The feasibility of leadless pacemaker implantation in nonagenarians: case report and literature review
2022-09-06ChunLingZHENGLiZHAOYuCHENZhengMingXULingLIULiLiWANGYingMingLIU
Chun-Ling ZHENG, Li ZHAO,✉, Yu CHEN, Zheng-Ming XU, Ling LIU, Li-Li WANG,Ying-Ming LIU✉
Department of Cardiology, the Sixth Medical Center, Chinese PLA General Hospital, Beijing, China*The authors contributed equally to this manuscript
In the aging populations of China, there is an increasing prevalence of various types of bradycardias that require pacing therapy. In 1958, the first transvenous pacemaker (TVPM) was implanted at Karolinska Hospital in Sweden, which unveiled the era of cardiac pacing therapy.[1]A TVPM includes transvenous electrodes and a pulse generator that is implanted subcutaneously. Despite its obvious benefits, the complication rate associated with TVPM could be as high as 10%,[2]including electrode dysfunction and device infection. Notably,reports show that among those who developed a device infection, the one-year mortality rate was 12%for a pocket infection and 31% for an endovascular infection, even after the device was removed.[3]
Senior patients, especially nonagenarians, have unique pathophysiological properties, including frailty, malnutrition, and dementia, which further increase the risk of developing complications associated with TVPMs.[4]The advent of leadless pacemakers (LLPMs), which are less than 1/10 the size of TVPMs, have no transvenous leads or need for subcutaneous pockets, greatly reduces the complications of pacing therapy. Here, we report the implantation of LLPMs in two seniors who are older than 90 years of age in the Sixth Medical Center, Chinese PLA General Hospital, Beijing, China, and review the recent advances in LLPM.
Case 1 A 94-year-old male received his first single chamber pacemaker (VVI, Philos II S, Biotronik)on March 24th, 2010, due to sick sinus syndrome. He had multiple comorbidities, including coronary heart diseases, hypertension, and diabetes mellitus. A routine programing control during the follow-up showed an elective replacement indicator in October 2021, which means the battery of the pacemaker was at the end of its life 11 years after implantation, and replacement was scheduled. However, the patient was skinny, with severe thinning of the skin above the original pocket, which was on the verge of bursting. As the original device model was no longer available, a different model with a different shape pulse generator would increase the tension of the skin and increase the risk of skin ulceration. After discussing these differences and risks with the patient and his family, an LLPM (Micra VR, Medtronic)was successfully implanted under local anesthesia on October 20th, 2021. The Micra capsule was steadily fixed to the right ventricular septal wall after the release, while the original TVPM electrode was left aside, as shown on the X-ray image (Figure 1). The original pulse generator was kept untouched and closely followed.
Case 2 A 92-year-old male was hospitalized due to severe atrioventricular conduction blockage on November 15th, 2021. He had hypertension, diabetes mellitus, history of ischemic stroke and severe dementia. The electrocardiogram showed a seconddegree atrioventricular block, with 2:1 atrioventricular conduction (Figure 2). A pacemaker was indicated according to current guidelines.[5]There were concerns that his poor cognitive function would increase the risk of a pocket infection due to uncontrolled scratching of the operation area, so an LLPM was selected after discussing the concerns and risks with the patient’s family. An LLPM (Micra VR, Medtronic) was successfully implanted under general anesthesia on November 23rd, 2021. The pacemaker functioned normally after implantation, with ventricular pacing signals at set pacing intervals, and this patient resumed his normal activities the day after the operation.
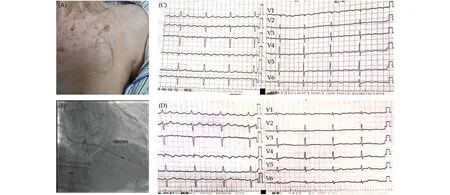
Figure 1 Periprocedural pictures of Case 1. (A): Pocket for the original pacemaker of the patient, where the skin was ultrathin; (B):the Micra VR pacemaker after release, with the original lead left aside; and (C & D): the electrocardiograms, both of which showed atrial flutter with 4:1 conduction.
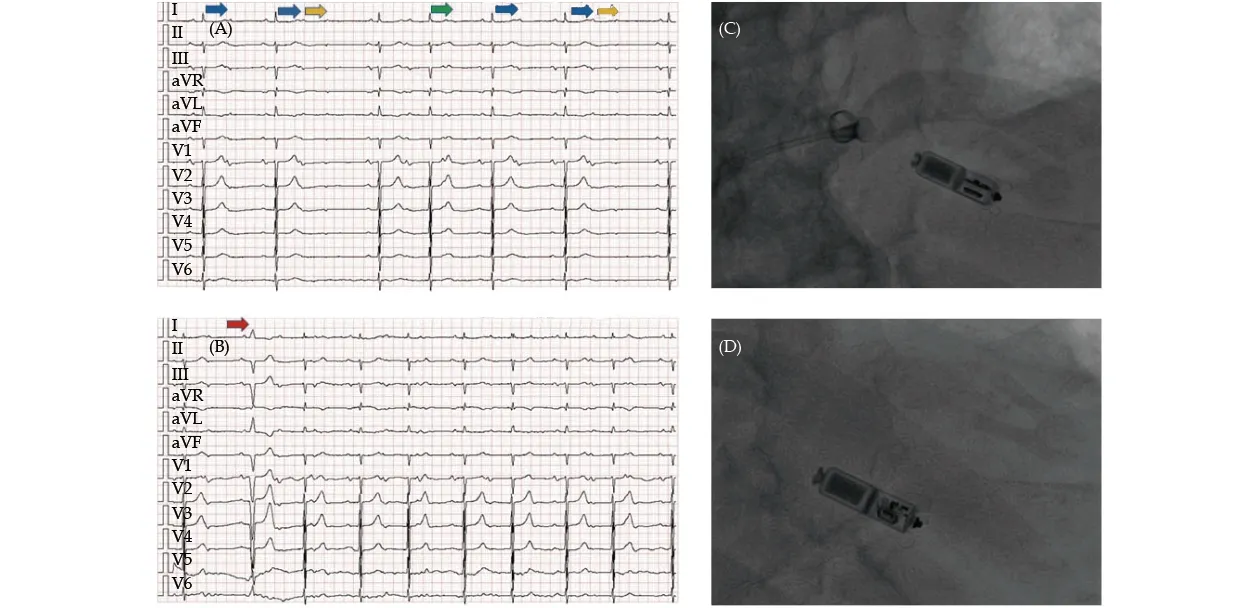
Figure 2 Periprocedural pictures of Case 2. (A): Preprocedural electrocardiogram, which showed second-degree (3:2, green arrow;2:1, blue arrows) atrioventricular block with blocked premature atrial beats (yellow arrows); (B): postprocedural electrocardiogram,which showed ventricular pacing in VVI mode (at pacing threshold of 45 beats/min) (red arrow); (C): attempted release of Micra VR;and (D): Micra VR after release.
Both procedures were successfully performed, the patients had a fast recovery after the procedure and no procedure-related complications were observed at the three-month follow-up, and programming at the follow-up showed that both LLPMs functioned normally. The periprocedural care was simple, with no restrictions on posture or daily activities, and the in-hospital stay was short.
These two patients are part of the East Asia nonagenarian population, and a large proportion of them are thin, frail, and complicated with multiple comorbidities or decreased cognitive function. Driven by an increasing life expectancy, the demand for pacing therapy and the challenges associated with pacing therapy among the super-aged population are rapidly increasing.[6]Herein, the implantation of LLPMs in nonagenarians at high risk of complications with traditional TVPMs was safe, feasible and beneficial for this population.
The miniaturization of high-density power, electronic circuits and advanced communication technology made it possible to design miniaturized electronic devices directly implanted into the heart.[7,8]There have been two commercial LLPMs, the Nanostim Leadless Cardiac Pacemaker (St. Jude Medical)and the Micra Transcatheter Pacing System (Micra VR, Medtronic) (Table 1). Nanostim, which received European Conformite Europeenne certification in 2013, had a major recall in 2016 due to early battery depletion and interface button disengagement, leading to the discontinuation of its implantation. Micra VR has been available since 2015 and is, currently, the mainstream LLPM; Micra VR measures approximately 1/10 the size of traditional TVPMs and reduces the amount of nonbiological materials within the body by 93%.[9]In addition, it is compatible with nuclear magnetic resonance imaging up to 3.0T, which is advantageous for those who need magnetic resonance imaging scans after pacemaker implantation.
A survey showed that common reasons for choosing LLPM included expected vascular access difficulties (91%), history of TVPM complications (87%), permanent atrial fibrillation with a pacing indication(83%) and an expected high risk of infection (70%).[10]LLPMs could be considered in seniors with high risks of poor compliance and device-related infection.[11,12]LLPM could also be considered in patients who want a perfect appearance and a higher quality of life.[13]In addition, for end-stage renal failure patients undergoing dialysis,[14]LLPM decreases the risk of complications related to infection and, at the same time,preserves the central veins and peripheral veins for potential future procedures.[15]
LLPMs have comparable effectiveness and superior safety in select patients when compared with TVPMs. Although there were no randomized controlled trials comparing LLPMs with TVPMs, observational studies showed that the total complication rate was lower for LLPMs than for traditional TVPMs,and the rate of heart perforation and tamponade was higher for LLPMs.[16]However, the cardiac perforation rate is expected to decrease as implantation techniques improve. From the Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers, compared with the transvenous VVI pacemakers, the complication rate was 23% lower in the Micra group at the six-month follow-up,[17]and 31% lower at the two-year follow-up, with a 38% lower risk of reinterventions.[18]In another report, the rare cases of infection after Micra implantation were sensitive to antibiotic therapy, which obviated the need for device removal.[19]
Surgeons are becoming more experienced in LLPM implantation for elderly patients at high risk of TVPM-associated complications. A recent study in super-aged patients (over 85 years) showed that the total complication rates were 7.4% in LLPM patients and 11.4% in TVPM patients.[12]Niu, et al.[20]reported 23 senior patients (average age: 80 ± 10 years)who successfully received Micra transcatheter pacing system, with no periprocedural complications,and the devices functioned normally in an average follow-up of 1.3 years. Ling, et al.[21]reported 15 pa-tients who underwent LLPM implantation, with an average age of 70 ± 11 years, and latter 5 of the 15 patients had markedly shorter procedural times when compared to the earlier 10 patients (23 ± 5 min vs.48 ± 23 min, P = 0.03), which indicated the overall short procedural time and a rapid learning curve for starting centers.
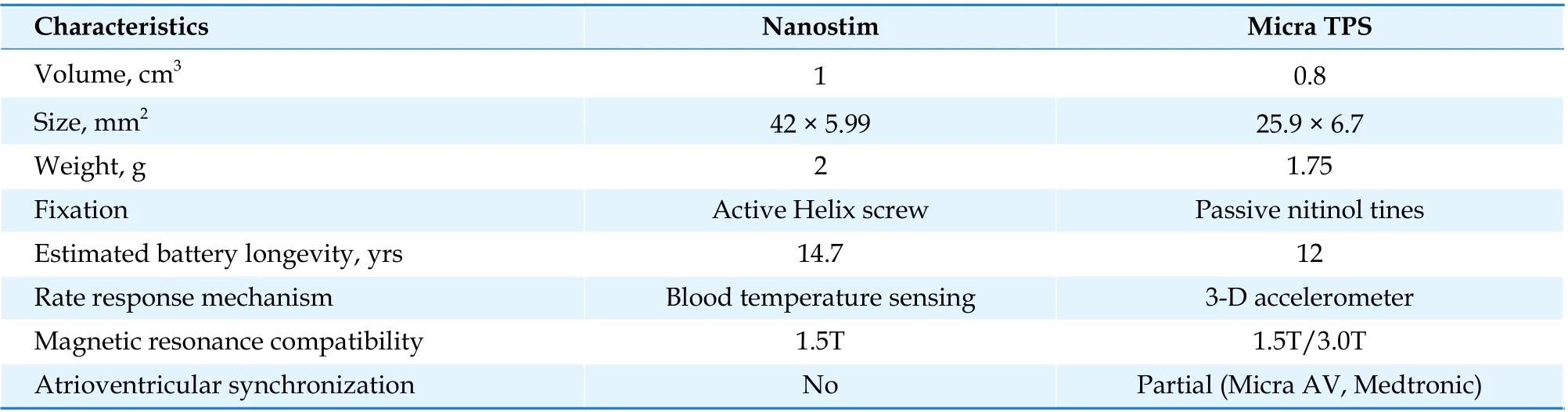
Table 1 Comparison of leadless pacemakers.
Despite its advantage in safety, the first-generation LLPM (Micra VR, Medtronic) was only feasible for 16% of patients with indications for cardiac pacing. The main limitation of the first-generation LLPM is its restricted right ventricular pacing and sensing, hence no atrioventricular synchronization. To circumvent the primary limitation of the first-generation LLPM, the second-generation LLPM (Micra AV, Medtronic), first implanted in 2020, was programmed to monitor atrial contraction by a 3-axis accelerometer, which enabled atrioventricular synchronous pacing (VDD mode).[22]With these improvements, the second-generation LLPM was suitable for 40% of all patients with pacing indications.In the Micra Atrial tRacking using a Ventricular accELerometer 2 study, the VDD pacing mode of Micra AV improved atrioventricular synchronization from 26.8% to 89.2% without pacing complications.[23]
Successful implantation and satisfactory performance of LLPMs in nonagenarians confirmed their safety and effectiveness in the increasing super-aged population. LLPMs can reasonably receive higher level recommendations in future guidelines as a preferable choice for select super-aged patients with increased susceptibility to TVPM-related complications.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Effect of uninterrupted dabigatran or rivaroxaban on achieving ideal activated clotting time to heparin response during catheter ablation in patients with atrial fibrillation
- Inflammation-based different association between anatomical severity of coronary artery disease and lung cancer
- Long-term outcome of percutaneous or surgical revascularization with and without prior stroke in patients with threevessel disease
- Relationship between arterial stiffness and cognitive function in outpatients with dementia and mild cognitive impairment compared with community residents without dementia
- Serum triglycerides concentration in relation to total and cardiovascular mortality in an elderly Chinese population
- The predictive value of triglyceride-glucose index for in-hospital and one-year mortality in elderly non-diabetic patients with ST-segment elevation myocardial infarction
