 245-251 [PMID: 2358777 DOI: 10.1084/jem.172.1.245]
104 Connolly DT, Stoddard BL, Harakas NK, Feder J. Human fibroblast-derived growth factor is a mitogen and chemoattractant for endothelial cells.
1987; 144: 705-712 [PMID: 3579937 DOI:10.1016/s0006-291x(87)80022-0]
105 Kurokawa M, Nakamura H, inventors; Polypeptide with epidermal regeneration accelerating minimal amino acid sequences, a polyalkylenepolyamine and/or polyarylenepolyamine, and a sheet. United States patent 7576051. 2009 August 18.
106 Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an antivascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders.
1997; 57: 4593-4599 [PMID: 9377574]
107 Zhou K, Ma Y, Brogan MS. Chronic and non-healing wounds: The story of vascular endothelial growth factor.
2015; 85: 399-404 [PMID: 26138626 DOI: 10.1016/j.mehy.2015.06.017]
108 Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators.
2007; 13: 2825-2830 [PMID: 17504979 DOI: 10.1158/1078-0432.CCR-06-2416]
109 Hardwicke JT, Hart J, Bell A, Duncan R, Thomas DW, Moseley R. The effect of dextrin-rhEGF on the healing of fullthickness, excisional wounds in the (db/db) diabetic mouse.
2011; 152: 411-417 [PMID: 21435363 DOI: 10.1016/j.jconrel.2011.03.016]
110 Brown DL, Kane CD, Chernausek SD, Greenhalgh DG. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice.
1997; 151: 715-724 [PMID: 9284820]
111 Bruhn-Olszewska B, Korzon-Burakowska A, Gabig-Cimińska M, Olszewski P, Węgrzyn A, Jakóbkiewicz-Banecka J.Molecular factors involved in the development of diabetic foot syndrome.
2012; 59: 507-513 [PMID:23251910]
112 Bhora FY, Dunkin BJ, Batzri S, Aly HM, Bass BL, Sidawy AN, Harmon JW. Effect of growth factors on cell proliferation and epithelialization in human skin.
1995; 59: 236-244 [PMID: 7543631 DOI:10.1006/jsre.1995.1160]
113 Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing.
2002; 92:12-18 [PMID: 11796794 DOI: 10.7547/87507315-92-1-12]
114 Lobmann R, Pap T, Ambrosch A, Waldmann K, König W, Lehnert H. Differential effects of PDGF-BB on matrix metalloproteases and cytokine release in fibroblasts of Type 2 diabetic patients and normal controls in vitro.
2006; 20: 105-112 [PMID: 16504839 DOI: 10.1016/j.jdiacomp.2005.05.013]
115 Gary Sibbald R, Woo KY. The biology of chronic foot ulcers in persons with diabetes.
2008;24 Suppl 1: S25-S30 [PMID: 18442179 DOI: 10.1002/dmrr.847]
116 Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells.
2009; 12: 359-366 [PMID:19929258 DOI: 10.1089/rej.2009.0872]
117 Yang M, Sheng L, Zhang TR, Li Q. Stem cell therapy for lower extremity diabetic ulcers: where do we stand?
2013; 2013: 462179 [PMID: 23586040 DOI: 10.1155/2013/462179]
118 Chong HC, Chan JS, Goh CQ, Gounko NV, Luo B, Wang X, Foo S, Wong MT, Choong C, Kersten S, Tan NS.Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice.
2014; 22: 1593-1604 [PMID: 24903577 DOI: 10.1038/mt.2014.102]
119 Laing T, Hanson R, Chan F, Bouchier-Hayes D. Effect of pravastatin on experimental diabetic wound healing.
2010; 161: 336-340 [PMID: 20031169 DOI: 10.1016/j.jss.2009.01.024]
120 Bagheri M, Jahromi BM, Mirkhani H, Solhjou Z, Noorafshan A, Zamani A, Amirghofran Z. Azelnidipine, a new calcium channel blocker, promotes skin wound healing in diabetic rats.
2011; 169: e101-e107 [PMID: 21571319 DOI:10.1016/j.jss.2011.02.039]
121 Leu JG, Chiang MH, Chen CY, Lin JT, Chen HM, Chen YL, Liang YJ. Adenine accelerated the diabetic wound healing by PPAR delta and angiogenic regulation.
2018; 818: 569-577 [PMID: 29162431 DOI:10.1016/j.ejphar.2017.11.027]
122 Lee Y, Chang JJ, Yang MC, Chien CT, Lai, WF Acceleration of wound healing in diabetic rats by layered hydrogel dressing.Carbohydr Polym 2021; 88: 809–819 [DOI: 10.1016/j.carbpol.2011.12.045]
123 Aly UF. Preparation and evaluation of novel topical gel preparations for wound healing in diabetics.
2012; 4: 76-77
124 Moon KC, Lee JS, Han SK, Lee HW, Dhong ES. Effects of human umbilical cord blood-derived mesenchymal stromal cells and dermal fibroblasts on diabetic wound healing.
2017; 19: 821-828 [PMID: 28462822 DOI:10.1016/j.jcyt.2017.03.074]
125 Dixon D, Edmonds M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing.
2021; 81: 29-56[PMID: 33382445 DOI: 10.1007/s40265-020-01415-8]
126 Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, Flores-Morales V, Martinez-Fierro ML. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments.
2019; 8 [PMID: 31652990 DOI: 10.3390/antibiotics8040193]
127 Kavitha KV, Tiwari S, Purandare VB, Khedkar S, Bhosale SS, Unnikrishnan AG. Choice of wound care in diabetic foot ulcer: A practical approach.
2014; 5: 546-556 [PMID: 25126400 DOI: 10.4239/wjd.v5.i4.546]
128 Tecilazich F, Dinh TL, Veves A. Emerging drugs for the treatment of diabetic ulcers.
2013;18: 207-217 [PMID: 23687931 DOI: 10.1517/14728214.2013.802305]
129 Karri VV, Kuppusamy G, Talluri SV, Yamjala K, Mannemala SS, Malayandi R. Current and emerging therapies in the management of diabetic foot ulcers.
2016; 32: 519-542 [PMID: 26643047 DOI:10.1185/03007995.2015.1128888]
130 Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, Driver VR, Frykberg R, Carman TL,Marston W, Mills JL Sr, Murad MH. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine.
2016; 63: 3S-21S [PMID: 26804367 DOI: 10.1016/j.jvs.2015.10.003]
131 Everett E, Mathioudakis N. Update on management of diabetic foot ulcers.
2018; 1411: 153-165[PMID: 29377202 DOI: 10.1111/nyas.13569]
132 Nayak C, Singh V, Singh K, Singh H, Gupta J, Ali MS, Ponnam HB. A prospective observational study to ascertain the role of homeopathic therapy in the management of diabetic foot ulcer.
2012; 6: 22-31
133 Ponnam HB, Gupta J. Role of silicea in diabetic foot ulcer: A retrospectively analysed case series.
2020; 33: 53-58 [DOI: 10.1055/s-0040-1705143]
134 Huang YY, Lin CW, Cheng NC, Cazzell SM, Chen HH, Huang KF, Tung KY, Huang HL, Lin PY, Perng CK, Shi B, Liu C, Ma Y, Cao Y, Li Y, Xue Y, Yan L, Li Q, Ning G, Chang SC. Effect of a Novel Macrophage-Regulating Drug on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial.
2021; 4:e2122607 [PMID: 34477854 DOI: 10.1001/jamanetworkopen.2021.22607]
135 Bagher ALM, Ramin H, Bahrami A, Delshad H, Ranjbar Omrani G, Mohammad K, Heydarpour R, Mohajeri Tehrani MR, Kamali K, Farhadi M, Gharib Doust F, Madani SH. Effects of intravenous semelil (angiparstm) on diabetic foot ulcers healing: A multicenter clinical trial.
2008; 16: 35-40
136 Nasiri M, Fayazi S, Jahani S, Yazdanpanah L, Haghighizadeh MH. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran.
2015; 14: 38 [PMID: 25969821 DOI: 10.1186/s40200-015-0167-9]
137 Viswanathan V, Kesavan R, Kavitha KV, Kumpatla S. A pilot study on the effects of a polyherbal formulation cream on diabetic foot ulcers.
2011; 134: 168-173 [PMID: 21911968]
138 Masoompour SM, Bagheri MH, Borhani Haghighi A, Novitsky YA, Sadeghi B, Gharibdoust F, Larijani B, Ranjbar Omrani G. Effect of ANGIPARS™, a new herbal drug on diabetic foot ulcer: A phase 2 clinical study.
2008; 16:31-34
139 Mohammadi MH, Molavi B, Mohammadi S, Nikbakht M, Mohammadi AM, Mostafaei S, Norooznezhad AH, Ghorbani Abdegah A, Ghavamzadeh A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A singlearm clinical trial.
2017; 56: 160-164 [PMID: 27839965 DOI: 10.1016/j.transci.2016.10.020]
140 Prakasam N, Prabakar MS, Reshma S, Loganathan K, Senguttuvan K. A clinical study of platelet rich plasma
conventional dressing in management of diabetic foot ulcers.
2018; 5: 3210-3216 [DOI:10.18203/2349-2902.isj20184069]
141 Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers.
2006; 56: 394-8; discussion 399 [PMID: 16557070 DOI: 10.1097/01.sap.0000198731.12407.0c]
142 Viswanathan V, Juttada U, Babu M. Efficacy of Recombinant Human Epidermal Growth Factor (Regen-D 150) in Healing Diabetic Foot Ulcers: A Hospital-Based Randomized Controlled Trial.
2020; 19: 158-164 [PMID: 31878810 DOI: 10.1177/1534734619892791]
143 Rangaswamy P, Rubby SA, Prasanth K. Prospective study of platelet derived growth factor in wound healing of diabetic foot ulcers in Indian population.
2017; 4: 194-199 [DOI: 10.18203/2349-2902.isj20164428]
144 Samuel S, Mahajan A, Mam MK, Prakash JS. Platelet derived growth factor in diabetic lower extremity ulcer: A randomized, double blind, placebo controlled study in Indian condition.
2016; 7: 3887-3892 [DOI:10.13040/IJPSR.0975-8232.7(9).3887-92]
145 UCHI H, IGARASHI A, URABE K, KOGA T, NAKAYAMA J, KAWAMORI R, TAMAKI K, HIRAKATA H,OHURA T, FURUE M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer.
2009; 19: 461-468 [PMID: 19638336 DOI: 10.1684/ejd.2009.0750]
146 Serena TE, Bullock NM, Cole W, Lantis J, Li L, Moore S, Patel K, Sabo M, Wahab N, Price P. Topical oxygen therapy in the treatment of diabetic foot ulcers: a multicentre, open, randomised controlled clinical trial.
2021; 30:S7-S14 [PMID: 33979229 DOI: 10.12968/jowc.2021.30.Sup5.S7]
147 Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes.
2010; 33: 998-1003 [PMID: 20427683 DOI: 10.2337/dc09-1754]
148 Maranna H, Lal P, Mishra A, Bains L, Sawant G, Bhatia R, Kumar P, Beg MY. Negative pressure wound therapy in grade 1 and 2 diabetic foot ulcers: A randomized controlled study.
2021; 15: 365-371 [PMID:33524646 DOI: 10.1016/j.dsx.2021.01.014]
149 Alamdari NM, Mehraneroodi B, Gholizadeh B, Zeinalpour A, Safe P, Besharat S. The efficacy of negative pressure wound therapy compared with conventional dressing in treating infected diabetic foot ulcers: a randomized controlled trial.
2021; 41: 664-668 [DOI: 10.1007/s13410-021-00941-9]
150 Kaviani A, Djavid GE, Ataie-Fashtami L, Fateh M, Ghodsi M, Salami M, Zand N, Kashef N, Larijani B. A randomized clinical trial on the effect of low-level laser therapy on chronic diabetic foot wound healing: a preliminary report.
2011; 29: 109-114 [PMID: 21214368 DOI: 10.1089/pho.2009.2680]
151 Salvi M, Rimini D, Molinari F, Bestente G, Alberto Bruno M. D. Effect of low-level light therapy on diabetic foot ulcers:a near-infrared spectroscopy study.
2017; 22: 038001 [DOI: 10.1117/1.JBO.22.3.038001]
152 Omar MT, Alghadir A, Al-Wahhabi KK, Al-Askar AB. Efficacy of shock wave therapy on chronic diabetic foot ulcer: a single-blinded randomized controlled clinical trial.
2014; 106: 548-554 [PMID: 25451894 DOI:10.1016/j.diabres.2014.09.024]
153 Jeppesen SM, Yderstraede KB, Rasmussen BS, Hanna M, Lund L. Extracorporeal shockwave therapy in the treatment of chronic diabetic foot ulcers: a prospective randomised trial.
2016; 25: 641-649 [PMID: 27827284 DOI:10.12968/jowc.2016.25.11.641]
154 Maksimova N, Krasheninnikov M, Zhang Y, Ponomarev E, Pomytkin I, Melnichenko G, Lyundup A. Early passage autologous mesenchymal stromal cells accelerate diabetic wound re-epithelialization: A clinical case study.
2017; 19: 1548-1550 [PMID: 28986173 DOI: 10.1016/j.jcyt.2017.08.017]
155 Qin HL, Zhu XH, Zhang B, Zhou L, Wang WY. Clinical Evaluation of Human Umbilical Cord Mesenchymal Stem Cell Transplantation After Angioplasty for Diabetic Foot.
2016; 124: 497-503 [PMID:27219884 DOI: 10.1055/s-0042-103684]
156 Edmonds ME, Bodansky HJ, Boulton AJM, Chadwick PJ, Dang CN, D'Costa R, Johnston A, Kennon B, Leese G,Rajbhandari SM, Serena TE, Young MJ, Stewart JE, Tucker AT, Carter MJ. Multicenter, randomized controlled,observer-blinded study of a nitric oxide generating treatment in foot ulcers of patients with diabetes-ProNOx1 study.Wound Repair Regen 2018; 26: 228-237 [PMID: 29617058 DOI: 10.1111/wrr.12630]
157 Rullan M, Cerdà L, Frontera G, Masmiquel L, Llobera J. Treatment of chronic diabetic foot ulcers with bemiparin: a randomized, triple-blind, placebo-controlled, clinical trial.
2008; 25: 1090-1095 [PMID: 19183313 DOI:10.1111/j.1464-5491.2008.02527.x]
158 Imran M, Hussain MB, Baig M. A Randomized, Controlled Clinical Trial of Honey-Impregnated Dressing for Treating Diabetic Foot Ulcer.
2015; 25: 721-725 [PMID: 26454386 DOI: 10.2015/JCPSP.721725]
159 Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis II JC. A multicenter, blinded, randomized controlled clinical trial evaluating the effect of omega-3–rich fish skin in the treatment of chronic, nonresponsive diabetic foot ulcers.Wounds 2021; 33: 169-177 [DOI: 10.25270/wnds/2021.169177]
160 Del Pino-Sedeño T, Trujillo-Martín MM, Andia I, Aragón-Sánchez J, Herrera-Ramos E, Iruzubieta Barragán FJ, Serrano-Aguilar P. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis.
2019; 27:170-182 [PMID: 30575212 DOI: 10.1111/wrr.12690]
161 Golledge J, Thanigaimani S. Novel therapeutic targets for diabetes-related wounds or ulcers: an update on preclinical and clinical research.
2021; 25: 1061-1075 [PMID: 34873970 DOI:10.1080/14728222.2021.2014816]
162 Mahdipour E, Sahebkar A. The Role of Recombinant Proteins and Growth Factors in the Management of Diabetic Foot Ulcers: A Systematic Review of Randomized Controlled Trials.
2020; 2020: 6320514 [PMID: 32733969 DOI: 10.1155/2020/6320514]
163 Ma C, Hernandez MA, Kirkpatrick VE, Liang LJ, Nouvong AL, Gordon II. Topical platelet-derived growth factor
placebo therapy of diabetic foot ulcers offloaded with windowed casts: a randomized, controlled trial.
2015; 27:83-91 [PMID: 25855851]
164 Jaiswal SS, Gambhir RP, Agrawal A, Harish S. Efficacy of topical recombinant human platelet derived growth factor on wound healing in patients with chronic diabetic lower limb ulcers.
2010; 72: 27-31 [PMID: 23133200 DOI:10.1007/s12262-010-0005-8]
165 Liu Y, Liu Y, Deng J, Li W, Nie X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects.
2021; 12: 744868 [PMID: 34721299 DOI: 10.3389/fendo.2021.744868]
166 Richard JL, Parer-Richard C, Daures JP, Clouet S, Vannereau D, Bringer J, Rodier M, Jacob C, Comte-Bardonnet M.Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot,randomized, double-blind, placebo-controlled study.
1995; 18: 64-69 [PMID: 7698050 DOI:10.2337/diacare.18.1.64]
167 Xu J, Min D, Guo G, Liao X, Fu Z. Experimental study of epidermal growth factor and acidic fibroblast growth factor in the treatment of diabetic foot wounds.
2018; 15: 5365-5370 [PMID: 29904416 DOI:10.3892/etm.2018.6131]
168 Borys S, Hohendorff J, Frankfurter C, Kiec-Wilk B, Malecki MT. Negative pressure wound therapy use in diabetic foot syndrome-from mechanisms of action to clinical practice.
2019; 49: e13067 [PMID: 30600541 DOI:10.1111/eci.13067]
169 Liu S, He CZ, Cai YT, Xing QP, Guo YZ, Chen ZL, Su JL, Yang LP. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: systematic review and meta-analysis.
2017; 13: 533-544 [PMID:28458556 DOI: 10.2147/TCRM.S131193]
170 Liu Z, Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, Peinemann F. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus.
2018; 10: CD010318[PMID: 30328611 DOI: 10.1002/14651858.CD010318.pub3]
171 Huang J, Chen J, Xiong S, Huang J, Liu Z. The effect of low-level laser therapy on diabetic foot ulcers: A meta-analysis of randomised controlled trials. Int Wound J 2021; 18: 763-776 [DOI: 10.1111/iwj.13577]
172 Huang Q, Yan P, Xiong H, Shuai T, Liu J, Zhu L, Lu J, Shi X, Yang K. Extracorporeal Shock Wave Therapy for Treating Foot Ulcers in Adults With Type 1 and Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.
2020; 44: 196-204.e3 [PMID: 31515158 DOI: 10.1016/j.jcjd.2019.05.006]
173 Yu Q, Qiao GH, Wang M, Yu L, Sun Y, Shi H, Ma TL. Stem Cell-Based Therapy for Diabetic Foot Ulcers.
2022; 10: 812262 [PMID: 35178389 DOI: 10.3389/fcell.2022.812262]
174 Ahmed R, Augustine R, Chaudhry M, Akhtar UA, Zahid AA, Tariq M, Falahati M, Ahmad IS, Hasan A. Nitric oxidereleasing biomaterials for promoting wound healing in impaired diabetic wounds: State of the art and recent trends.
2022; 149: 112707 [PMID: 35303565 DOI: 10.1016/j.biopha.2022.112707]
175 Kong M, Xie K, Lv M, Li J, Yao J, Yan K, Wu X, Xu Y, Ye D. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise.
2021; 133: 110975 [PMID:33212375 DOI: 10.1016/j.biopha.2020.110975]
176 Carvalho MTB, Araújo-Filho HG, Barreto AS, Quintans-Júnior LJ, Quintans JSS, Barreto RSS. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action.
2021; 90: 153636[PMID: 34333340 DOI: 10.1016/j.phymed.2021.153636]
177 Oguntibeju OO. Medicinal plants and their effects on diabetic wound healing.
2019; 12: 653-663 [PMID:31327900 DOI: 10.14202/vetworld.2019.653-663]
178 Gallelli G, Cione E, Serra R, Leo A, Citraro R, Matricardi P, Di Meo C, Bisceglia F, Caroleo MC, Basile S, Gallelli L.Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study.
2020; 17: 485-490 [PMID: 31876118 DOI: 10.1111/iwj.13299]
179 Majumdar K, Dtta NK, Dastidar SG, Motohashi N, Shirataki Y. Phytochemical isoflavones against diabetic foot bacteria.
2004; 4: 261-266 [DOI: 10.3742/OPEM.2004.4.4.261]
180 Thanganadar Appapalam S, Paul B, Arockiasamy S, Panchamoorthy R. Phytofabricated silver nanoparticles: Discovery of antibacterial targets against diabetic foot ulcer derived resistant bacterial isolates.
2020; 117: 111256 [PMID: 32919626 DOI: 10.1016/j.msec.2020.111256]
杂志排行
World Journal of Diabetes的其它文章
- Different nutrient compositions in diet and taking hypoglycemic drugs can modulate gut microbial flora
- Mapping the global research landscape on insulin resistance:Visualization and bibliometric analysis
- Role of insulin in pancreatic microcirculatory oxygen profile and bioenergetics
- Relationship between age of pregnant women with gestational diabetes mellitus and mode of delivery and neonatal Apgar score
- Hyperglycemia and reduced adiposity of streptozotocin-induced diabetic mice are not alleviated by oral benzylamine supplementation
- Effectiveness and safety of COVID-19 vaccines in patients with diabetes as a factor for vaccine hesitancy
