PREPARATION,CHARACTERIZATION AND ELECTROCHEMICAL PROPERTIES OF A CU(II)COMPLEX CONTAINING 5-NITRO-ISOPHTHALIC ACID AND PHEN LIGANDS
2022-08-31WANGZhijunHUANGWeiYIXiuguangZHONGFanYIZhiqiang
WANG Zhi-jun,HUANG Wei,YI Xiu-guang,ZHONG Fan,YI Zhi-qiang,2
(1.School of Chemistry and Chemical Engineering,Jinggangshan University,Ji’an,Jiangxi 343009,China;2.Ji’an Central People’s Hospital,Ji’an,Jiangxi 343000,China)
Abstract : A copper complex with mixed ligands, [CuLPhen]n (HL = 5-Nitro-isophthalic acid, Phen =1,10-phenanthroline) was synthesized by a hydrothermal approach and its structure was determined by single-crystal X-ray crystallography. The title complex crystallizes in monoclinic space group P21/c with the crystal data:C20H11N3CuO6,Mr=106.57,ɑ=10.6619(3),b=12.5760(4),c=13.0550(3)Å,α=90,β=95.463(3),γ = 90 °, V= 1742.50(9)Å3, Z = 4, T = 293(2)K, Dc= 1.726 g/cm3, μ(MoKα) = 0.301 mm-1, F(000)= 51.0, R =0.0380, wR = 0.0907, and GOF = 1.026. When used as electrode materials of super-capacitor, the prepared complex showed high specific capacitance, good cycle stability and excellent rate performance. Specifically, the maximum specific capacitance could achieve 225 F/g in 1 mol/L KOH solution.At the current density of 5 A/g,the retention of specific capacitance was 67.31%after 5000 cycles.
Key words:preparation;characterization;electrochemical properties;copper complex
1 INTRODUCTION
Facing the serious environmental pollution and the gradual depletion of fossil fuels, people urgently need to develop and utilize new clean energy. Considering the intermittent and regional characteristics of solar energy and wind energy, in order to make full use of the electric energy generated by them,efficient energy storage devices are essential[1-4]. In recent years, as an energy storage device, super-capacitor has the characteristics of high power density, long cycle life and rapid charge-discharge, which has attracted considerable attention[5-6].
In recent years, metal organic frameworks(MOFs) and porous coordination polymers have attracted great attention, because of their special chemical and physical properties and many potential applications, such as catalysis, gas storage and separation, sensors, lithium-ion batteries, magnetic and optical properties. The application of MOFs has also begun to appear in the field of super-capacitors[7-12].
Compounds containing carboxylic acid groups are good organic ligands,in which the carboxylic acid group is a flexible multi-coordination mode group.For example, Professor Yi’s team took quinoline carboxylic acid as the main organic ligand to form mononuclear monodentate complexes, mononuclear bidentate complexes and binuclear monodentate complexes with transition metal atoms Zn(II), Cu(II),Ni(II), Cd(II) and Na(I), respectively. They studied a series of optical properties and found that there are good luminescent materials and potential semiconductor materials[13-17].
Base on the special interest in carboxylic acid derivatives, we synthesized a MOFs material with 3D structure and investigated its electrochemical properties as super-capacitor electrode materials. The title complex electrode has high specific capacitance,good cycle stability and magnification performance.In 1 mol/L KOH solution, when the current density is 1 A/g,the specific capacitance of the electrode reaches 225 F/g.
2 EXPERIMENTAL
2.1 General procedure
All reactants of A.R. grade were commercially obtained and used without further purification. The infrared spectrum was measured on a PE Spectrum-One FT-IR spectrophotometer over the frequency range 4000~400 cm-1by using the KBr pellet technique.
All electrochemical tests were carried out at room temperature using CHI660D electrochemical workstation produced by Shanghai Chenhua Co., Ltd.Using a conventional three electrode system,the foam nickel electrode was used as the working electrode,the Graphite rod as the auxiliary electrode(the counter electrode), the Ag/AgCl as the reference electrode,and the electrolyte is the 1 mol/L KOH solution. The electrodes were tested by cyclic voltammetry (CV) at a scanning rates from 5 to 100 mV/s in the potential range of 0.2 ~0.6 V (vs Ag/AgCl); In the potential range of 0 ~ 0.6 V, the electrode was tested by constant current charge discharge (GCD) at different current densities of 1 ~20 A/g, and the long-term cycle stability was tested for 5000 cycles at a current density of 5 A/g using a battery testing system(CT3001A, LAND, China); The AC impedance is tested at open circuit potential with an amplitude of 5 mV and in the frequency from 100 mHz to 100 kHz.The specific capacity of the electrode material is calculated by the following formula(1):C=IΔt/(mΔV)(1),where C is the specific capacity(F/g), and I is the discharge current of the electrode active material current (A),tis the discharge duration of constant current charge and discharge (s), and m is the mass of the copper base of the material of the active electrode(g),ΔV is the potential window(V).
2.2 Preparation of the title complex
The title copper complex was prepared by mixing CuCl2·2H2O (2 mmol, 340 mg), 5-Nitro-isophthalic acid(2 mmol,422 mg),Phen(3 mmol,540 mg),Et3N(4 mL)and distilled water(15 mL)were mixed in a 25 mL Teflon-lined stainless-steel autoclave.The mixture was heated to 393 K and kept at this temperature for three days. After cooling the mixture slowly down to room temperature, bluish crystals suitable for X-ray analysis were collected and washed. Yield: 90.5%(based on copper). IR (KBr, cm-1): 3427 (vs), 3047(w), 2929 (w), 2863 (w), 1627 (s), 1519 (s), 1423 (s),1386 (w), 1149 (m), 1099 (m), 850 (s), 725 (s), as presented in Fig.1.

Fig.1 FTIR spectra of the title complex
The strong absorption peak of the compound inσ= 3300-3500 cm-1is 3427 cm-1, which is the characteristic peak of the N-H bond and the associated oxygen-h bond. In the region of 2800~3000 cm-1,there are two peaks,namely 2929 cm-1and 2863 cm-1,which are the stretching vibration peak of the C-H bond. In the fingerprint region, i.e. the region with wave number less than 1600 cm-1, the strong absorption peak inσ= 1600-1800 cm-1region is the light absorption frequency of the stretching vibration of the double bond, which mainly includes C-O double bond, C-N double bond and C-C double bond.Aromatic compounds: generally, there are four peaks with different intensities at 1600 cm-1, 1580 cm-1,1500 cm-1and 1450 cm-1, and the compound has corresponding wave numbers near these four peaks.Combined with the stretching vibration peaks of C-H bonds on aromatics at 3100~3000 cm-1,There are two peaks of 850 cm-1and 720 cm-1in the region of 880~680 cm-1, which are the changes in the number and position of substituent groups on the benzene ring in the out-of-plane bending vibration absorption of C-H bond. Combined with Figure 1, it is not difficult to infer that the compound contains benzene ring structure.Atσ= 1080~1300 cm-1andσ= 1180~1360 cm-1, there are multiple asymmetric stretching vibration wave numbers. At 1099 cm-1,σ=1080~1300 cm-1, and at 1149 cm-1,σ= 1180~1360 cm-1, carbon and oxygen single bonds can be identified. 1386 cm-1in the region ofσ= 600~1500 cm-1,it can be determined that the compound contains carbon-carbon single bond.
2.3 X-ray structural determination
The diffraction data were collected on a SuperNova CCD X-ray diffractometer using carefully selected single crystals of the title complex.The X-ray source was graphite monochromated Mo-Kαradiation(λ = 0.71073 Å) and ω scan method was employed.The reduction and empirical absorption correction of diffraction data were carried out with the CrystalClear software. Using Olex2[18], the structures of the title complex were solved with the ShelXT[19],the structure solution program using Intrinsic Phasing and refined with the ShelXL[20]refinement package using Least Squares minimization.All of the non-hydrogen atoms were generated based on the subsequent Fourier difference maps and were refined anisotropically.The hydrogen atoms, except for the lattice water, were located theoretically and ride on their parent atoms.Reflections measured are 8847; the finalR= 0.0380 for 271 parameters and 4064 observed reflections withI>2σ(I)andwR=0.0907,index ranges are-13≤h≤13,-16≤k≤17,-17≤l≤16,S=1.026,(Δσ)max=0.52 and(Δσ)min=-0.50 e/Å3. The selected bond distances and bond angles are shown in Table 1.
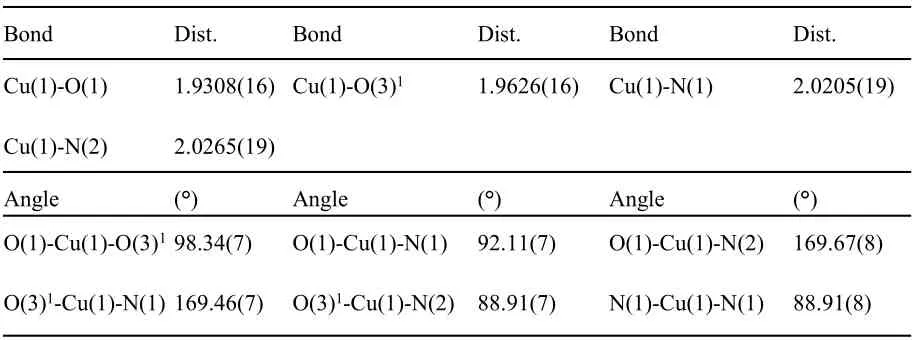
Table 1 Selected Bond Lengths(Å)and Bond Angles(°)
3 RESULTS AND DISCUSSION
Single-crystal X-ray diffraction analysis revealed the title complex is a neutral molecule that crystallizes in theP21/cspace group, monoclinic system. The asymmetric unit contains one Cu (II) ion, one 5-nitro-isophthalic acid molecule and one Phen molecule, as shown in Fig. 2. The Cu2+ion is coordinated by two oxygen atoms and two nitrogen atoms, of which two oxygen atoms are from two 5-nitro-isophthalic acid ligands and two nitrogen atoms are from one Phen ligand. The copper ions are connected to each other by oxygen atoms to form a one dimensional(1D)Cu-O chain extending along the c axis. The carboxylate and Phen act as the monodentate and bidentate ligands coordinated to the copper metal center,as shown in Fig.3.The following bond distances were observed:Cu(1)-O(1)1.9308(16)Å, Cu(1)-O(3)11.9626(16) Å, Cu(1)-N(1) 2.0205(19)Å, Cu(1)-N(2) 2.0265(19) Å. These are comparable with those reported in the literature[21-22].Additionally,there are abundant offset face-to-face π···π stacking interactions betweenCg1···Cg1(symmetry codes:1-x,1-y, 2-z),Cg1···Cg3 (symmetry codes: 1-x, 1/2+y,3/2-z),Cg2···Cg2 (symmetry codes: -x, 1-y, 1-z),Cg2···Cg4 (symmetry codes:-x, 1-y,1-z), (Cg1 is the ring consisting of C2 to C7;Cg2 is C12 to C14, and C18 to C20;Cg3 is N1,C9 to C13;Cg4 is N2,C14 to C18). The centroid-centroid distance ofCg1···Cg1 is 3.728 Å,with a slippage distance of 1.344 Å and with a dihedral angle of 0°. The centroid-centroid distance ofCg1···Cg3 is 3.804 Å, with a slippage distance of 1.631 Å and with a dihedral angle of 12.108°. The centroid-centroid distance ofCg2·Cg2 is 3.536 Å,with a slippage distance of 0.767 Å and with a dihedral angle of 0°.The centroid-centroid distance ofCg2···Cg4 is 3.886 Å, with a slippage distance of 1.684 Å and with a dihedral angle of 2.433°,as shown in Fig. 4. The intramolecular hydrogen bonds can be found between the carbon atom, carboxyl oxygen atoms (C3-H3·O2; C9-H9·O1; C9-H9···O4;C15-H15···O3).Some intermolecular hydrogen bonds like C17-H17·O5, C19-H19·O2, C20-H20···O6, as shown in Table 2 and Fig. 5. In the title complex,there are π···π stacking interactions, van der Waals and hydrogen bonds attraction yielding the 3-Dsupramolecular structure, the crystal packing is presented in Fig.6.

Table 2 Hydrogen Bond Lengths(Å)and Bond Angles(°)

Fig.2 The crystal structure of the title complex with 50%thermal ellipsoids
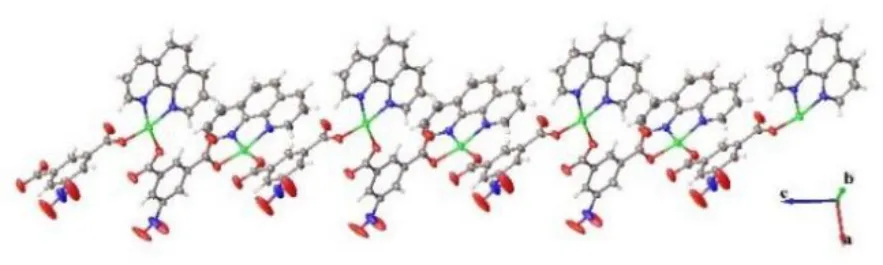
Fig.3 A 1-D chain of the title complex viewed along the c axis

Fig.4 The π···π stacking interaction diagram of the title complex.Hydrogen atoms are omitted for clarity
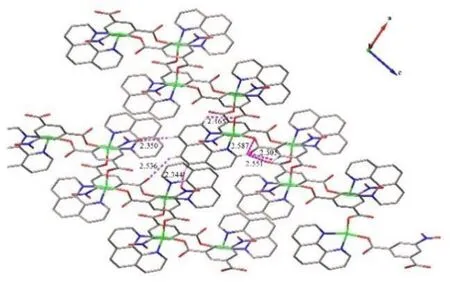
Fig.5 The hydrogen bond diagram of the title complex.Hydrogen atoms were omitted for charity

Fig.6 The packing diagram of the title complex
Base on the consideration, increasing attention has been paid to the electrochemical properties of coordination complexes, we conducted CV and GCD analysis in the three electrode system. In 1 mol/L KOH solution, a pair of obvious redox peaks appear in the CV curve of the electrode when the scanning speed is 5 mV/s, indicating that this a pseudo capacitance behavior, as shown in Fig. 7a. The pseudo capacitance behavior mainly comes from the redox reaction on the sample surface,as show in 2-3 (where subscript s represents solid state and ad represents adsorption).Similar precesses have been reported in the other MOFs based electrical materials[KCo7(OH)3(ip)6(H2O)4]·12H2O[23]. Fig. 7b is the CV curves at the scanning rate of 5,10,20,50, 100 mV/s. It can be seen from the Fig. 7b.That the positions of the oxidation and reduction peaks move to the positive and negative directions respectively with the increase of the scanning rate, which may be related to the increase of the internal resistance of the electrode. When the scanning rate increases to 50 mV/s, the oxidation peak disappears, which may be that the redox process of the electrode changes from diffusion control to charge transfer control or the mixed control diffusion and charge transfer.
At the same time, we investigated the GCD curves of the electrode in 1 mol/L KOH solution, 0 ~0.6 V charge discharge potential range and different current densities (2 ~10 A/g), as shown in Fig 7c.As can be seen from Fig. 7c, each discharge curve has a slope,indicating that the electrode has undergone redox reaction and generated pseudo capacitance.
The specific capacitance of the title complex electrode calculated according to the discharge curve under different current densities in shown in Fig.7d.When the current density is 1 A/g, the title complex has a high specific capacitance (225 F/g). Even at 10 A/g,the specific capacitance is about 96.7 F/g,showing excellent magnification performance.Fig. 7d shows that the specific capacitance decreases with the increase of current density,mainly because the effective interaction between electrolyte ions and electrode materials decreases[24-25].

Fig.7 Electrochemical properties of Cu-based the title complex in 1 mol/L KOH
Cycle stability is also an important index to investigate the practical application of super-capacitors.When the current density was 5 A/g, the copper based complex electrode was cycled for 5000 times,the capacitance retention was 67.31%, indicating that the electrode material has good cycling performance, as shown in Fig.8a.
Electrochemical impedance spectroscopy(EIS) is also an important index to evaluate electrochemical performance[26-28].Fig. 8b is an EIS spectrum under open circuit voltage,which is composed of a semicircle in the high frequency region and a straight line in the low frequency region generated by Faraday reaction.From the semicircle and real axis intercept in the high frequency region, we can know that the internal resistance Rs of the electrode is about 0.1 Ω, indicating that it has a small internal resistance at the open circuit potential.The internal resistance is caused by the ion resistance of the electrolyte, the internal resistance of the active material and the contact resistance between the active material and the collector.


Fig.8 (a)Cycle performance of Cu-based title complex electrode;(b)EIS spectrum of Cu-based electrode.
4 CONCLUSION
In summary, a copper compound has been prepared through a hydrothermal reaction and characterized by single-crystal X-ray diffraction. Crystal data analysis shows: the title complex crystallizes in monoclinic space groupP21/c, and exists as a 1-Dchain-like structure along thecaxis. In the title complex,there are π···π stacking interactions, van der Waals and hydrogen bonds attraction, yielding the 3-Dsupramolecular structure.
The complex shows high specific capacitance, good cycle stability and excellent rate performance. Specifically, the maximum specific capacitance can achieve 225 F/g in 1 mol/L KOH solution.At the current density of 5 A/g, the retention of specific capacitance was 67.31%after 5000 cycles.
Our laboratory interest is to synthesize more inorganic-organic hybrid complexes(inorganisms dominated by transition metal elements and organisms containing carboxylic acid functional groups). Firstly, in the preparation process, complexes with different coordination modes will be prepared by adjusting the experimental process conditions,such as, solvent, temperature, pressure, pH value and the second ligand, etc. Secondly, the electrochemical and optical properties of the complexes under different coordination modes will be tested. Finally, an optimal relationship between preparation and structure of the complexes will be sought, and the essential relationship between structure and properties will be discussed.
