Effect of an ionic antineoplastic agent Cytoreg on blood chemistry in a Wistar rat model
2022-09-10KatiuscaVillasanaWilliamQuinteroYepsysMonteroCristianPinoOscarUzcateguiGeizonTorresMariangelPradaLewisPozoWilliamBautaWilliamJimenez
Katiusca Villasana,William Quintero,Yepsys Montero,Cristian Pino,Oscar Uzcategui,Geizon Torres,Mariangel Prada,Lewis Pozo,William Bauta,William Jimenez
1 Biochemistry Laboratories,Department of Pathology,University of the Andes,Mérida,Venezuela
2 Department of Biology,Faculty of Science,University of the Andes,Mérida,Venezuela
3 Vivarium,University of the Andes,Mérida,Venezuela
4 Department of Technical and Experimental Surgery,Faculty of Medicine,University of the Andes,Mérida,Venezuela
5 Vitalis,Clinical Laboratory,Smart Health Laboratory,Merida,Venezuela
6 Cytorex de Venezuela SA,Maracaibo,Venezuela
7 Cytorex Biosciences,Inc.,Weston,FL,USA
Abstract Cytoreg is an ionic therapeutic agent comprising a mixture of hydrochloric,sulfuric,phosphoric,hydrofluoric,oxalic,and citric acids.In diluted form,it has demonstrated efficacy against human cancers in vitro and in vivo.Although Cytoreg is well tolerated in mice,rats,rabbits,and dogs by oral and intravenous administration,its mechanism of action is not documented.The acidic nature of Cytoreg could potentially disrupt the pH and levels of ions and dissolved gases in the blood.Here,we report the effects of the intravenous administration of Cytoreg on the arterial pH,oxygen and carbon dioxide pressures,and bicarbonate,sodium,potassium,and chloride concentrations.Our results demonstrate that Cytoreg does not disturb the normal blood pH,ion levels,or carbon dioxide content,but increases oxygen levels in rats.These data are consistent with the excellent tolerability of intravenous Cytoreg observed in rabbits,and dogs.The study was approved by the Bioethics Committee of the University of the Andes,Venezuela (CEBIOULA) (approval No.125) on November 3,2019.
Key words:acid-base homeostasis;arterial blood chemistry;Cytorex;electrolyte balance;ion therapy;oxygen pressure;tissue oxygenation;Warburg effect
INTRODUCTION
In 1908,the eminent biochemist Lawrence J.Henderson defined the fundamental relationship between the acidic and basic components of living systems,which must exist in harmony (i.e.,equilibrium) for proper physiological function.Because the concentration of hydrogen ions [H+] influences all the systems of an organism,this concentration must be precisely regulated1;changes in [H+] can wholly alter an organism’s endocrine,hematological,and metabolic functions.A variety of factors can disturb a living system’s acid-base balance,which prompts the organism to avoid acidosis or alkalosis by rebalancing pH through three lines of defense that regulate the concentration of [H+] in physiological fluids.These include 1) acid-base buffers,which act in seconds,to counter deviations in acidity;2) the respiratory system,which,within minutes,regulates the elimination of carbon dioxide (CO2;by way of carbonic acid,H2CO3) from the extracellular fluid;and 3) the renal system,which excretes acids or bases in the urine to achieve a [H+] level of 4 × 10–6M or pH 7.40,as opposed to an arterial pH ≤ 7.35 in acidosis or pH ≥ 7.55 in alkalosis.The renal response,which can be measured through the concentration of HCO3–,reacts more slowly in comparison to the other rebalancing defenses,i.e.,on the order of hours,but is nevertheless the most powerful system as a regulator of acids and bases.2Cytoreg is an ionic therapeutic agent consisting of low-molecular-weight organic and inorganic acids (a mixture of hydrochloric,sulfuric,phosphoric,hydrofluoric,oxalic,and citric acids).In diluted form,it has demonstrated efficacyin vitroagainst several cancer cell lines,as well as several dermatological conditions(viral,bacterial,and fungal infections).Its tolerability has been investigatedin vivoin animal models (rats,mice,rabbits,and dogs).However,the pH of the concentrated drug is less than 1,and its principal active component is hydrofluoric acid.3,4Owing to its wide-ranging and attractive pharmacological effects in various studies,it is necessary to investigate whether the physicochemical properties of Cytoreg alter the acid-base equilibrium in an animal,and so infer the compatibility of the drug in human patients.Arterial blood gas analysis is one of the most useful techniques for measuring acid-base equilibria in human and animal subjects.5,6This respiratory monitoring technique permits the measurement of pH,partial pressures of oxygen and carbon dioxide (pO2and pCO2),oxygen saturation (SatO2),hemoglobin content,total carbon dioxide(TCO2),and bicarbonate concentrations [HCO3–] in a sample of arterial blood.7Using arterial blood gas analysis,we sought to determine the acid-base equilibrium responses in male Wistar rats before and after intravenous administration of Cytoreg.
It is important to note that the pharmacodynamics and pharmacokinetic properties of an investigational drug contribute to its safety and successful clinical outcomes.8An understanding of the impact of Cytoreg administration on acidbase equilibria will offer insight on its mechanism of action through its biochemical and physiological effects on arterial blood in the Wistar rat model.
MATERIALS AND METHODS
The design and protocol for the present study were evaluated and approved by the Bioethics Committee of the University of the Andes,Venezuela (CEBIOULA) (protocol number 125)on November 3,2019.
Experimental animals
Ten male Wistar rats (weight,350 g;3 months of age) were obtained from the vivarium of the University of the Andes,Venezuela,and were acclimated to pathogen-free enclosures withad libitumaccess to food and water.
During the course of the experiment,the same environmental conditions were maintained to keep results consistent.The rats were individually housed in polycarbonate Seal safe cages(Tecniplast,Xenoplus,University of Los Andes,Bioterio BIOULA,Mérida,Venezuela) for the duration of the study.The general procedures for the care and housing of animals were in accordance with the Guide for the Care and Use of Laboratory Animals.9Ambient temperature and humidity were maintained in accordance with the Animal Welfare Laws of the United States Department of Agriculture (USDA) with a light period of timer-controlled illumination.10
One flask of Cytoreg (0.105 mL/kg;Cytorex Biosciences,Inc.,Weston,FL,USA;50 mL) was used as the medication in the study.Cytoreg is a solution of mixed strong and weak acids,constituting a complex of ions with individual functions,among which hydrofluoric acid (55 mg/kg) is the active component.The test article was stored at ambient temperature in polypropylene containers and diluted in physiological saline(0.9% w/v) before intravenous administration.
Cytoreg solution (0.105 mL/kg) was administered intravenous under the Surgical Procedure protocol.Diluted Cytoreg solution was prepared by mixing concentrated Cytoreg solution(0.036 mL) with isotonic (0.9%) saline solution (0.464 mL).Once the animals weighed 350 g,the diluted Cytoreg solution was administered.Experiments were performed diurnally at 20 ± 2°C at an altitude of 1690 meters above sea level;importantly,the circadian rhythms of the animals and the altitude influence the pH and pO2levels.11
Arterial blood samples from the ten animals were taken as follows.Prior to drawing the sample (time,0 minute,T0′),each animal was anesthetized with ketamine (100 mg/kg;University of Los Andes,Bioterio BIOULA,Mérida,Venezuela)/xylazine(5 mg/kg;University of Los Andes) intraperitoneal.From the abdominal aorta,blood (0.7 mL) was removed with an insulin syringe containing heparin sodium (0.2 mL,20%,University of Los Andes).Arterial gas analysis was immediately performed with an OPTIMedical arterial blood gas analyzer (OPTITMCCA-TS,Bioula,Mérida,Venezuela).
The diluted Cytoreg solution (0.105 mL/kg) was then administered intravenously.Thirty minutes post-treatment (T30′),arterial blood (0.70 mL) was collected from the sedated animals and analyzed as before.After 60 minutes (T60′),the rats were again anesthetized with ketamine/xylazine (100 mg/kg),and arterial blood was collected and analyzed as before.
Surgical procedure
Each rat was weighed,and the dose of anesthesia calculated(xylazine (5 mg/kg) and ketamine (75 mg/kg).Antisepsis was applied in a supine position using betadine in alcohol (70%v/v).The abdominal wall was incised with Metzenbaum scissors (Bioula) until the abdominal viscera were exposed.The viscera were parted until the abdominal aorta was exposed and the dissection proceeded.An arterial blood sample was withdrawn from the abdominal aorta with an insulin syringe(impregnated with 0.2 mL heparin 20%) prior to treatment with Cytoreg solution.Cytoreg (0.5 mL,0.105 mL/kg) was administered intravenously into the abdominal vena cava.At 30 minutes (T30′) post-treatment,a sample of arterial blood(0.7 mL) was collected as before,and again after 60 minutes(T60′).The visceral organs were replaced in the abdominal cavity and hemostasis was confirmed by observing the absence of bleeding or clots.The muscle layer was closed with continuous catgut 3-0 sutures,followed by the skin with simple 3-0 nylon sutures.The surgical area was treated with antiseptic(betadine in alcohol,70% v/v).Animals were kept sedated for 1 hour during the procedure using ketamine/xylazine intraperitoneally (100 mg/kg).
Post-surgical procedure:The sedated animals were euthanized after the final sampling with an overdose of inhaled enflurane 100% (Bioula) in a closed chamber.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 22 (IBM SPSS Inc.,Chicago,IL,USA).To determine the significance of the averages between the control group (T0′),and treatment groups (T30′ and T60′)was used the repeated measurement analysis of variance for the following variables:pH,PCO2,pO2,[HCO3–],TCO2,and electrolytes (sodium,Na+;potassium,K+:and chloride,Cl–).Then,Tukey’s honest significant difference post hoc test was used to analyze differences between T0′ and T30′,and T0′and T60′.The significance level was 95% and thePvalue was 0.05 (P<0.05).
RESULTS
pH before and after Cytoreg treatment
Prior to treatment with Cytoreg,the mean arterial pH of the 10 Wistar male rats was 7.45 ± 0.046.Our data are comparable with those from other studies on the same species and strain of rat (Table 1).12-16The average pH values determined at T0′(pre-treatment),T30′,and T60′ are 7.45 ± 0.046,7.25 ± 0.11,and 7.22 ± 0.09,respectively (Figure 1A).Values which differ significantly from the initial pH (T0′) (P<0.05) are still within the tolerated range for the organism,since they can be compensated by physiological buffers.However,only two rats(20% of the animals) presented low pH values,at 7.17 (T30′)and 7.16 (T60′).It is worth emphasizing that,between the average pH values at T30′ and T60′,there are no statistically significant differences (P>0.05).
pCO2 before and after Cytoreg treatment
The measurement of the pCO2in each animal before treatment afforded an average value of 27.5 ± 4.22 mmHg,which is comparable to the value of 24.97 mmHg obtained independently by Dvořáček et al.17and Schultz et al.18In the blood,carbon dioxide is present partially in the forms of bicarbonate(HCO3–) and carbonic acid (H2CO3),as well as in its free form(CO2).19Given that gaseous CO2exerts pressure on blood vessels (i.e.,the arteries,in this case),its levels can measured by the electrode in the OPTIMedical analyzer in terms of the pCO2,which can be converted into the quantity of carbon dioxide in circulation.Comparing the pCO2value (Figure 1B) before and after Cytoreg administration reveals that it remains within the initial range (T0′) of 27.5 ± 4.22 mmHg(range 19–33 mmHg),falling post-treatment to 25.5 ± 9.22 mmHg (range 12–43 mmHg) at T30′ and 23 ± 3.37 mmHg(range 15–28 mmHg) at T60′.This is indicative of respiratory stability,or alveolar ventilation,wherein the elimination of CO2is realized without difficulty in an adequate length of time (60 minutes after treatment).
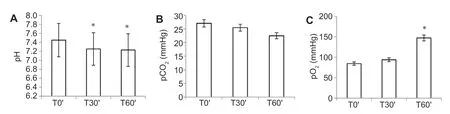
Figure 1:Effects of Cytoreg (0.105 mL/kg) on the acid-base regulatory mechanisms in abdominal arterial blood from 10 male Wistar rats at different time points.
pO2 and hemoglobin before and after Cytoreg treatment
The amount of oxygen found in arterial blood depends on its pressure (pO2) in solution,as well as on the content of the oxygen transport protein,hemoglobin,and the integrity of respiratory mechanisms.12In our study,we obtained an average pO2value of 81.6 ± 7.97 mmHg before treatment with Cytoreg,with post-treatment values of 94.1 ± 19.3 and 147.3 ± 9.59 mmHg at T30′ and T60′,respectively (Figure 1C).Comparing these values with reported data for Wistar rats in similar studies,in which the lowest pO2was 51 mmHg,16with the ranges reported in Table 1,the rats present a state of normoxia at the start of our study,14,16and then exhibit significant increases (P<0.05) at T30′ and T60′ post-treatment.Quantitation of the hemoglobin content reveals a slight decline post-treatment,with values of 13.16 ± 2.31,11.5 ± 2.52,and 11.6 ± 1.49 g/dL at T0′,T30′,and T60′,respectively.However,despite a decrease of ± 0.93 g/dL,we can conclude that our results are within range of the control (as observed at T0′).
[HCO3–] before and after Cytoreg treatment
Among the mechanisms of physiological pH adjustment,the primary action is exerted by bicarbonate ion (HCO3–) in equilibrium with carbonic acid,via H2CO3H++ HCO3–.20In our study,the Wistar rats presented a plasma HCO3–concentration of 18.31 ± 2.82 mM (16.9–22.7 mM) before treatment.Upon Cytoreg administration,concentrations of 11.3 ± 4.10 mM(3.7–17.9 mM) were observed after 30 minutes and 7.94–2.92 mM (4.5–11.1 mM) after 60 minutes.Buffering action is the first of the lines of defense against a change in pH.21In our study,we saw a decrease in the plasma [HCO3–] after Cytoreg treatment,in agreement with the observed decrease in pH from its initial level (7.45) to 7.25 1 hour later.These results demonstrate the expected “bicarbonate buffer” compensation for the change in [H+] as the first line of defense against a change in pH.22The values obtained were significant (P<0.05) for each time point (i.e.,T30′ and T60′,with respect to T0′).
TCO2 before and after Cytoreg treatment
Total CO2,also known as the reserve alkalinity,represents the amount of CO2present in the bloodstream.It comprises the sum of CO2contained in the forms of HCO3–(~98%),dissolved CO2,and carbonic acid (H2CO3).Since almost all the CO2corresponds to plasma bicarbonate,the term “reserve alkalinity” represents the amount of CO2present in plasma as HCO3–.23The TCO2value in the Wistar rats was 19.14 ± 2.96 mM (range 12.2–23.8 mM) before treatment.After Cytoreg treatment,these values varied widely,decreasing on average to 12.06 ± 4.33 mM (4.1–19.2 mM) at T30′ and 8.54 ± 2.31 mM (5–11.9 mM) at T60′.There was a decrease in TCO2over time proportional to the bicarbonate buffer to compensate for the [H+] entering the organism.
Seeing that the TCO2was diminished in our experiments,we inferred metabolic acidosis,by which the organism compensates for excess acid.23Since these data have utility if they complement the pH status and levels of potassium and chloride ion,we also measured the electrolyte ions,Na+,K+,and Cl–.
Sodium is the most important cation in extracellular fluids,as it establishes the ionic pressure of interstitial liquids.24Its determination is indispensable in the study of electrolyte balance.In our study,the Wistar rats presented stable concentrations with respect to T0′ (prior to Cytoreg administration),without significant differences (P>0.05) at the different measurement times:157.5 ± 6.51 mM at T0′,150.4 ± 3.13 mM at T30′,and 139.6 ± 11.24 mM at T60′,with a variation of ±9.05 mM over the duration of the experiment.The level of potassium,as the intracellular cation that predominates in striated muscle cells,together with that of sodium,is indicative of electrolyte balance,and its decrease or increase is important in electrocardiographic studies as well as in acid-base equilibrium.24In our study,the Wistar rats had a basal K+concentration (T0′) of 2.53± 0.51 mM,with the value rising slightly to 3.03 ± 1.09 mM at T30′ and further to 4.29 ± 1.52 mM at T60′,with significant differences (P<0.05) at the different measurement times.
The chloride ion constitutes the predominant electrolytic ion in the extracellular space.Found in the organism in its free form as an ion,Cl–maintains cellular integrity via its influence on osmotic pressure,acid-base equilibrium,and water balance.25Its concentration is generally found to increase in the acidosis process and diminishes in alkalosis.The concentration of Cl–in the plasma of the Wistar rats was 119.4 mM,and remained fairly constant after treatment (P>0.05,not significant) with a value of 114.2 mM.
DISCUSSION
pH
Rats are useful models for the study of acid-base mechanisms and respiratory parameters in preclinical and toxicological assays.Data considered to be normative for arterial blood gases and electrolytes help to identify healthy animals for experiments.26Based on our results in the Wistar rats,we found that pH decreases significantly (P<0.05) after Cytoreg administration,from 7.45 to 7.25 after T30′ and 7.22 after T60′.Despite the observed changes,these results conform to the reference ranges reported in other study16(7.2–7.51 ± 0.3).It is known that general anesthetics (phenobarbital,ketamine/xylazine,and Zoletil®) have a great influence on arterial blood pH.16In general,anesthetics produce a more acidic internal environment in humans and in animal models (rodents),causing respiratory system depression;affecting cardiac rhythm and locomotor function in rodents;and also affecting the mechanisms of pH regulation.16In our study,we used intraperitoneal ketamine/xylazine (100 mg/kg) to anesthetize the rats,and our results indicate that ionic treatment with Cytoreg does not disrupt such acid/base mechanisms.
pCO2
All discussions on gas exchange should begin with CO2,given that it is the only gas that affords information about ventilation,oxygenation,and acid-base equilibrium.23In this study,the values for pCO2remained constant (P>0.05),or in a state of normocapnia,throughout the 1 hour treatment,indicating stable respiration or alveolar ventilation,wherein the proper elimination of CO2is realized without difficulty in an adequate time (T60′).As indicated previously,the second line of defense against changes in [H+],i.e.,the respiratory system,acts within a few minutes to eliminate CO2by way of H2CO3from the organism.Our results demonstrate that Cytoreg does not affect this mitigation system.
pO2
The values obtained before and after Cytoreg treatment for the pO2and hemoglobin content,which determine the saturation and transport of oxygen in experimental animals,demonstrate the physiological integrity of the respiratory mechanism.By obtaining pO2data,we can determine whether an individual is capturing oxygen and in what proportion;this data has great importance in all the processes by which we evaluate respiratory capacity and the transport of oxygen to the tissues.In this work,the pO2of the Wistar rats increases after the intravenous administration of Cytoreg,which suggests better oxygen capture in the rat tissues.As the final acceptor of electrons in the electron transport chain coupled to the production of ATP through oxidative phosphorylation,O2plays a critical role in cellular function at the mitochondrial level for the generation of energy,a vital process in the organism.For cells such as neurons,such functions are dependent on mitochondrial metabolism,in which oxygen has an indispensable function.Many neurodegenerative diseases involving progressive neuronal death (e.g.,Parkinson’s disease,Alzheimer’s disease,Huntington’s disease) and immunological diseases (e.g.,lupus,multiple sclerosis) have their origin in a failure of mitochondrial activity.27,28For example,proper aerobic metabolism is very important for the vitality of the brain,in which the oxygen consumption of ~3.4 mol per day is much higher compared to other human tissues.29Similarly,the activation,differentiation,and production of antibodies or immunoglobulins by leukocytes,the principal cells of the immune system,depend on proper mitochondrial metabolism.The therapies for these diseases are directed to the improvement of oxygen capture through techniques such as hyperbaric chamber and coenzyme Q treatments.30,31Thus,the principal strategy underlying hyperbaric oxygen treatment is to increase the oxygen level in tissues.Furthermore,since a small increase in the pO2in ischemic areas increases the bactericidal activity of leukocytes,Cytoreg,as a therapeutic ionic agent,could play an important role in the regulation of these immune cells.Additionally,experiments have been performed on the effects of oxygen levels on cancer cells,indicating that hypoxia (insufficient oxygen to meet the metabolic demands of the cell)is a factor which favors the growth of cancer cells,referred to as the Warburg effect.32The role of hypoxia in metabolic pathways specific to cancer–including changes in gene expression (genes inducible by HIF) and the proteome,genomic instability,the regulation of angiogenesis (formation of blood vessels),and the evasion of apoptotic processes33–has driven the attention of scientists towards the creation of therapies that alter cellular metabolism such that the cell shifts from hypoxic to oxygen-rich.16,29,32,34On observing the effects of Cytoreg on arterial oxygen (P<0.05),we see a significant increase at T30′ and T60′.The simultaneous decrease in blood pH (±0.02) increases the liberation of oxygen from hemoglobin to the tissues,a phenomenon known as the Bohr effect,35without affecting the acid-base mechanisms in the organism.One of the reported therapeutic effects of Cytoreg is the inhibition of the growth and proliferation of many cancer cell lines.36Our findings indicate that Cytoreg has an important effect on cellular metabolism by increasing mitochondrial activity,and suggest a mechanism for cancer cell apoptosis through the generation of reactive oxygen species,which may be considered a therapeutic mechanism of action that alters cellular metabolism from a hypoxic,oxygen-deficient state with an initial pO2of 84.6 ± 7.97 mmHg to a totally aerobic environment 147.3 ± 10.1 mmHg 60 minutes after administration.This mechanism of action not only influences the growth of neoplastic tissue but also affects the aforementioned illnesses,which are related to proper mitochondrial function.Our results are even more significant,given the studies on the hypoxic effects of general anesthesia on murine animal models (with hypercapnia,or increased pCO2,34which significantly influence pO2readings.Despite the fact that the animals in this study were intraperitoneally anesthetized with ketamine/xylazine,and that arterial gas measurements were made at an altitude of 1690 m above sea level and a temperature of 20 ± 2°C (low atmospheric pressure and temperature are factors that influence pO2),23no hypoxia was observed upon administration of the medication,but rather,an increase in pO2.
HCO3–
The acid-base equilibrium in bodily fluids depends on the concentrations of H+and HCO3–ions.In healthy and awake mammals,including humans,there are compensatory mechanisms to maintain the necessary acid-base equilibrium for normal enzymatic activity,electrolyte diffusion,hemoglobin saturation,and coronary contraction,all of which contribute to the normal functioning of vital organs.11Plasma bicarbonate is an important factor in directing the metabolic equilibrium in metabolic states of acidosis or alkalosis.Our data show a difference in [HCO3–] before (18.33 ± 2.82 mM) and after treatment(7.94 ± 2.22 mM).We infer a state of metabolic acidosis (pH 7.24 ± 0.09),and in this setting,bicarbonate functions as the primary buffer to re-establish the normal acid-base equilibrium.At the human clinical level,these variations are physiologically corrected via the renal system without compromising the proper functioning of the organism.The observed decrease in bicarbonate concentration in our study demonstrates its role in compensating for the [H+] introduced into the organism.This is likewise observed in humans,where the TCO2is found to decrease during metabolic acidosis to compensate for excess acid in the organism,a fact which is useful if complemented with information about pH and K+and Cl–levels.
Electrolytes
The electrolytes Na+,K+,and Cl–play important roles in acidbase equilibrium.Although arterial pH is the principal factor that determines the secretion of acid,its excretion also depends on the concentrations of K+,Cl–,and aldosterone.The intracellular concentration of K+and the secretion of H+are related in a reciprocal fashion:the depletion of K+increases the secretion of H+and,as a consequence,alters acid-base homeostasis.When the extracellular pH varies,changes in the acid-base equilibrium influence the plasma concentration of K+.During metabolic acidosis,as provoked by other causes besides the accumulation of organic acids (as from renal insufficiency),60% or more of the excess H+is absorbed in the interior of cells.Electroneutrality is maintained through the movement of K+and Na+.25Observing the effect of Cytoreg on electrolytes(Na+and Cl–) in the blood,we see that the concentrations are constant relative to their pre-treatment levels (T0′),indicating a stable acid-base system.Given that Cytoreg contains hydrochloric acid (HCl) as part of its chemical composition37;our data suggests the capture of Cl–by a specific tissue,which could possibly explain its therapeutic effects.We know that,in our experiment,the increase in K+(P<0.05) from 2.56 ± 0.51 mM (T0′) to 4.26 ± 1.52 mM (T60′) might be a physiological response to a change or decrease in [H+] (and likewise a decrease in [HCO3–]) in the blood,where its concentration is primarily mediated by renal excretion,which takes hours or days to exercise its effect despite being most powerful acidbase regulatory system.38We observed the effect of Cytoreg on the acid-base equilibrium one hour post-treatment,and for this reason,it would be interesting to monitor the effect over several days of constant-dose administration,and thus deduce the effects of the renal regulatory system and the concentrations of electrolytes in the urine.
We consider that the only limitations we had were regarding the availability of a greater number of animals to include in the study.We had in our plan to include a greater number of animals,but the surgical procedure to be performed on each animal in the operating room was limiting due to the unavailability in the country to provide those resources to the research and development center of the Universidad de Los Andes in the city of Merida,where we performed the surgical activity.This single limitation meant that we could not include a greater number of animals in the study.This limited us to only 10 animals.
● Cytoreg is an ionic antineoplastic agent which,despite its physicochemical characteristic of low pH owing to its organic and inorganic acid composition,does not affect the acid-base equilibrium in blood because of the immediate compensatory response by plasma buffers which do not permit significant variation of pH in the blood.Our study confirmed that the principal buffer,HCO3–,behaves in the same manner in the rat model as it does in humans.
● We may conclude from this study using the Wistar rat model that the partial pressures of CO2(pCO2),the principle gas that affords information on the levels of ventilation,oxygenation,and acid-base equilibrium,remain constant.This behavior of pCO2levels shows that the ionic therapeutic Cytoreg does not alter respiratory stability or alveolar ventilation,such that the adequate elimination of CO2is realized in an appropriate time without difficulty.
● The results also demonstrate that the ionic therapeutic Cytoreg does not compromise the physiological integrity of the organs (lung and kidney) responsible for maintaining acid-base equilibrium.
● With respect to electrolyte behavior,we conclude that treatment with Cytoreg maintains hydration and the electrolyte balance in Wistar rats.
● This study also demonstrates that the pO2increases after the administration of Cytoreg.This could result in improved oxygen capture by tissues and have a beneficial therapeutic effect in diseases characterized by hypoxia.
Acknowledgements
We thank William Quintero MSc for his critical observations and assistance with preparing the manuscript.We thank to Vitalis Clinical Analysis Laboratory for providing the equipment and reagents for quantification of the hemoglobin content.
Author contributions
KV,LP and WJ participated in research design;KV,GT and MP conducted experiments;CP,YM and OU contributed new reagents or analytic tools;WQ,PL,WJ and WB wrote or contributed to the writing of the manuscript.All authors contributed to this study and approved the final manuscript.
Conflicts of interest
Cytorex actively participates with its resources,in support of the research team for the development of research through its contribution in the design,coordination and direction of its districts scientific studies so that they achieve the development objectives established in the Cytorex Plans.All the authors declare no conflicts of interest.
Financial support
This work was financially supported by Cytorex Biosciences,Inc.
Institutional review board statement
This study was approved by the Bioethics Committee of the University of the Andes,Venezuela (CEBIOULA) (approval No.125) on November 3,2019.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Medical Gas Research的其它文章
- Effects of nitrous oxide on end-tidal carbon dioxide measurements in spontaneously breathing patients under general anesthesia
- A special oropharyngeal oxygenation device to facilitate apneic oxygenation in comparison to high flow oxygenation devices
- Xenon as a transdermal enhancer for niacinamide in Strat-MTM membranes
- Comparing the effect of xenon and sevoflurane anesthesia on postoperative neural injury biomarkers:a randomized controlled trial
- Potential role of hydrogen sulfide in central nervous system tumors:a narrative review
- Role of hyperbaric oxygen in glioma:a narrative review
