Anti-tumour activity and toxicological studies of combination treatment of Orthosiphon stamineus and gemcitabine on pancreatic xenograft model
2022-09-14AshwaqHamidSalemYehyaAyappaSubramaniamMuhammadAsifGurjeetKaurAminAbdulMajidChernEinOon
Ashwaq Hamid Salem Yehya, Ayappa V Subramaniam, Muhammad Asif, Gurjeet Kaur, Amin M S Abdul Majid, Chern Ein Oon
Abstract
Key Words: Pancreatic cancer; Orthosiphon stamineus; C5EOSEW5050ESA; Gemcitabine; Complementary medicine
lNTRODUCTlON
Pancreatic cancer is one of the deadliest cancers globally and has the most unfavourable survival rate of any cancer[1]. Chemotherapy, radiation, surgery, and molecular targeting agents are common strategies to treat pancreatic cancer. However, the majority of these systemic treatments are associated with severe side effects[1]. Gemcitabine, a nucleoside analogue of cytidine, is the first line of treatment used to treat pancreatic cancer[2]. However, its overall success rates are poor. In addition, combination treatments of gemcitabine with other chemo-drugs, such as capecitabine, irinotecan, oxaliplatin, and cisplatin, may cause multiple adverse reactions and drug resistance, leading to a reduction of drug efficacy[3].
Herbal products have been traditionally used to treat many diseases in Asian countries[4]. Data from pre-clinical and clinical studies have also highlighted that these natural herbs, when combined with conventional radio- or chemotherapies, can sensitise tumour cells to treatments to improve cancer patients' survival and quality of life[5,6]. Nevertheless, this is not always the case, as studies have also shown that herbal medicines, when combined with chemotherapies, may instead enhance the toxicity potential of chemo drugs, leading to increased incidences of severe side effects[7].
Therefore, understanding the interactions of herbal-chemo drugs is necessary for the appropriate use of these combinations to prevent the emergence of toxicity and therapeutic failure in cancer patients.Orthosiphon stamineus(O.s) is a traditional Asian herbal medicine used to treat various diseases,including cancers[8]. The safety profile ofO.shas already been established globally by numerous research groups usingin vivoandin vitromodels[3,9]. Our research group has already established the antitumor efficacy ofO.sagainst colon cancer[9,10]. C5OSEW5050ESA(NuvastaticTM) is a proprietary extract ofO.sthat completed a phase 2/3 clinical study for cancer fatigue in cancer patients with solid tumours receiving chemotherapy[11]. However, no study has reported the toxicity profile of C5OSEW5050ESA in a pancreatic cancer xenograft model as a stand-alone or in combination withgemcitabine. We have previously reported that the combination treatment ofO.sand gemcitabine showed no toxicity in mice, either as a stand-alone or in combination[3]. Furthermore,O.ssignificantly sensitised Panc-1 towards gemcitabinein vitro[4]. Consequently, the present study was designed to evaluate the toxicological effects of NuvastaticTMand gemcitabine either alone or in combination using the pancreatic cancer nude mice model.

Table 1 Treatment conditions for 28 d
MATERlALS AND METHODS
Plant materials and chemicals
Orthosiphon stamineusextract (C5EOSEW5050ESA) was purchased from NatureCeutical Sdn Bhd,Malaysia. Gemcitabine (Catalogue No. S1149) was obtained from Selleckchem, Houston, TX, United States. C5EOSEW5050ESA was dissolved in sterile distilled water and filtered by a membrane filter unit(0.22 µm). C5EOSEW5050ESA was administered orally to mice, while gemcitabine was givenviaintraperitoneal injection after being dissolved in phosphate buffer saline (PBS).
Animals
Male athymic nude mice (procured from iDNA, Malaysia) were kept in filter-top cages under controlled atmospheric conditions at the EMAN Testing and Research laboratory, School of Pharmaceutical Sciences, USM. Autoclaved food, water, and bedding of cages were changed every 48 h. The animal study was approved and conducted under strict guidelines according to USM Animal Ethics Committee(Reference #: USM/Animal Ethics Approval/2016/(97) (746).
Cell lines
Panc-1, a pancreatic cancer cell line, was purchased from American Type Culture Collection (ATCC).The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Scientific, United States) supplemented with 10%fetal bovine serum (Biowest, United States) and 100 units/mL penicillin-streptomycin (Nacalai Tesque, United States). Cells were kept at 37°C in a humidified incubator with 5%CO2atmosphere.
In vivo assessment of tumour growth in a nude mouse xenograft model:Panc-1 cells (20 × 106cells suspended in 200 μL of a mixture of DMEM and matrigel in a 1:1 ratio) were injected subcutaneously on the dorsal side of each mouse. Treatment with gemcitabine andEt. O.sin different combinations or as a stand-alone was initiated when at least three of the tumours in any group reached 100 mm3.
Experimental treatment design
Mice were randomly divided into six groups of 6 mice each (n= 6) and given different treatments for 28 d, as mentioned in Table 1. The body weight of all mice and tumour size was measured every 3rdday. At the end of the study, all the mice were euthanised with a combination of ketamine and xylazine when the tumour volumes from the untreated group reached 1000 mm3. The blood samples were collected for haematological and serum biochemical tests. Organs (liver, kidney, and spleen) were harvested and weighed to observe the changes in the organ weights of treated animals compared to the untreated group. Tumour tissues and organs were collected and fixed with 10%buffered formalin. Paraffin blocks were prepared, and sections of 5 μm were stained with haematoxylin and eosin (HE). Ten microscopic fields per slide were examined at ×20 and ×40 magnification, and photomicrographic images were captured using a digital camera.
Blood parameters and biochemical tests
Blood samples were used to measure different haematological parameters, including haemoglobin (Hb)levels, total blood count, differential counting of white blood cells, packed cell volume (PCV), mean cell volume (MCV), mean cell haemoglobin (MCH), mean cell haemoglobin concentration (MCHC), red cell distribution width (RDW), creatinine, urea, uric acid, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin, cholesterol (low and high-density cholesterol), triglycerides, total protein, albumin, globulin,albumin/globulin ratio, and minerals (sodium, potassium, and chloride).
Histopathological examination
Key organs (liver, kidney, and spleen) were harvested and fixed in a 10%buffered formaldehyde solution. An automated tissue processing machine then processed the fixed tissues and embedded them in paraffin wax to prepare sample blocks.
Haematoxylin and eosin staining
Tissue sections of 5 mm thickness were cut and prepared at 60°C. The slides were dewaxed in xylene and rehydrated through graded alcohol. Slides were rinsed and incubated with haematoxylin for 4 min.After rinsing, eosin was added to the slides for 1 min. Next, slides were rinsed in tap water and briefly air-dried at room temperature. Slides were then mounted with glycerol (Sigma, United States). Stained slides were examined and scored for the percentage of necrosis within the tumour areas by a pathologist(GK) under a light microscope.
Immunohistochemistry staining
The formalin-fixed-paraffin-embedded (FFPE) tissue sections were prepared at 60°C, dewaxed in xylene, and rehydrated through graded alcohol. Antigens were retrieved using Dako's antigen retrieval buffer (1 ×, pH 9.0) and then microwaved on high for 15 min. Slides were cooled, washed and then blocked with hydrogen peroxidase and BSA. Then slides were treated with primary antibody Ki67(Dako, Glostrup, Denmark) and left to incubate at 4°C overnight at 1:50 dilution. The next day, slides were treated with secondary antibody goat anti-mouse (Dako, Glostrup, Denmark) for 1 h at 1:500 dilution. Later, avidin-biotin complex (Vector Laboratories, United States) was added to tissues for 1 h.After that, Dab stain (Dako, Glostrup, Denmark) and haematoxylin were added to the slides. Finally,slides were air-dried at room temperature and mounted with glycerol (Sigma, United States). Using ImageJ, the Ki-67 score was determined as the percentage of tumour cells that showed brown nuclear staining over the total number of nuclei from five random fields per tumour section.
Statistical analysis
Graphing software Excel (Microsoft, United States) and Prism (GraphPad, United States) were used for statistical analysis. Data were presented as mean ± SD whereas the parametric data analysis was performed using a one-way analysis of variance(ANOVA).Two-way ANOVA was used to understand if there is an interaction between the two independent variables on the dependent variable. The Tukey’s honest significant difference (HSD) post hoc test was used to assess the significant differences among all groups. Analysis for non-parametric data was performed using Kruskal-Wallis ANOVA. A value ofP<0.05 was considered significant when compared to values in the respective untreated group.
RESULTS
Effect of treatment on mouse body weight and key organs
C5EOSEW5050ESA demonstrated no toxicity in mice:The average body weight within the untreated group increased compared to other treatment groups (Table 2). However, there was no notable difference in body weight between untreated groups and other treatment groups (Table 2). In addition,the weight of vital organs, including the kidney, liver, and spleen, remained unchanged post-treatment with either C5EOSEW5050ESA and/or gemcitabine (Table 3).
Haematological-biochemical parameters and electrolyte profiles:No significant changes were observed in the Hb level, total blood cells count, or differential counting of WBC, PCV, MCV, MCH,MCHC, and RDW when compared with the corresponding parameters in the untreated group (Table 4).No significant changes were noted in serum parameters,i.e.creatinine, urea, uric acid, AST, ALT, ALP,GGT, total bilirubin, total protein, albumin, globulin, and albumin/globulin ratio, which were observed in animal groups treated with C5EOSEW5050ESA at (200 or 400 mg/kg per day) alone or in combination with gemcitabine (10 mg/kg per 3 d) after 28 d treatment when compared to the untreated group (Table 5). Normal ALT, ALP, and AST levels in the serum indicate that there is no damage in hepatocytes. Similarly, urea and total bilirubin levels were also within the normal range, indicating the lack of toxicity in the kidneys when treated with C5EOSEW5050ESA and gemcitabine either alone or incombination treatment (Table 5). Electrolytes were within the normal ranges in all the groups, with no significant changes observed compared to the untreated group (Table 5).
Table 2 Effect of C5EOSEW5050ESA and gemcitabine alone or in combination on mouse body weight

Table 3 Effect of C5EOSEW5050ESA and gemcitabine alone or in combination on mouse key organ weight
Histopathology analysis: Histopathological examination of formalin-fixed paraffin-embedded tissues of the spleen (Figure 1), kidney (Figure 2), and liver (Figure 3) of all treatment groups, as well as the untreated group, revealed normal histology without pathological evidence of inflammation or necrosis.In addition, the liver did not exhibit an accumulation of neutral fats or triglycerides within the liver cells. However, the liver cells demonstrated mild swelling in all groups except for C5EOSEW5050ESA(200 mg/kg per day) treatment group (Figure 3).
C5EOSEW5050ESA and gemcitabine combination synergistically inhibited pancreatic tumour growth in the xenograft model
Single treatments with gemcitabine at 10 mg/kg and C5EO-SEW5050ESA at 200 mg/kg or 400 mg/kg inhibited tumour growth and reduced tumour weight compared to the vehicle untreated group (Figures 4A and B). However, compared to a single treatment, no additive effect in inhibiting tumour growth and tumour weight was obtained with 200 mg/kg C5EOSE-W5050ESA combined with gemcitabine(Figure 4A and B). However, C5EOSEW5050ESA at 400 mg/kg combined with gemcitabine significantly reduced tumour growth and tumour weight compared to individual treatment groups (Figures 4A and B).
Table 4 Haematological parameters in different treatment groups
C5EOSEW5050ESA and gemcitabine combination synergistically inhibited pancreatic tumour necrosis in the xenograft model:A single treatment with gemcitabine at 10 mg/kg and C5EOSEW5050ESA at 200 mg/kg or 400 mg/kg reduced tumour necrosis compared to untreated tumour cells(Figure 5). No additive effect in reducing tumour necrosis was obtained with C5EOSEW5050ESA at 200 mg/kg and gemcitabine combination compared to a single treatment (Figure 5D). However,C5EOSEW5050ESA at 400 mg/kg and gemcitabine combination significantly reduced tumour necrosis compared to single treatment groups of the respective doses (Figure 5).
C5EOSEW5050ESA combined with gemcitabine synergistically inhibited Ki67 protein expression in pancreatic tumour tissue:Ki67 proliferative marker protein was highly expressed in the untreated group compared to all treatment groups (Figure 6). Compared to a single treatment, no additive effect in reducing Ki67 protein expression was obtained with 200 mg/kg C5EOSEW5050ESA and gemcitabine combination (Figure 6). However, 400 mg/kg C5EOSEW5050ESA and gemcitabine combination synergistically inhibited the expression of Ki67 protein compared to single treatment and other treatment groups (Figure 6).

Table 5 Blood biochemical parameters and electrolyte profiles in different treatment groups
DlSCUSSlON
Studies have shown that therapeutic combinations containing low-dose marketed drugs and plant extracts/isolated compounds or essential oils may improve the safety profile of marketed drugs and produce synergistic actions[12,13]. The safety profile of C5EOSEW5050ESA has previously been established[14,15], with its LD50values reported to be greater than 5000 mg/kg[15]. Chemo-herbal combinations are one of the possible therapeutic options which can be employed to improve the efficacy of a drug, reduce adverse drug effects and increase disease-free intervals and overall survival rates in cancer patients. The majority of chemotherapeutic drugs destroy tumours and retard cancer growth but may also damage healthy tissues. Thus, new chemo-herbal combinations are anticipated to play an essential role in developing more effective and safer strategies to inhibit cancer progress with minimal side effects[4].
We have previously shown the synergistic anti-cancer effects of combined C5EOSEW5050ESA and gemcitabine treatment on Panc-1 and MiaPaCa-2 pancreatic cancer cell lines[3]. Moreover, no toxicity was observed in mice in an acute toxicity study[3]. In the current study, the combination treatment in a pancreatic tumour xenograft model revealed no abnormal signs in any of the treatment groups (Figures 1-3). Moreover, no significant difference in organ weight change was noted when compared to the untreated group, supporting the relatively safe nature of selected chemo-herbal combinations (Table 3).
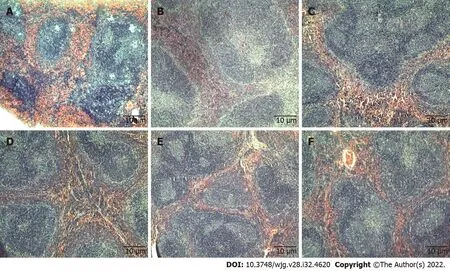
Figure 1 Tissue sections of mice spleen stained with haematoxylin and eosin. Tissue sections from untreated and treated groups showed normal spleen architecture. A: Untreated; B: Gemcitabine (10 mg/kg per 3 d); C: C5EOSEW5050ESA (200 mg/kg per day); D: C5EOSEW5050ESA (200 mg/kg per day) and gemcitabine (10 mg/kg per 3 d) combination; E: C5EOSEW5050ESA (400 mg/kg per day); F: C5EOSEW5050ESA (400 mg/kg per day) and gemcitabine (10 mg/kg per 3 d) combination.
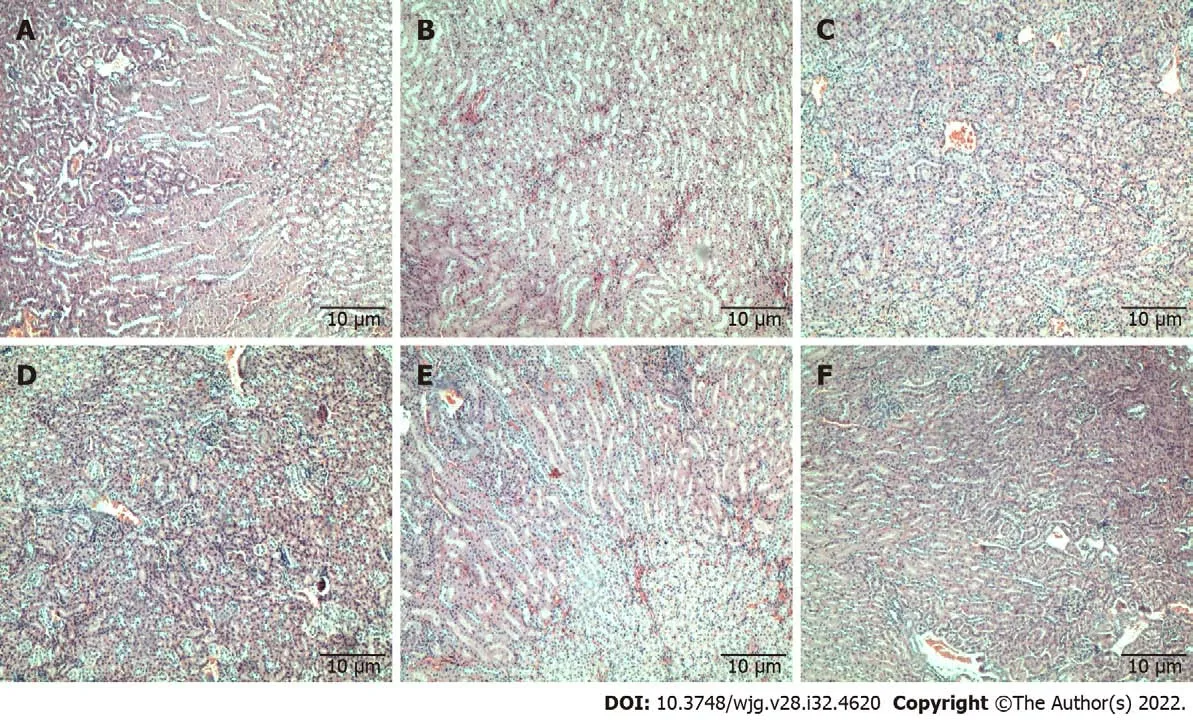
Figure 2 Tissue sections of mice kidneys stained with haematoxylin and eosin. Tissue sections showed normal glomeruli and tubules in all treated groups and untreated. A: Untreated; B: Gemcitabine (10 mg/kg per 3 d); C: C5EOSEW5050ESA (200 mg/kg per day); D: C5EOSEW5050ESA (200 mg/kg per day)and gemcitabine (10 mg/kg per 3 d) combination; E: C5EOSEW5050ESA (400 mg/kg per day); F: C5EOSEW5050ESA (400 mg/kg per day) and gemcitabine (10 mg/kg per 3 d) combination.

Figure 3 Tissue sections of mice liver stained with haematoxylin and eosin. Liver sections showed normal architecture with distinct hepatic cells,sinusoidal spaces, and a central vein in all treatment groups and untreated; mild hepatocellular swelling is seen in all groups except the C5EOSEW5050ESA (200 mg/kg per day) group where no swelling is present. A: Untreated; B: Gemcitabine (10 mg/kg per 3 d); C: C5EOSEW5050ESA (200 mg/kg per day); D:C5EOSEW5050ESA (200 mg/kg per day) and gemcitabine (10 mg/kg per 3 d) combination; E: C5EOSEW5050ESA (400 mg/kg per day); F: C5EOSEW5050ESA(400 mg/kg per day) and gemcitabine (10 mg/kg per 3 d) combination.
Clinical biochemistry and haematological analyses play a significant role in evaluating the signs of toxicity induced by drugs[16]. No significant alterations in haematological parameters were observed in the animal blood samples treated with C5EOSEW5050ESA and gemcitabine combination, indicating that these combinations did not damage the blood cells (Table 4). Increased serum bilirubin concentration and occurrence of tissue haemolysis are commonly seen when the liver is impaired[17]. The absence of these observations in the current study demonstrated the non-toxic effects of the C5EOSEW5050ESA gemcitabine combination on haemoglobin metabolic pathways (Table 4). A slight drop in white blood count and platelets and mild myelosuppression have been reported with extended treatment duration and high doses of gemcitabine[18]. In 2013, the MPACT phase III trial, which included over 800 patients, showed a significant overall survival benefit with the combination of nanomolecular albumin-bound (nab)-paclitaxel and gemcitabine (nabPGem) over gemcitabine monotherapy, with acceptable toxicity. The overall toxicity of nabPGem is lower than in the triple chemotherapy 5-FU, leucovorin, irinotecan and oxaliplatin, especially haematotoxicity and rates of neutropenic fever[19,20]. However, the current study and our previous toxicity study revealed no reduction in white blood cells and platelets (Table 4)[3]. In fact, C5EOSEW5050ESA and gemcitabine combination at the higher dose enhanced its efficacy at reducing tumour growth without causing any side effects, at least in this short term study.
Kidneys are particularly vulnerable to high doses of drugs as they eradicate many drugs and their metabolites. Therefore, renal function tests measure various substances, including serum urea, serum creatinine, and albumin, to determine the current health of the kidneys[21]. In the present study, there was no nephrotoxicity observed, indicating the safe nature of chemo-herbal combinations. The results showed normal level concentrations of urea, creatinine, and albumin and were supported by the typical renal architecture of kidney sections (Figure 2).
Liver function tests, for such markers as ALT, AST, ALP, and GGT, are used to measure hepatocellular damage during illnesses[22]. There were no significant changes in the serum levels of AST, ALT,ALP, and GGT in all treatment groups (Table 5). In addition, the hepatocellular architecture appeared normal in all treatment groups (Figure 3).
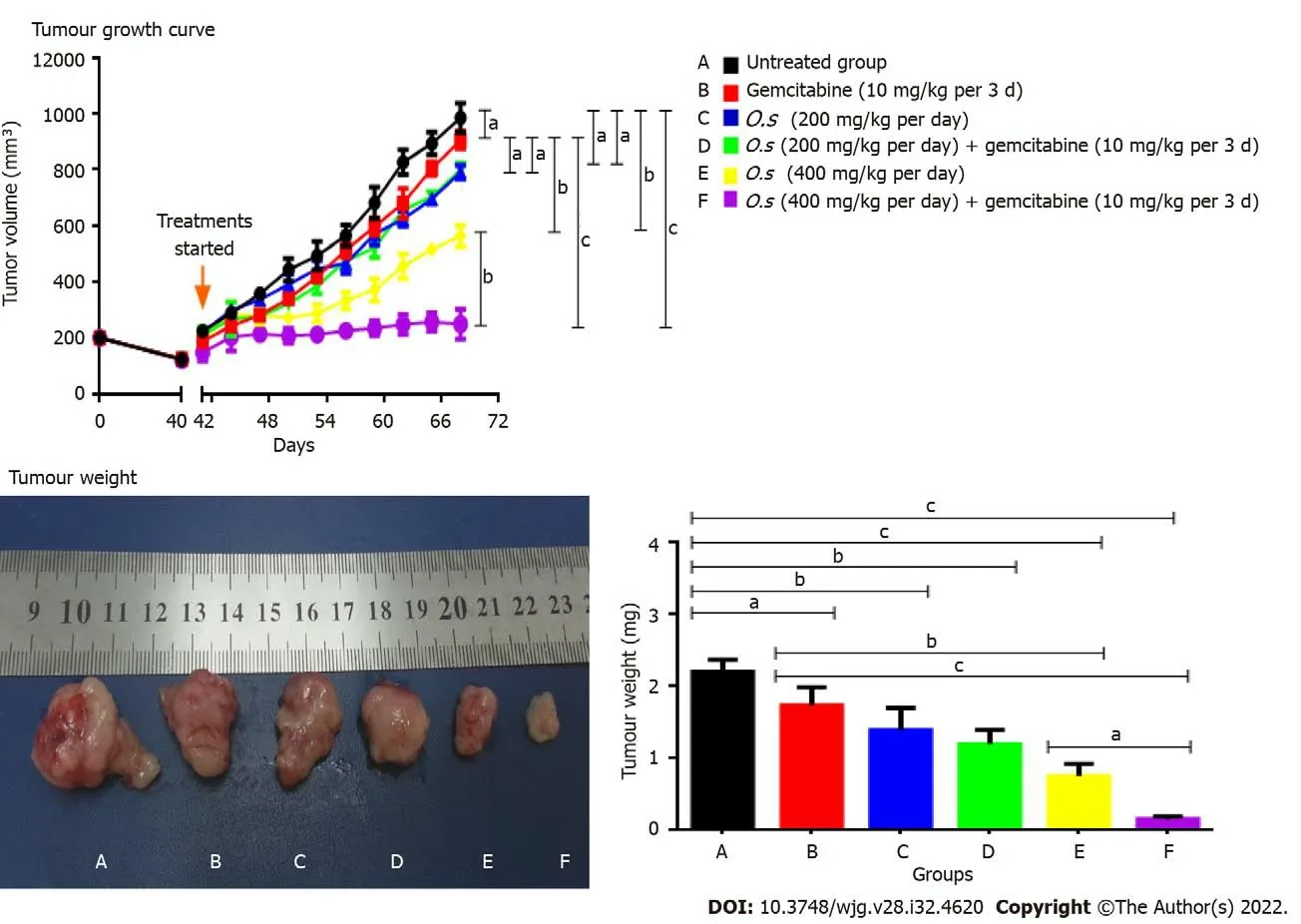
Figure 4 Tumour growth curve of Panc-1 cells in subcutaneous pancreatic tumour growth in nude mice and tumour weight. Treatments were given when the tumours reached 100 mm3. A: Untreated; B: Gemcitabine (10 mg/kg per 3 d); C: C5EOSEW5050ESA (200 mg/kg per day); D:C5EOSEW5050ESA (200 mg/kg per day) and gemcitabine (10 mg/kg per 3 d) combination; E: C5EOSEW5050ESA (400 mg/kg per day); F: C5EOSEW5050ESA(400 mg/kg per day) and gemcitabine (10 mg/kg per 3 d) combination. Combination treatment of 400 mg/kg C5EOSEW5050ESA and gemcitabine (F) synergistically reduced the tumour growth compared to a single treatment. The combination treatment of 400 mg/kg C5EOSEW5050ESA and gemcitabine (F) significantly reduced tumour weight compared to a single treatment. Error bars represent standard deviations. Statistics analysis (aP < 0.05; bP < 0.01; cP < 0.001, two-way ANOVA for tumour growth, one-way ANOVA with Tukey’s HSD posthoc test for tumour weight, n = 6 animals per group) using SPSS and GraphPad Prism 6.0 software.
As a single treatment, gemcitabine at 10 mg/kg and C5EOSEW5050ESA at 200 mg/kg or 400 mg/kg inhibited tumour growth compared to untreated mice (Figure 4). Compared to a single treatment, no additive inhibition of tumour growth was obtained with 200 mg/kg C5EOSEW5050ESA and gemcitabine combination (Figure 4). However, C5EOSEW5050ESA at 400 mg/kg and gemcitabine combination significantly reduced tumour growth compared to the single treatment groups of the respective doses (Figure 4). Further studies investigating how combination treatment affects pancreatic cancer cell proliferation and apoptosis or necrosis will shed light on their mode of action. In our previous study, C5EOSEW5050ESA and gemcitabine combination inhibited the survival and proliferation of Panc-1 cellsin vitro[23]. Also, the combination of C5EOSEW5050ESA and gemcitabineinduced apoptosis and reduced EMT markers and multidrug-resistant genes (MDR-1,MRP-4,andMRP-5), and the Notch signalling pathway[24]. In the current study, the tumour necrosis percentage was estimated in tissue sections stained by HE. Single treatment of gemcitabine and C5EOSEW5050ESA either at low or high dose reduced the tumour percentage compared to untreated (Figure 5). There were no additional effects in the reduction of tumour necrosis post-treatment with a low dose of C5EOSEW5050ESA and gemcitabine combination compared to a single treatment (Figure 5). However,the high dose of C5EOSEW5050ESA and gemcitabine combination reduced tumour necrosis compared to a single treatment (Figure 5).
The combination treatment of C5EOSEW5050ESA at a high dose and gemcitabine significantly reduced Ki67 protein expression in tumour cells compared to a single treatment (Figure 6), indicating reduced cell proliferation in the former group. The epigallocatechin-3-O-gallate (EGCG) polyphenol is enriched in C5EOSEW5050ESA extract. Others have reported EGCG to inhibit tumour growth and induce apoptosis in mice implanted with head, neck, and lung cancers by decreasing the Ki67 expression in tissues from the xenograft model and downregulating caspase-3, caspase-8, caspase-9, and Bax in mouse vascular smooth muscle cells[25,26]. Another study showed that resveratrol inhibited Ki67 expression in pancreatic cancer cellsin vitroandin vivoby sensitising pancreatic cancer cells to gemcitabine treatment[25]. It is postulated that the polyphenols in C5EOSEW5050ESA may act synergistically with gemcitabine in the pancreatic tumour xenograft model. C5EOSEW5050ESA worked in synergy with gemcitabine to reduce pancreatic tumour growth and tumour necrosis in mice compared to a single treatment of either C5EOSEW5050ESA or gemcitabine. Thus, based on current findings, it is proposed that 50%ethanol extract ofO.shas the potential to be used in combination with gemcitabine to treat pancreatic cancer.

Figure 5 Tumour necrosis percentage in tumour tissues stained with haematoxylin and eosin. A: Untreated group; B: Gemcitabine (10 mg/kg per 3 d); C: Orthosiphon stamineus (O.s) (200 mg/kg per day); D: O.s (200 mg/kg per day) + gemcitabine (10 mg/kg per 3 d); E: O.s (400 mg/kg per day); F: O.s (400 mg/kg per day) + gemcitabine (10 mg/kg per 3 d); G: Estimated marginal means of necrosis. Combination treatment of 400 mg/kg C5EOSEW5050ESA with gemcitabine synergistically inhibited tumour necrosis compared to a single treatment. Arrows indicate the necrotic area in tumour sections. Error bars represent standard deviations. Statistics analysis (two-way ANOVA, n = 6 animals per group) using SPSS software. O.s: Orthosiphon stamineus.
CONCLUSlON
This study provides preliminary scientific evidence about the safety profile ofO.sderived extract C5EOSEW5050ESA present in NuvastaticTMin combination with gemcitabine in an athymic nude mice model. Furthermore, this work demonstrated that C5EOSEW5050ESA extract in combination with gemcitabine is relatively safe in the acute toxicity study. Furthermore, the clinical efficacy and safety of NuvastaticTMin cancer asthenia have been evaluated in phase III clinical trials (ClinicalTrials.gov,Identifier: NCT04546607). Therefore, NuvastaticTMhas the potential to be employed as a complementary treatment with gemcitabine to treat pancreatic cancer.
ARTlCLE HlGHLlGHTS
Research background
Gemcitabine is the cornerstone for pancreatic cancer but demonstrates adverse effects in patients.Orthosiphon stamineus(O.s) has been traditionally used to treat various diseases. C5OSEW5050ESA(NuvastaticTM) is a proprietary extract ofO.sthat completed a phase 2/3 clinical study for cancer fatigue in cancer patients with solid tumours receiving chemotherapy.
Research motivation
No study has reported the toxicity profile of C5OSEW5050ESA in a pancreatic tumour animal model either as a stand-alone or in combination with gemcitabine.
Research objectives
To determine the anti-tumour activity and potential toxicity of NuvastaticTMand gemcitabine combination on pancreatic xenograft model.
Research methods
Human pancreatic cancer cells were injected subcutaneously into athymic nude mice.C5EOSEW5050ESA (200 or 400 mg/kg per day) was administered orally, while gemcitabine (10 mg/kg/3 d) was given intraperitoneally either alone or in combination treatment. Histopathological analyses of key organs, tumour tissues, and incidence of lethality were determined.
Research results
C5EOSEW5050ESA at 200 mg/kg and gemcitabine combination had no additive antitumor effects compared to a single treatment. A comparably greater response in a reduction in tumour growthviaa reduction of Ki-67 protein expression, and a decrease in necrosis was also demonstrated by 400 mg/kg of C5EOSEW5050ESA and gemcitabine combination compared to that of individual agents. No signs of organ toxicities were observed in any treatment group.
Research conclusions
The combination of C5EOSEW5050ESA with gemcitabine significantly reduced the pancreatic tumour growth in mice compared to a single treatment. This study provides valuable insights into using C5EOSEW5050ESA as a complementary treatment to gemcitabine for pancreatic cancer.
Research perspectives
Findings from this study may provide the basis for product formulation and manufacturing of botanical drugs (NuvastaticTM) to be used as complementary medicine for the treatment of pancreatic cancer patients.
ACKNOWLEDGEMENTS
Ashwaq Yehya was supported by TWAS-USM (The Academy of Sciences for the Developing World,Italy-Universiti Sains Malaysia).
FOOTNOTES
Author contributions:Yehya AHS and Subramaniam AV carried out the experiments; Yehya AHS drafted the manuscript; Yehya AHS, Asif M, and Kaur G analysed the data; Abdul Majid AMS conceived and planned the experiments; Oon CE designed the experiments, derived the model, and supervised the project; All authors have read and approved the final manuscript.
Supported bythe NKEA Research Grant Scheme (NRGS) by the Ministry of Agriculture Malaysia, No.304/CIPPM/650736/k123.
lnstitutional animal care and use committee statement:The animal study was approved and conducted under strict guidelines according to USM Animal Ethics Committee (Reference #: USM/Animal Ethics Approval/2016/(97)(746).
Conflict-of-interest statement:Amin Malik Shah Abdul Majid has a commercial interest in C5EOSEW5050ESAO.sextract of NuvastaticTM. All the other authors declare no financial and non-financial competing interests.
Data sharing statement:No additional data are available.
ARRlVE guidelines statement:The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Malaysia
ORClD number:Ashwaq Hamid Salem Yehya 0000-0001-7464-1618; Ayappa V Subramaniam 0000-0002-3302-6704;Muhammad Asif 0000-0001-8989-9945; Gurjeet Kaur 0000-0002-6232-5703; Amin MS Abdul Majid 0000-0001-7499-2258;Chern Ein Oon 0000-0002-4685-6408.
S-Editor:Ma YJ
L-Editor:Filipodia
P-Editor:Ma YJ
杂志排行
World Journal of Gastroenterology的其它文章
- The mechanism of Yinchenhao decoction in treating obstructivejaundice-induced liver injury based on Nrf2 signaling pathway
- Anoctamin 5 regulates the cell cycle and affects prognosis in gastric cancer
- Effects of Granule Dendrobii on chronic atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats
- Machine learning predicts portal vein thrombosis after splenectomy in patients with portal hypertension: Comparative analysis of three practical models
- Sirolimus increases the anti-cancer effect of Huai Er by regulating hypoxia inducible factor-1α-mediated glycolysis in hepatocellular carcinoma
- lnternational patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis
