Enhanced electrode kinetics and properties via anionic regulation inpolyanionic Na3+xV2(PO4)3-x(P2O7)x cathode material
2022-08-30MeiYiWngXinXinZhoJinZhiGuoXueJioNieZhenYiGuXuYngXingLongWu
Mei-Yi Wng,Xin-Xin Zho,Jin-Zhi Guo,Xue-Jio Nie,Zhen-Yi Gu,Xu Yng,Xing-Long Wu,,
a National & Local United Engineering Laboratory for Power Batteries,Faculty of Chemistry,Northeast Normal University,Changchun,Jilin,130024,China
b Key Laboratory for UV Light-Emitting Materials and Technology of Ministry of Education,Northeast Normal University,Changchun,Jilin,130024,China
Abstract Mixing polyanion cathode materials are promising candidates for the development of next-generation batteries,owing to their structural robustness and low-volume changes,yet low conductivity of polyanion hinders their practical capacity.Herein,the anion-site regulation is proposed to elevate the electrode kinetics and properties of polyanionic cathode.Multivalent anionis selected to substitute thein Na3V2(PO4)3 (NVP) lattice and regulate the ratio of polyanion groups to prepare Na3+xV2(PO4)3-x(P2O7)x (NVPPx,0 ≤x ≤0.15) materials.The optimal Na3.1V2(PO4)2.9(P2O7)0.1(NVPP0.1)material can deliver remarkably elevated specific capacity(104 mAh g-1 at 0.1 C,60 mAh g-1 at 20 C,respectively),which is higher than those of NVP.Moreover,NVPP0.1 exhibits outstanding cyclic stability(91%capacity retention after 300 cycles at 1 C).Experimental analyses reveal that the regulation of anions improves the structure stability,increases the active Na occupancy in the lattice and accelerates the Na+migration kinetics.The strategy of anion-site regulation provides the researchers a reference for the design of new high-performance polyanionic materials.
Keywords: Sodium-ion batteries;Cathode;Polyanion materials;Anionic substitution;Electrode kinetics
1.Introduction
With the rapid development of the sustainable and intelligent technology society,the demand for smart power grid and large-scale energy storage increases by years.New battery systems with low cost,long life,high capacity and high safety need to be exploited urgently to alleviate the of lithium-ion batteries (LIBs),caused by the lithium resource shortage on the present obstacles earth [1,2].Sodium-ion batteries (SIBs)have returned to the researchers'perspective due to the similar working mechanism with LIBs and abundant sodium resources,thus have the potential to apply for low-cost and large-scale stationary electrochemical energy storage system[3-5].However,the development of SIBs system faces more challenges,compared with LIBs system,which have been commercially produced.The radius of Na (1.02 Å) is bigger than that of Li (0.76 Å),so Na+migration kinetics is more sluggish than that of Li+in the same host material[6,7].As is known to us,the energy density of the battery system depends on the specific capacity and average voltage of the electrode materials [8,9].The atomic weight of sodium is higher than that of lithium (22.99 g mol-1vs.6.94 g mol-1) and the standard electrode potential of lithium (-3.04 V vs.SHE) is 330 mV lower than that of sodium (-2.71 V vs.SHE),which makes the specific capacity and energy density of SIBs lower than that of LIBs [10-12].However,due to the cost advantages and environmental friendliness of SIBs,SIBs are expected to be the next generation of large-scale energy storage systems,so we still need to sequentially explore and develop high-performance SIBs electrode materials [13,14].
For the current situation of SIBs researches,advanced cathode materials are the “short cask” for developing SIBs,compared to high-capacity anode materials [15].The instability and complex phase transition of 2D layered oxides materials promote the researches of polyanionic compound NaxMy(XO4)n(X=B,P,S,Si etc.),which exhibits higher lattice stability [16-18].In addition,polyanion structure greatly enhances the redox potential for the same redox pairs based on molecular orbital theory [19].NASICON-structured polyanionic materials with 3D Na+transport channel possess excellent kinetics and electrochemical properties,which are ideal candidates of SIBs cathode materials.Na3V2(PO4)3(NVP) as a typical NASICON-structured material has been favored by researchers [19,20].However,the poor conductivity caused by its structure characteristics affects its electrochemical performances and hinders its practical application.Foreign ion lattice doping or substitution is proved to be an effective modification strategy,which can improve the electrochemical performance by adjusting the composition and structure of materials to a certain extent and then affecting the physical and chemical properties[21,22].The ions selected in recent reports mainly concentrate on: i) Larger radius ion doping,which regulates the crystal structure and then enhances the Na+diffusion kinetics in the lattice [23,24].ii)Heterovalent ion,would adjust carrier transport characteristics[25-28].Compared with the ionic substitution on active Na or V site,the inactive anionsite can not only regulate the structure properties of materials but improve active Na content with the increase of theoretical capacity.In recent reports,a series of Na3(VO1-xPO4)2F1+2x(0 ≤x ≤1)cathode materials were investigated by the substitution of monatomic anion(F-)in the NVP crystal lattice,and this kind of materials show higher reversible specific capacity and better electrochemical performance compared with matrix material NVP[29,30].The volatilization of VF3during the synthesis process makes it difficult to control the ratio of V to F in the final product.Moreover,the substitution of multivalent anions is more efficient and easier to regulate during the synthesis process.For example,[31] and[32,33] have been introduced into NVP lattice,which show excellent electrochemical properties,Na+diffusion kinetics and structural stability as SIBs cathode materials.
2.Experimental details
2.1.Materials preparation
The NASICON structured Na3+xV2(PO4)3-x(P2O7)x(0 ≤x ≤0.15)samples were synthesized by a feasible sol-gel method.NH4VO3,NH4H2PO4,Na4P2O4,Na2CO3,and citric acid were used as raw materials.Stoichiometric amounts of NH4VO3were dispersed in the deionized water,then citric acid was dissolved into it,which is used to ensure completely chelate with the metal ions and to form the gels.Violently stir the solution at 80°C and a transparent solution was obtained.Next,stoichiometric amounts of NH4H2PO4,Na4P2O4,and Na2CO3were added while stirring.Sol was converted into gel by evaporating deionized water at 80°C.Then the wet gel was dried for 5 h in a vacuum oven at 120°C to form dry gel,which was heat-treated at 400°C for 5 h in an Ar atmosphere.Cool to room temperature and slightly ground the precursor.Finally,the powder samples were annealed at 800°C for 12 h under Ar atmosphere and obtained the final compound Na3+xV2(PO4)3-x(P2O7)x.
2.2.Materials characterization
X-ray diffraction (Rigaku SmartLab) is used to collect XRD data of as-prepared power samples using Cu Kαradiation(λ=1.5418 Å) in the scanning range (2θ) of 5-60°.Scan electron microscopy (SEM,Hitachi SU 8000) is employed to explore the size and morphology.The images of transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HR-TEM) were collected on the JEOL-2100 F.The Fourier transform infrared spectrum (FT-IR Nicolet 6700) and Microscopes Raman Spectrometer (In Via)were used to analyze the chemical bond.
2.3.Electrochemical measurement
The electrodes consist of 70 wt% active material,20 wt%acetylene black and 10 wt% sodium carboxymethylcellulose(CMC).Mix with deionized water and coat the slurry on Al foil,and dry in vacuum oven at 60°C for 10 h.The coin cells(CR2032) were assembled in the argon-filled atmosphere glovebox.The electrolyte composition was 1 mol L-1NaClO4with the solvent propylene carbonate (PC) and the additive 5 vol% fluoroethylene carbonate (FEC).The glassfiber was used as separator.The Neware testing system was performed galvanostatic charge/discharge tests at the voltage window of 2.3-4.1 V.CHI600E electrochemical workstation was carried on testing cyclic voltammetry at different scanning rates.Electrochemical impedance data was measured by P4000 electrochemical workstation.
3.Results and discussions
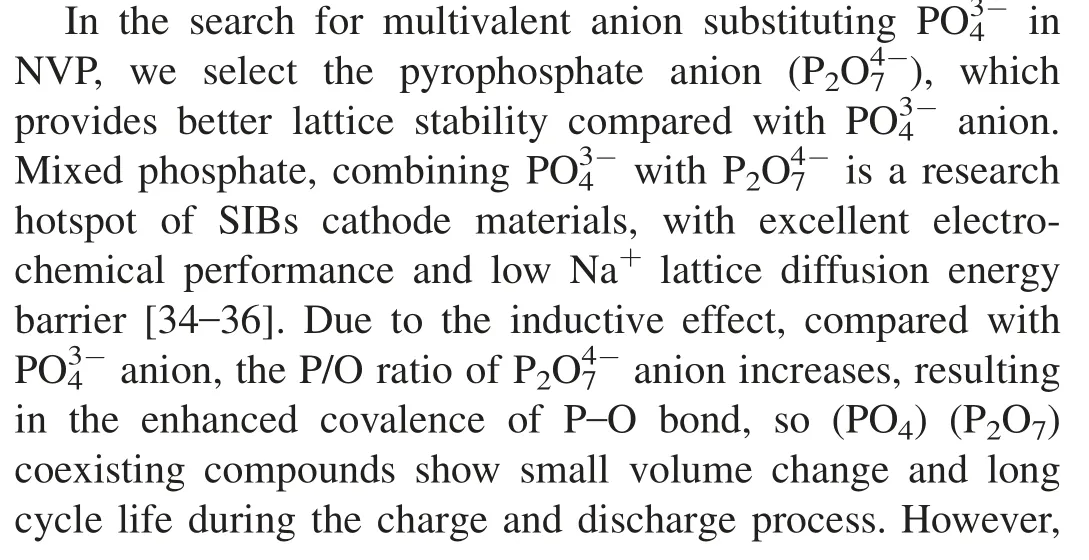
Fig.1.(a) The comparison of XRD patterns collected by NVPPx (0 ≤x ≤0.15) samples;(b) FTIR spectra of NVPPx samples;(c) Raman spectra of NVPPx samples.
NVPPxmaterials synthesized by sol-gel method were characterized by XRD to analyze the structure and composition.Fig.1a compares the XRD patterns of NVP,NVPP0.05,NVPP0.1and NVPP0.15four substitution quantity materials.Through the XRD,it can be seen that the diffraction peaks of NVP,NVPP0.05and NVPP0.1materials all correspond well to the orthorhombic system(R-3c space group),and the standard PDF card (JCPDS No.62-0345) of NVP [37],indicating that the introduction ofanion has not changed the original crystal phase of NVP material when the substitution quantity was less than 0.1.However,the XRD of NVPP0.15material contains small traces of the other phase material,as well as the principal NVP phase.By comparing with the standard PDF cards,we confirm that the other phase material is mixed phosphate Na7V4(P2O7)4PO4material,with tetragonal system and P421c space group [38].In addition,we find that as the amount ofanion substitution increases,the strength of XRD diffraction peak gradually increases,indicating the substitution ofanion improves the crystallinity of NVPbased materials.Meanwhile,the addition ofcauses the cor111responding (300) crystal plane diffraction peak shift to the lower angle with the increase of the substitution amount(Fig.S1,Supporting information),which indirectly confirmed the introduction ofincreases the layer spacing along the a-axis direction.At the same time,the broadening diffraction peak indicated that the grain size decreases with the increase of the substitution amount,which indirectly reduced the Na+transmission path and was beneficial to the performance of the material.While the material with x=0.15 did not get better performance with the increase of the substitution amount due to the existence of impurity phase.
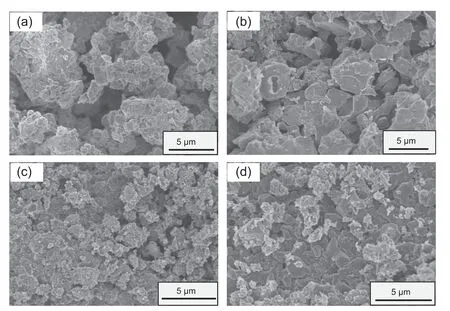
Fig.2.SEM images of (a) NVP,(b) NVPP0.05,(c) NVPP0.1 and (d) NVPP0.15 samples.
Raman spectroscopy was used to further analyze the influence ofsubstitution on the chemical bond characteristics.As shown in Fig.1c,there are obvious D-band and Gband peaks of carbon materials in NVPPxmaterials,located around 1359 and 1585 cm-1,respectively [42],which is attributed to the addition of citric acid during the synthesis process.As the chelating agent,the citric acid decomposed during the high-temperature calcining process,and produced carbon material coated on the particle surface.The peak around 1150 cm-1corresponds to the characteristic peaks of the stretching vibration inside thegroup.With the increase ofsubstitution,the blue shift has taken place in the characteristic peak,which is caused by the stronger polarity of P2O7group than that of PO4group [39].When the amount of substitution reaches 0.15,a wide and strong characteristic peak appears on the Raman spectra of NVPP0.15sample,which is the result of the co-existence of P2O7and PO4group,and is consistent with the XRD results aforementioned new phase Na7V4(P2O7)4PO4.The above structural characterizations prove thatanion has replacedanion in NVP lattice.
The synthetic materials were characterized by SEM(Fig.2)and TEM (Fig.S3).It can be seen from the SEM or TEM figures that the four materials are all constituted by the accumulation of micron-scale particles.According to the mapping in the Fig.S2,all of the elements in the sample(x=0.1)are evenly dispersed throughout the material.At the same time,according to Fig.S3,it can be seen that the introduction ofmakes the morphology of the material more complete.According to its cor111responding HR-TEM,it can be seen that thesubstitution material still maintains the crystal of the original NVP material.In addition,the particle size decreases aftersubstitution,indicating thatsubstitution inhibits the growth of NVP-based materials,and the reduced particle size can shorten the transmission distance of Na+in the electrode materials,thus improving the electrode kinetic characteristics of NVPPxelectrode material and the electrochemical performance.In addition,when the amount of substitution reaches 0.15,it can be observed that the material is a two-phase mixed material (in Fig.2d),which is consistent with the results of our previous XRD analysis.
In order to explore the influence ofsubstitution on the electrochemical performance of NVP-based electrode materials,galvanostatic charge-discharge(GCD)and CV tests were carried out on four materials.Fig.3a is the GCD curve comparison of the four materials at the rate of 0.1C in the voltage window of 2.3-4.1 V (vs.Na+/Na).Through the comparison of the specific capacity,NVPP0.1material shows the highest discharge specific capacity of 103.5 mAh g-1,significantly higher than that of NVP 93.8 mAh g-1.When the amount of substitution reaches 0.15,the NVPP0.15material has a higher charge specific capacity,however,the discharge specific capacity of it is only 90.2 mAh g-1,the low initial coulomb efficiency indicates the presence of another phase Na7V4(P2O7)4PO4affecting the reversibility of the material.In addition,it can be seen from the GCD curves of the four materials there are a pair of long and flat reversible voltage platforms,which correspond to the two-phase conversion of Na3V2(PO4)3to NaV2(PO4)3,and belong to the V3+/V4+redox reaction [43].Besides this voltage platform,we found that NVPPxmaterials also exhibit short voltage platforms in the high voltage range.The charging curve at about 3.9 V of NVPP0.1cathode material appears a short voltage platform,which corresponds to the V3+/V4+redox reaction after thesubstitution,which increases the potential of V3+/V4+due to the stronger induction effect of pyrophosphate[44-46].In the charge and discharge curves of NVPP0.15,a pair of short and reversible voltage platform at around 3.8 V appeared,which belongs to redox reaction of another phase Na7V4(-P2O7)4PO4materials[38,47,48],this is consistent with the CV test results discussed next.The results preliminarily indicate thatsubstitution can improve the electrochemical performance of NVP-based materials.
In order to further study the effect ofsubstitution on electrode reaction of NVP-based materials,CV tests were performed in the voltage range of 2.3-4.1 V and at the scanning rate of 0.1 mV s-1.Fig.3b shows the comparison of the NVPPxmaterials’ CV curves.In the process of Na+extraction/insertion,the CV curves of the samples all appeared a pair of strong oxidation/reduction peaks at about 3.4 V,which corresponds to the reversible transformation of Na3V2(PO4)3to NaV2(PO4)3[49].In addition,we also analyzed the redox peak in high voltage region as shown in the illustration of Fig.3b.Except for NVP electrode,the other three electrodes all show a pair of redox peaks at 4.0 V,cor111responding to the transformation of V3+/V4+,indicatingsubstitution in the local coordination environment promoted the redox reaction of V3+/V4+to the high potential.Meanwhile,the CV curve of NVPP0.15material exhibits a pair of redox peaks at around 3.85 V,which is due to the existence of another phase Na7V4(P2O7)4PO4and consistent with the results of XRD and GCD curves[38].In addition,during the discharging process,the reduction peak of the NVPPxsplits into two peaks.This split has been attributed to the transfer of Na+from Na (1) to Na(2)sites,which have been reported by the published papers[50,51].
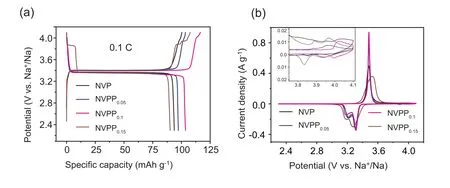
Fig.3.Electrochemical properties of the NVPPx(x=0,0.05,0.10,0.15)materials in the voltage range of 2.3-4.1 V(vs.Na+/Na)(a)the initial charge/discharge curves at 0.2 C;(b) CV curves collected at a scanning rate of 0.1 mV s-1.
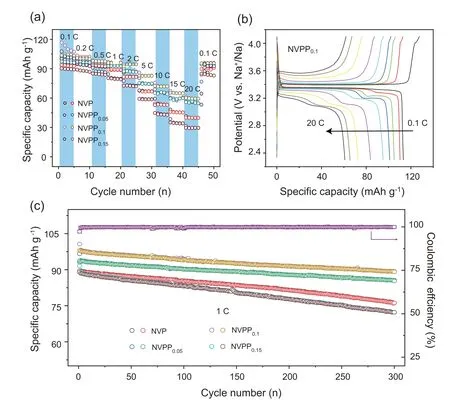
Fig.4.(a)Rate performances of NVPPx(x=0,0.05,0.10,0.15)material;(b)the charging and discharging curves of NVPP0.1 at different rate from 0.1 C to 20 C;(c) the comparison of cycling performances of NVPPx.
To evaluate the electrochemical properties of the NVPPxas cathode materials for SIBs,the rate and cycle performances of the four materials were tested.Fig.4a compares the rate performance of NVPPxmaterials in the rate range from 0.1 C to 20 C.At the rate of 0.1 C,0.2 C and 0.5 C,the difference of charge/discharge specific capacity is not obvious,but NVPP0.1material shows the highest charge/discharge specific capacity compared with the other three materials.At the rate of 0.1 C,the discharge capacity of NVPP0.1can reach about 104 mAh g-1,which is significantly higher than that of NVP 93 mAh g-1.At higher rates,it was obviously observed that the charge and discharge specific capacity of NVPP0.05and NVPP0.1materials was higher than that of NVP.Under the rate of 20 C,the discharge specific capacity of NVPP0.1still remained 60 mAh g-1,while NVP only kept 40 mAh g-1.When the rate returns to 0.1 C,the specific capacity of NVPP0.1is 94% of that in the first cycle.Yet the specific capacity of NVPP0.15at high rate is lower than that of NVP,indicating that an appropriate amount ofsubstitution will improve the high-rate performance of NVP-based materials.Fig.4b shows the GCD curve of NVPP0.1from 0.1 C to 20 C.With the increase of the rate,the polarization of NVPP0.1in SIBs becomes larger and larger [52].
Fig.4c compares cycling performance of NVPPxmaterials under 1 C through 300 cycles.NVPP0.05and NVPP0.1electrodes show a relatively good cycling performance.After 300 cycles,the capacity retention rate of NVPP0.05and NVPP0.1is up to 91%,the average capacity loss of each cycle is only 0.03%.Meanwhile,the discharge specific capacity of NVPP0.1electrode is still remaining 86.1 mAh g-1,which is more than that of NVPP0.0585.3 mAh g-1.And the comparison of charge-discharge curves every 50 cycles in Fig.S4,shows that the curve is not much different and the difference in discharge capacity is also very small in 300 cycles at 1 C.The capacity retention rate of NVP is only 85%,and the specific discharge capacity is only 76.2 mAh g-1after 300 cycles.Compared with NVPP0.05and NVPP0.1,due to the presence of another phase Na7V4(P2O7)4PO4,the cycling performance of NVPP0.15electrode is worse than that of NVP.In a word,an appropriate amount ofsubstitution will improve the high-rate and cycling performance of NVP-based materials.
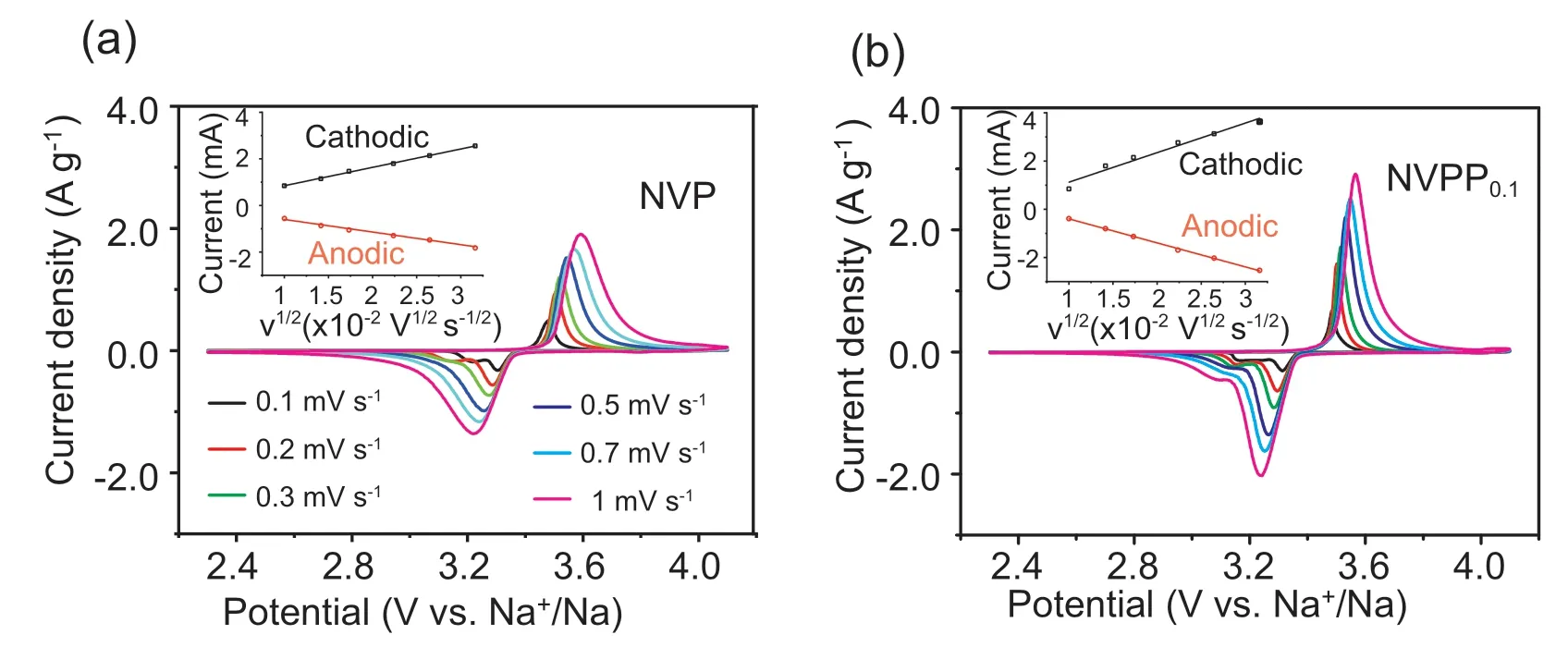
Fig.5.Studies of kinetics properties of Na+insertion/deinsertion in NVP and NVPP0.1 electrodes:CV profiles at various scan rates from 0.1 to 1.0 mV s-1 in the potential window of 2.3-4.1 V versus Na+/Na of(a)NVP and(b)NVPP0.1;insets of(a)and(b)are the linear fitting profiles obtaining from ip and v1/2 of NVP and NVPP0.1.
In order to evaluate the influence ofsubstitution on the Na+migration kinetics,the apparent Na+diffusion coefficient (Dapp,Na) was calculated.Fig.5a and b show the CV curves of NVP and NVPP0.1electrodes measured at varied scanning rates (0.1,0.2,0.3,0.5,0.7 and 1 mV s-1) respectively.It can be seen from the curves that the peak current(ip)of the redox peak gradually increases with the increase of scanning rate (v).Compared with the CV curves of NVP,the CV curves of NVPP0.1electrode show higher peak current value and larger area of redox peak curve,indicating thatsubstitution can reduce polarization,and increase the specific capacity of NVP-based materials.In addition,the peak current evolution of CV curves at varied scanning rates is related to the dynamic characteristics of Na+extraction/insertion.According to Eq.(1),the apparent Na+diffusion coefficient (Dapp,Na) can be calculated from the peak current value (ip) and the square root of the scanning rate (v1/2) [53]:

where A,n and C0represent the electrode area,the number of transmissible electrons (two electrons are transferred from each NVP electrode in the redox reaction),and the concentration of Na+,respectively.
As shown in the illustration of Fig.5a and b,the linear fitting curves of NVP and NVPP0.1electrodes show good linear correlation,indicating that the Na+extraction/insertion process is the diffusion-controlling behavior.According to the results of calculation,the Dapp,Naof NVP and NVPP0.1in Na+extractionprocessare1.760×10-10and 4.241 × 10-10cm2s-1,respectively.Meanwhile,the Dapp,Nain the Na+insertion process is 8.266 × 10-11and 2.798×10-10cm2s-1,respectively.According to the analysis data,the Dapp,Naof NVPP0.1is 2.4 and 3.3 times more than these of NVP in the process of Na+extraction/insertion process,respectively.The results of varied scanning rates CV fitting indicatesubstitution can improve the apparent Na+diffusion coefficient of NVP-based material.Therefore,the reason for the improvement of the high-rate and cycling performance is that appropriate amount ofsubstitution can improve the internal Na+diffusion kinetics characteristics of the NVP-based materials.
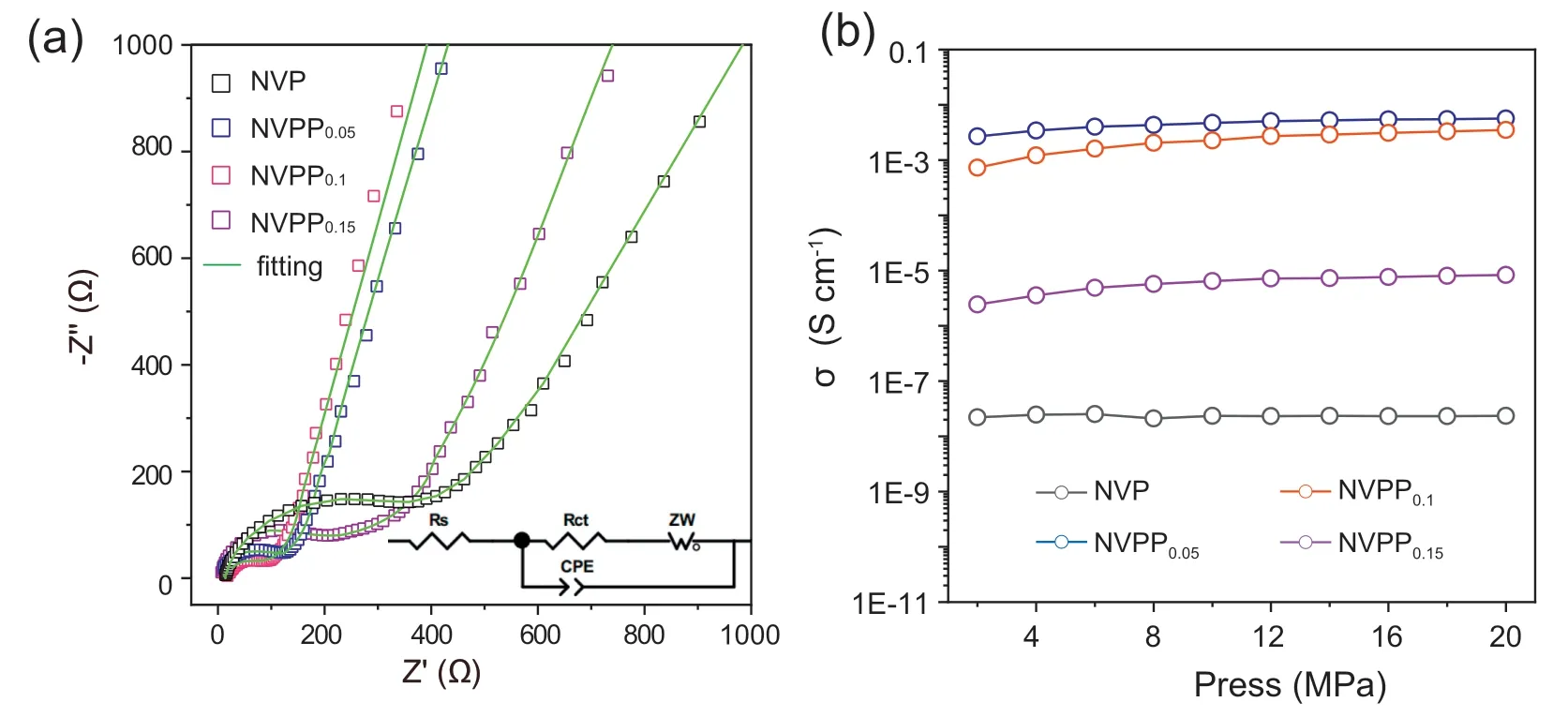
Fig.6.(a) AC impedance diagram of the NVPPx (x=0,0.05,0.10,0.15) samples;(b) pressure -electronic conductivity curves.
EIS can be used to study the electrode kinetics of NVPPxsamples.Fig.6a shows the AC impedance spectra of the four samples and the cor111responding equivalent circuit diagram.The impedance spectra consist of the half circle in high frequency region representing the charge transfer(electrode reaction kinetics) and the linear in low frequency region representing the electrode reaction controlled by the diffusion.The illustration in Fig.6 is the equivalent circuit diagram used to fit the impedance spectrum.Rsrepresents the internal resistance of the battery,Rctrepresents the charge transfer resistance,CPE represents the two-layer capacitance of the surface layer,and Warburg impedance Zwrepresents the diffusion in the low-frequency region [54].The charge transfer resistance fitting NVPP0.05and NVPP0.1respectively 149,117 Ω,which is lower than of NVP 439 Ω,so Na+migration kinetics is fastest in NVPP0.1particle,suggesting that the right amount ofsubstitution can improve the dynamic characteristics of NVP-based materials in the SIBs.The electronic conductivity of NVPPxmaterials was tested by high resistance powder meter with the RST-4 linear four-point probe method.As shown in Fig.6b,the electronic conductivity has obviously enhanced after the introduction of,and the electronic conductivity of NVPP0.05and NVPP0.1is five orders of magnitude higher than that of NVP.This result further proves the introduction ofcan improve the electronic conduction,accelerate the diffusion of Na+and enhance the electrochemical properties of the NVP-based material.
4.Conclusions
In this paper,we have successfully controlled a series of different contentssubstituted NVPPx(x=0,0.05,0.10,0.15)as SIBs cathode materials using a simple sol-gel method.Among the four materials,NVPP0.1shows the most excellent rate and cycling performance.NVPP0.1delivers the specific discharge capacity of 104 mAh g-1,which is significantly higher than that of matrix material(NVP 93 mAh g-1,under the rate of 0.1 C).When the rate reaches 20 C,the specific discharge capacity of NVPP0.1material is higher 20 mAh g-1than that of NVP.The capacity retention rate of NVPP0.05and NVPP0.1can reach 91%after 300 cycles at 1 C,while the capacity retention rate of NVP is only 75%.The substitution ofanions increases the active Na occupancy of NVP-based materials,which improves the specific capacity of NVP-based materials.In addition,the substitution ofreduces the particle size of NVP-based materials and shortens the transmission distance of Na+,thus improves electrochemical performance of occupancy as cathode in SIBs.In addition,the study on the reaction kinetics of the electrode also finds that the substitution ofwould improve the apparent Na+diffusion coefficient of the materials in SIBs and promote the transfer of charge in particles and electrolyte,which is another reason for the improvement of the electrochemical performance of NVP-based materials.The method of multivalent anion regulation provides a new research idea to design the excellent performance polyanionic cathode materials.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No.91963118),Science Technology Program of Jilin Province (No.20200201066JC),“13th Five-Year” Science and Technology Research from the Education Department of Jilin Province (No.JJKH20201179KJ),and the 111 Project (No.B13013).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.11.026.
杂志排行
Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
