Multivariate MOF for optimizing atmospheric water harvesting
2022-08-30AoMaHengjiangCongHexiangDeng
Ao Ma,Hengjiang Cong,Hexiang Deng,d
a College of Chemistry and Molecular Sciences,Wuhan University,Wuhan,430072,China
b Hubei Yangtze Memory Laboratories,Wuhan,430205,China
c Engineering Research Center of Organosilicon Compounds and Materials-Ministry of Education,College of Chemistry and Molecular Sciences,Wuhan University,Wuhan,430072,China
d Key Laboratory of Biomedical Polymers-Ministry of Education,College of Chemistry and Molecular Sciences,Wuhan University,Wuhan,430072,China
Abstract Atmospheric water harvesting offers a powerful and promising solution to address the problem of global freshwater scarcity.In the past decade,significant progress has been achieved in utilizing hydrolytically stable metal-organic frameworks as recyclable water-sorbent materials under low relative humidity,especially in those arid areas.Recently,Yaghi's group has employed a combined crystallographic and theoretical technique to decipher the water filling mechanism in MOF-303,where the polar organic linkers rather than the inorganic units of MOF are demonstrated as the key factor.Hence,the hydrophilic strength of the water-binding pocket in MOFs can be optimized through the approach of multivariate modulations,resulting in enhanced water harvesting properties.
Keywords: Atmospheric water harvesting;Metal-organic framework;Multivariate strategy;Single-crystal X-ray diffraction;Water-sorption dynamics
Water,the most essential natural resource on our planet,now faces severe challenges: Due to rapid population growth,environmental pollution,and climate change,the overall storage of freshwater falls to be less than 3% and unevenly distributed,albeit the large inaccessible forms of glaciers and groundwater [1].In this regard,techniques to produce clean water from alternative sources become an urgent need.Compared to the expensive desalination process or huge water diversion project,the atmospheric water generation technique is more energy-saving and environmentally beneficial,ideally suited for the arid regions,landlocked and underdeveloped countries [2,3].Normally,atmospheric water generation can be realized in three different ways: fog collection,cooling air below the dew point,and sorbent-assisted water capture[4,5].However,the first two methods have great limitations on their rigid geographic or climatic requirements.Hence,in the past decades,the strategy of sorbent-assisted water harvesting has been extensively studied.
In principle,an ideal sorbent-assisted water harvesting device should meet four key criteria: working at low relative humidity (RH) level,high water sorption working capacity(step-shaped isotherms with negligible hysteresis),running a considerable number of cycles per day (fast dynamic sorption properties),and less energy cost to regenerate(low enthalpy of water adsorption and desorption temperature) [5,6].Unfortunately,conventional absorbents on the market,such as hygroscopic salts,silica gel and zeolite,cannot yield suitably shaped water isotherms or suffer from sluggish kinetics of uptake and release cycles [6].Metal-organic frameworks(MOFs),assembled from inorganic secondary building units(SBUs) and organic linkers,have demonstrated excellent performance in gas adsorption and vapor uptakes due to their ultra-high porosity and versatile host-guest chemistry [7,8].Recently,some hydrolytically stable MOFs have been proposed and utilized as favorable sorbents for atmospheric water harvesting[9-14].It is timely and important to get an in-depth understanding of the water-filling mechanism in MOFs at the molecular level to guide material design and device development in the future.
An aluminum-based microporous MOF,MOF-303,exhibits record-keeping fast water adsorption and desorption kinetics showing each cycle within minutes under mild temperatures[14].However,an initial step with a slow uptake increase is observed in its water sorption isotherm at low RH,which largely affects the water-harvesting working capacity of this MOF (about 20 wt%).Recently,Yaghi group carefully examined the molecular mechanism of water-binding sequence in MOF-303 through a series of in situ single-crystal X-ray diffraction (SCXRD) experiments facilitated with density functional theory (DFT) calculations [15].Crystal structure analysis reveals that the small hydrophilic pocket located between a pair of polar PZDC2-linkers (PZDC2-=1-Hpyrazole-3,5-dicarboxylate) connecting two neighboring aluminum oxide SBU rods is the real active center for water adsorption (Fig.1a).Here,the whole adsorption sequence of water molecules is found to go through three consecutive stages,i.e.,seeding,clustering,and networking,before the formation of a three-dimensional water network.In the initial stage of seeding,the water molecules are captured one by one at sites I-IV.In the end,some polymeric water clusters are formed,which serve as a nucleus to trap additional water molecules in the following stages.Specifically,the binding with the first two water molecules (I-II) is identified as the strongest one due to their multiple H-bonds with the pyrazole groups.Therefore,both sites could be associated with the problematic S-shaped step of the water sorption isotherm of MOF-303 at low RH (Fig.1b).
The multivariate (MTV) approach is an important strategy in the reticular synthesis of MOFs,which could introduce considerable heterogeneity into one framework without altering its topology [16-18].Previous research has revealed that the unique structural characters of MTV-MOFs,such as distinct spatial arrangements of metals [19],infinite and continuous host-guest interaction energy levels [20],and sequence coding of heterometals [21],could favor improved macroscopic physical responses.In addition,the MTV approach has also been demonstrated as the key for tuning the water adsorption isotherm shape [22].Hence,with the aid of forming MTV-MOFs,the H-bonding geometry and strength between populated water molecules and the hydrophilic pocket could be optimized.MOF-333,isoreticular to MOF-303,is first synthesized by substituting the PZDC2-group with FDC2-group(FDC2-=2,4-furandicarboxylate).FDC2-group is known to be less acidic and less basic than PZDC2-group,cor111responding to the weaker H-bonds with water molecules.As Fig.1c shows,an ideally shaped sorption isotherm with a steep step was observed on MOF-333 at 22%RH,different with the S-shaped step of MOF-303.Based on this fact,a series of MTV-MOFs with nine different ratios of PZDC2-to FDC2-have been produced.It was found that with theincreasingofFDC2-content,theinitialS-shapedstepinwater sorption isotherm of MOF-303 has been alleviated (Fig.1d).More important,different from the emergent features first identified in MTV-MOF-5 [11],these MTV-MOFs exhibits a continuous change in their atmospheric water harvesting capabilities,where improved water adsorption enthalpies,desorption temperature (Fig.1e),as well as water productivity(the maximum working capacity increased up to 15%)have been realized without any compromise in other physical properties.
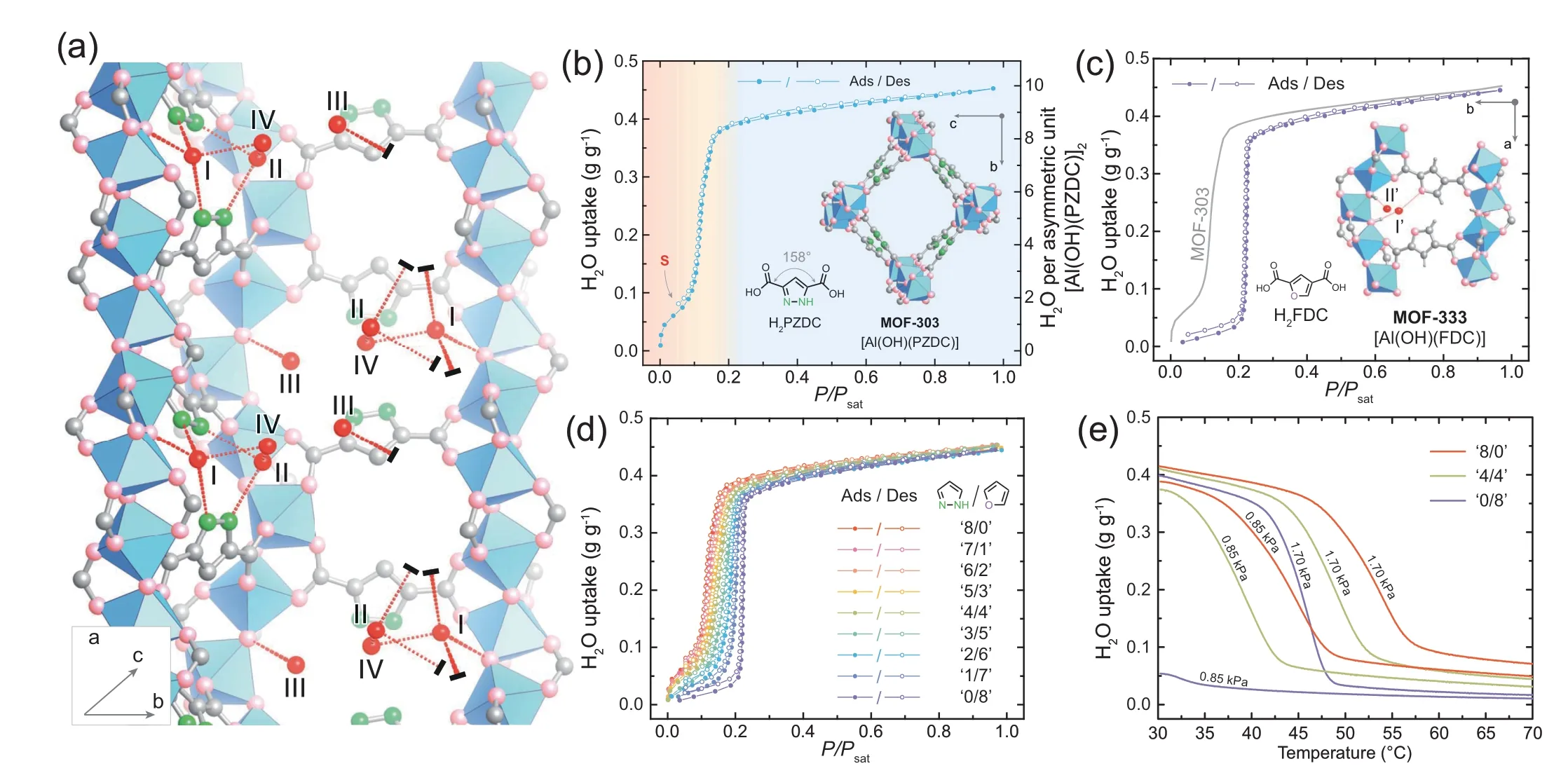
Fig.1.(a) Evolution of water-binding sites in MOF-303 during the seeding stage.(b and c) Water sorption isotherms of pure MOF-303 and MOF-333 at 25°C,where P is water vapor pressure and Psat is the saturation water vapor pressure.The organic linker and MOF crystal structure are shown in the inset.(d)Full-range of the water sorption analyses on the MTV-MOF series at 25°C.(e) Water vapor isobar measurements at 0.85 to 1.70 kPa for ‘8/0,’ ‘4/4,’ and ‘0/8.’ (The nonmenclature ‘n/m’ denotes the input ratio of PZDC2- to FDC2-).
In summary,this study not only provides fundamental mechanistic insights intowater filling dynamics in MOFs but elegantly demonstrates the power of MTV-MOFs in the application of atmospheric water harvesting.We believe this work will greatly encourage the future design of powerful MTV-MOF-based atmospheric water harvesting devices from atomic precision.
Conflict of interest
There are no conflicts to declare.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Grant Nos.21471118,21971199,22025106,51202127,91545205,and 91622103),National Key Research and Development Project of China(2018YFA0704000),Natural Science Foundation of Hubei Province (2016CFB382),and Fundamental Research Funds for the Central Universities(2042017kf0227,2042019kf0205).
杂志排行
Green Energy & Environment的其它文章
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
- Design and in-situ construct BiOI/Bi/TiO2 photocatalysts with metal-mediated heterostructures employing oxygen vacancies in TiO2 nanosheets
