Effect of probiotics on hemodynamic changes and complications associated with cirrhosis: A pilot randomized controlled trial
2022-09-01RomanMaslennikovIrinaEfremovaVladimirIvashkinMariaZharkovaElenaPoluektovaElenaShirokovaKonstantinIvashkin
Roman Maslennikov, Irina Efremova, Vladimir Ivashkin, Maria Zharkova, Elena Poluektova, Elena Shirokova,Konstantin Ivashkin
Roman Maslennikov, lrina Efremova, Vladimir lvashkin, Maria Zharkova, Elena Poluektova, Elena Shirokova, Konstantin lvashkin, Department of Internal Medicine, Gastroenterology and Hepatology, Sechenov University, Moscow 119435, Russia
Roman Maslennikov, Vladimir lvashkin, Elena Poluektova, The Scientific Community for Human Microbiome Research, Moscow 119435, Russia
Roman Maslennikov, Consultative and Diagnostic Center No. 2 of Moscow Health Department ,Moscow 107764, Russia
Abstract BACKGROUND Bacterial translocation exacerbates the hyperdynamic circulation observed in cirrhosis and contributes to a more severe disease course. Probiotics may reduce bacterial translocation and may therefore be useful to redress the circulatory imbalance.AIM To investigate the effect of probiotics on hemodynamic parameters, systemic inflammation, and complications of cirrhosis in this randomized placebocontrolled trial.METHODS This single-blind randomized placebo-controlled study included 40 patients with Child-Pugh class B and C cirrhosis; 24 patients received probiotics (Saccharomyces boulardii) for 3 mo, and 16 patients received a placebo over the same period. Liver function and the systemic hemodynamic status were evaluated pre- and postintervention. Echocardiography and simultaneous blood pressure and heart rate monitoring were performed to evaluate systemic hemodynamic indicators.Cardiac output and systemic vascular resistance were calculated.RESULTS Following a 3-mo course of probiotics in comparison to the control group, we observed amelioration of hyperdynamic circulation [a decrease in cardiac output (P = 0.026) and an increase in systemic vascular resistance (P = 0.026)] and systemic inflammation [a decrease in serum C-reactive protein levels (P = 0.044)], with improved liver function [an increase in serum albumin (P = 0.001) and a decrease in the value of Child-Pugh score (P = 0.001)] as well as a reduction in the severity of ascites (P = 0.022), hepatic encephalopathy (P = 0.048), and cholestasis[a decrease in serum alkaline phosphatase (P = 0.016) and serum gamma-glutamyl transpeptidase (P = 0.039) activity] and an increase in platelet counts (P < 0.001) and serum sodium level (P =0.048).CONCLUSION Probiotic administration was associated with amelioration of hyperdynamic circulation and the associated complications of cirrhosis.
Key Words: Gut; Gut-liver axis; Microbiota; Hemodynamics; Heart; Gut-heart axis; Saccharomyces boulardii; Portal hypertension
lNTRODUCTlON
Cirrhosis, which represents the culmination of chronic liver disease[1], is characterized by changes in liver morphology, reduced liver function, and the onset of portal hypertension. However, in addition to the liver, the intestine and its microbiota are affected by the pathophysiological derangements in cirrhosis. Cirrhosis is known to be associated with disturbances in the composition of the gut microbiota(gut dysbiosis[2-16]), expansion of the microbiota of the small intestine (small intestine bacterial overgrowth[17]), and increased permeability of the intestinal barrier[18], all of which result in bacterial translocation, which refers to the entry of bacteria and their components from the intestinal contents through the intestinal wall into the lymph, blood, and body tissues[19,20]. Bacterial translocation leads to systemic inflammation, which precipitates hemodynamic alterations [hyperdynamic circulation indicated by increased cardiac output and decreased systemic vascular resistance (SVR)] that contribute to liver decompensation[21-25]. This bidirectional association between the gut along with its microbiota and the liver is referred to as the gut-liver axis[26] or the gut-heart-liver axis[25]. Studies have shown that certain drugs that affect this axis can redress the hemodynamic imbalance and improve the clinical course in patients with cirrhosis. Among these drugs, probiotics are live microorganisms, which when administered in adequate amounts confer several health benefits on the host[27]. Although evidencebased research supports the role of probiotics in cases of hepatic encephalopathy, their effects on other symptoms and manifestations of cirrhosis remain unclear[28]. A non-controlled study reported that a 6-wk course of probiotics reduced the cardiac output and heart rate and increased the SVR and serum sodium levels in the study population[29].
Saccharomyces boulardii(S. boulardii), a probiotic yeast, has shown significant effectiveness for the treatment or prevention of diarrhea, inflammatory bowel disease, irritable bowel syndrome,Helicobacter pyloriinfection, and dyslipidemia, among other such conditions[30,31].S. boulardiiproduces pleiotropic effects; it reestablishes the gut microbiome after dysbiosis[32], strengthens the intestinal immune barrier[33], improves the trophic function of gut microbiota[34], restores the impaired gut barrier, and protects against bacterial translocation[35] in experimental models and in patients with gut diseases.S. boulardiiadministration in an experimental mouse model of cirrhosis led to correction of gut dysbiosis, decreased intestinal permeability, as well as reduced severity of liver inflammation and fibrosis[36]. However, the role of this probiotic is not known in humans with cirrhosis.
In this randomized placebo-controlled trial, we investigated the effect of probiotic administration (S.boulardii) on hemodynamic parameters, systemic inflammation, and complications of cirrhosis.
MATERlALS AND METHODS
Patients
In this single-blind randomized placebo-controlled trial, 198 consecutive patients with cirrhosis who underwent health check-ups at the Department of Hepatology’s Clinic for Internal Diseases, Gastroenterology, and Hepatology at Sechenov University were screened for inclusion. The study procedures were explained to potential participants, and written informed consent was obtained before enrollment.The study was approved by the Ethics Committee of Sechenov University and was registered at clinica ltrials.gov (NCT05231772). The research protocol can be accessed from this website.
Inclusion criteria were as follows: (1) Diagnosis of cirrhosis based on histopathological, or clinical,biochemical, and ultrasonographic findings; (2) Child-Pugh class B or C cirrhosis; (3) Age between 18 years and 70 years; and (4) Signed informed consent. Exclusion criteria were as follows: (1) Administration of lactulose, lactitol, or other prebiotics, probiotics, antibiotics, or metformin during the 6 wk preceding study commencement; (2) Alcohol consumption 6 wk preceding study commencement; or (3)Diagnosis of inflammatory bowel disease, cancer, or any other serious disease.
There are no data to calculate the required sample size.
Of the 198 patients initially screened for inclusion, 40 met the inclusion criteria and were enrolled in the study (Figure 1). Patients included in the study were randomized into the test and control arms(ratio 1.5:1). The Excel function RANDBETWEEN (1:5) was used as a random number generator; for numbers 1 to 3, patients were assigned to the test arm and for numbers 4 or 5, patients were assigned to the placebo group. Patients who prematurely discontinued ingestion of the experimental probiotic/placebo or were administered antibacterial drugs, other probiotics, or prebiotics during the follow-up period were excluded from the study.

Figure 1 CONSORT 2010 flow diagram.
Intervention and controls
Patients in the test arm receivedS. boulardiiat a dose of 250 mg twice a day for 3 mo and those in the control group received a placebo preparation at the same dose over the same period. Patients were not aware whether they were administered a placebo or the experimental drug. Additionally, all patients received standard of care treatment for cirrhosis. Drugs administered did not significantly differ between patient groups (Table 1). Patients were re-evaluated 3 mo after initiation ofS. boulardiior placebo treatment.

HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Outcomes
All patients underwent a standard medical check-up for evaluation of cirrhosis and for measurement of indicators of systemic hemodynamics before and 3 mo after initiation ofS. boulardiior placebo treatment(the first and second visit, respectively). There were no additional visits or examinations between these two time points. The outcomes included changes in cardiac output, SVR, the extent of systemic inflammation (represented by serum C-reactive protein levels), severity of ascites and hepatic encephalopathy,serum levels of liver biomarkers, and Child-Pugh scale scores.
Echocardiography was performed at rest based on the guidelines of the American Society of Echocardiography[37-40]. The systolic and diastolic blood pressure and heart rate were measured using an automatic oscillometric sphygmomanometer (A and D, Japan) simultaneously with measurement of the stroke volume. Table 2 shows the hemodynamic parameters calculated in this study[37-42].
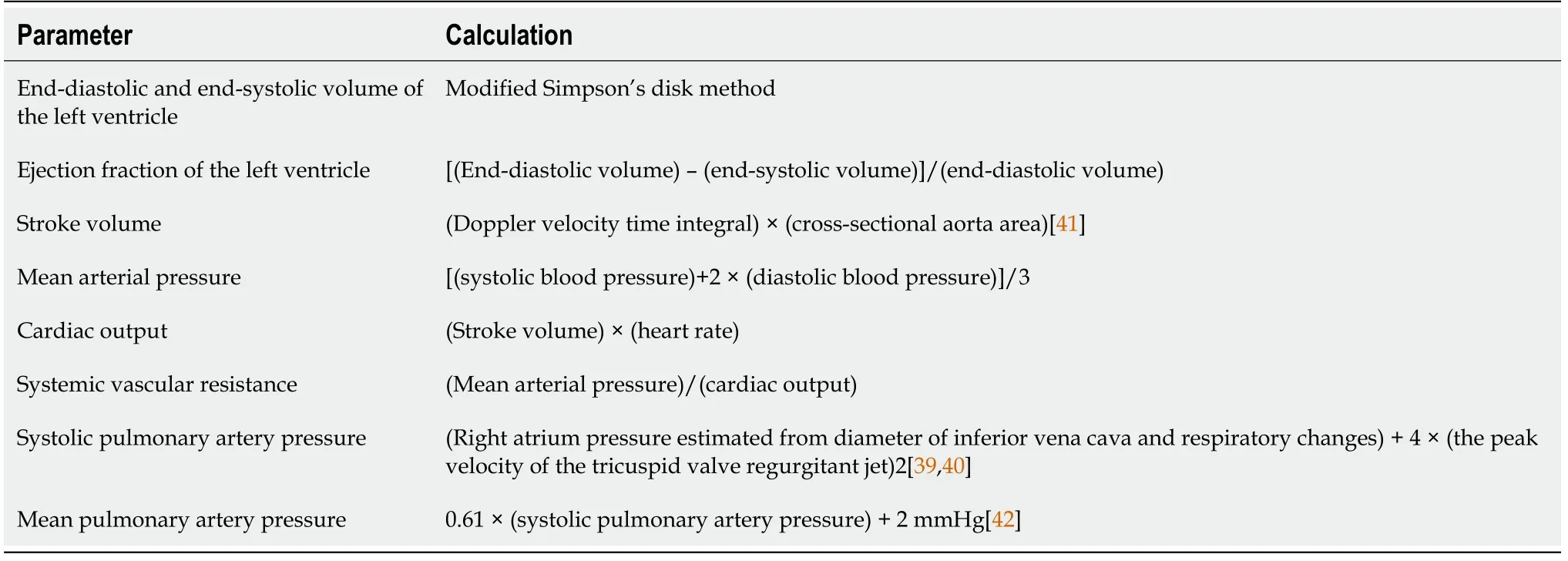
Table 2 Calculations of hemodynamic parameters
The degree of ascites was determined based on the International Ascites Club scale as follows: 0 = No ascites; 1 = Minimal ascites (measurable only with instrumental methods); 2 = Clinically significant ascites (determined on physical examination); and 3 = Gross ascites[43].
The degree of hepatic encephalopathy was determined based on the following scale: 0 = No hepatic encephalopathy; 1 = Minimal hepatic encephalopathy; and 2 = Overt hepatic encephalopathy[44].
Statistical analysis
Statistical analysis was performed with STATISTICA 10 (StatSoft Inc., Tulsa, OK, United States)software. The data were presented as medians interquartile ranges]. Differences between continuous variables were assessed with the Mann-Whitney test because many variables were not distributed normally. Fisher’s exact test was used to assess the differences between categorical variables.Pvalues ≤0.05 were considered as statistically significant. We performed per-protocol analysis.
RESULTS
The study included 40 patients [24 (test group) and 16 (control group)] (Figure 1). No significant differences were observed between the groups at the time of study inclusion (Table 1). All included patients completed the study. None of the patients were hospitalized between the visits.
After a 3-mo course of the probiotic in comparison to the control group, we observed evidence of amelioration of hyperdynamic circulation (a decrease in cardiac output and end-diastolic volume and an increase in SVR) and systemic inflammation (a decrease in serum C-reactive protein levels),improved liver function (an increase in serum albumin and cholinesterase levels and a decrease in the value of Child-Pugh score), regression of ascites and hepatic encephalopathy, increased serum sodium levels, as well as a reduction in the severity of cholestasis (a decrease in serum alkaline phosphatase and serum gamma-glutamyl transpeptidase activity), and hypersplenism (an increase in platelet count).However, in contrast to patients in the test group, those in the control group showed an increase in mean blood pressure. No significant changes were observed in the levels of other variables, including in the grade of esophageal varices and the international normalized ratio (Table 3).

Table 3 Changes of the main indicators of the included patients after treatment
In the test arm, an improvement in liver function (a decrease in the value of Child-Pugh score: -2 [-3-(-1)]vs-0.5 [-1-0];P= 0.042) and a decrease in the degree of ascites (-1[-1-(-1)]vs0 [0-0];P= 0.015) was observed only in those patients (n= 18) who had a decrease in cardiac output after the course of the probiotic.
No patient developed acute-on-chronic liver failure and bleeding esophageal varices during the study, and no patient died during the study period.
Only 1 patient in the test arm developed self-limited itching as an adverse effect.
DlSCUSSlON
This is the first randomized controlled study that investigated the effect of probiotics on hemodynamic disturbances in patients with cirrhosis. Our results concur with those reported by a previous uncontrolled study[29], which showed that these drugs reduce cardiac output and increase SVR. In our study, the reduced cardiac output was attributable to a decrease in the end-diastolic volume, which may indicate a reduction in the effective circulating blood volume.
The use of the probiotic also increased serum albumin and sodium levels and decreased in the degree of ascites. We assume that the probiotics inhibit bacterial translocation and thereby ameliorate hyperdynamic circulation, with a consequent reduction in the degree of ascites, which corrects hypoalbuminemia and hyponatremia. Unfortunately, we could not evaluate the indicators of intrahepatic hemodynamics (for example, the hepatic venous pressure gradient, among other variables).
Interestingly, this study highlights that probiotics reduced serum levels of biomarkers of cholestasis(alkaline phosphatase and gamma-glutamyl transpeptidase). This is the first study to report these findings; further studies are warranted to investigate the mechanisms underlying these changes. We observed probiotic-induced reduction in the severity of hepatic encephalopathy, which is consistent with the results reported by previous research[28]. In our study, probiotic ingestion did not affect the degree of esophageal varices and prothrombin levels (indicated by the international normalized ratio);our results were consistent with those reported by a previous study[28].
Probiotic use was associated with a significant improvement in liver function in our study; 33.3% of patients in the probiotic arm and only 6.3% of patients in the control arm showed Child-Pugh class A cirrhosis after a course of probiotic or placebo administration. We observed no significant probioticinduced adverse effects during the study. Overall, our study showed that probiotic administration may be a useful therapeutic strategy for correction of gut-heart-liver axis disturbances.
This is the first randomized placebo-controlled trial that confirms the role of probiotics in amelioration of hemodynamic disorders in cirrhosis, together with improvement in levels of several liver biomarkers, which serves as a strength of our study.
A limitation of our study is the fact that biomarkers of bacterial translocation and intestinal permeability, biomarkers of systemic inflammation in addition to C-reactive protein, as well as indicators of intrahepatic hemodynamics were not evaluated. Further research is warranted to overcome this challenge.
CONCLUSlON
Probiotic administration was associated with amelioration of hyperdynamic circulation and the associated complications of cirrhosis.
ARTlCLE HlGHLlGHTS
Research background
Bacterial translocation exacerbates the hyperdynamic circulation observed in cirrhosis and contributes to a more severe disease course.
Research motivation
Probiotics may reduce bacterial translocation and may therefore be useful to redress the circulatory imbalance.
Research objectives
To investigate the effect of probiotics on hemodynamic parameters, systemic inflammation, and complications of cirrhosis in this randomized placebo-controlled trial.
Research methods
This single-blind randomized placebo-controlled study included patients with Child-Pugh class B and C cirrhosis that received probiotics (Saccharomyces boulardii) or a placebo for 3 mo. Liver function and the systemic hemodynamic status were evaluated pre- and post-intervention. Echocardiography and simultaneous blood pressure and heart rate monitoring were performed to evaluate systemic hemodynamic indicators. Cardiac output and systemic vascular resistance were calculated.
Research results
Following a 3-mo course of probiotics in comparison to the control group, we observed amelioration of hyperdynamic circulation and systemic inflammation with improved liver function, reduction in the severity of ascites, hepatic encephalopathy, and cholestasis, and an increase in platelet counts.
Research conclusions
Probiotic administration was associated with amelioration of hyperdynamic circulation and the associated complications of cirrhosis.
Research perspectives
To study the changes in the levels of the biomarkers of bacterial translocation, intestinal permeability,and in indicators of intrahepatic hemodynamics after the use of the probiotic in decompensated cirrhosis.
ACKNOWLEDGEMENTS
The authors are grateful to the staff of the Department of Hepatology: Alexei Lapshin, Shauki Ondos,Petr Tkachenko, Igor Tikhonov, and others.
FOOTNOTES
Author contributions:Ivashkin V provided the research idea; Ivashkin V, Efremova I, and Roman Maslennikov R designed the study; Efremova I and Maslennikov R wrote the draft; Maslennikov R was the guarantor; All authors edited the draft and contributed to the research and data analysis.
Supported byBiocodex Microbiota Foundation: National Research Grant Russia 2019.
lnstitutional review board statement:The study was approved by the Ethics Committee of Sechenov University.
Clinical trial registration statement:The study was registered at https://clinicaltrials.gov (NCT05231772).
lnformed consent statement:The study procedures were explained to potential participants, and written informed consent was obtained before enrollment.
Conflict-of-interest statement:All authors report no relevant conflicts of interest for this article.
Data sharing statement:Data can be provided upon request to the corresponding author.
CONSORT 2010 statement:The authors have read the CONSORT Statement—checklist of items, and the manuscript was prepared and revised according to the CONSORT Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Russia
ORClD number:Roman Maslennikov 0000-0001-7513-1636; Irina Efremova 0000-0002-4112-0426; Vladimir Ivashkin 0000-0002-6815-6015; Maria Zharkova 0000-0001-5939-1032; Elena Poluektova 0000-0002-9038-3732; Elena Shirokova 0000-0002-6819-0889; Konstantin Ivashkin 0000-0002-5699-541X.
S-Editor:Ma YJ
L-Editor:Filipodia
P-Editor:Cai YX
杂志排行
World Journal of Hepatology的其它文章
- Long-term liver allograft fibrosis: A review with emphasis on idiopathic post-transplant hepatitis and chronic antibody mediated rejection
- Outcomes of patients with post-hepatectomy hypophosphatemia: A narrative review
- Simple diagnostic algorithm identifying at-risk nonalcoholic fatty liver disease patients needing specialty referral within the United States
- Higher cardiovascular risk scores and liver fibrosis risk estimated by biomarkers in patients with metabolic-dysfunction-associated fatty liver disease
- Prevalence of sarcopenia using different methods in patients with non-alcoholic fatty liver disease
- Metabolic-associated fatty liver disease is associated with low muscle mass and strength in patients with chronic hepatitis B
