The regulating effect of doping Cu on the catalytic performance of CO oxidative coupling to DMO on PdxCuy/GDY: A DFT study
2022-08-30JunZhoMinHnZhnhuiWngLixiLingRigungZhngBojunWng
Jun Zho,Min Hn,Zhnhui Wng,Lixi Ling,,Rigung Zhng,Bojun Wng,
a College of Chemistry and Chemical Engineering,Taiyuan University of Technology,Taiyuan,030024,China
b Key Laboratory of Coal Science and Technology of Ministry of Education and Shanxi Province,Taiyuan University of Technology,Taiyuan,030024,China
Abstract Rely on the density functional theory (DFT) calculation,the catalytic performance of PdxCuy/GDY (x=1,2,3,4;x+y ≤4) for CO oxidative coupling reaction was obtained.The Pdx/GDY(x=1,2,3,4)are not ideal catalyst for dimethyl oxalate(DMO)formation because byproduct dimethyl carbonate (DMC) is easily formed on Pd1/GDY and Pd2/GDY,and high activation energies are needed on Pd3/GDY and Pd4/GDY.Therefore the second metal Cu is doped to regulate the performance of Pdx/GDY(x=1,2,3,4).Doping Cu not only improve the activity of DMO formation,but more importantly,controlling the ratio of Cu:Pd can effectively regulate the selectivity of DMO.Thus taking into account the activity and selectivity of the reaction for the preparation of DMO by CO oxidative coupling,the Pd1Cu1/GDY and Pd1Cu2/GDY with the activation energies of 105.2 and 99.2 kJ mol-1 to generate DMO show excellent catalytic activity and high DMO selectivity,which are considered as good catalysts for CO oxidative coupling.The differential charge density analysis shows the decrease in the charge density of metal clusters is an important reason for improving the selectivity of the catalyst.
Keywords: GDY;Pd clusters;Cu:Pd ratio;DMO formation;Catalytic performance
1.Introduction
The CO gas phase coupling to generate dimethyl oxalate(DMO)is an efficient and clean reaction,and water is the only by-product during the entire reaction process,which is a green production route [1-4].Pd-based catalysts exhibit good activity and selectivity for CO oxidative coupling reaction,due to the limited precious metal resources and its high price,considering how to ensure catalytic performance and simultaneously reduce the amount of precious metal Pd becomes the focus of research [5-8].The Pd nanocrystals with exposed(111) and (100) facets through preferentially oriented facet growth technology were synthesized by Xu et al.[8],and the space-time yield of DMO on Pd (111) surface was much higher than Pd (100) which demonstrated Pd (111) was more favorable for CO oxidative coupling.The CO oxidative coupling to generate DMO on different crystal surfaces of Pd was studied by theoretical calculation,the results showed that the activity sequence of different surfaces of Pd was as follows: Pd (111) (120.6 kJ mol-1) [9] > Pd (100)(129.9 kJ mol-1) >Pd (110) (177.0 kJ mol-1) >Pd (211)(239.6 kJ mol-1) [10].Therefore,the Pd (111) is selected as judgment standard to screen the catalyst which will applied in DMO generation.
We all know that when the size of the metal particles is reduced to the ultra-small cluster with only a few atoms,the electronic properties of the metal particles will change strongly because of their discrete band structure,so the interactions with the reactants may be different,and the influence of the number of atoms on the catalytic performance is more significant [11-14].By adjusting the number of Fe atoms in the catalyst,He et al.precisely controlled the catalytic performance of CO2reduction reaction,because the number of Fe atoms can affect its affinity for the key intermediates such as *CO,*CHO,*OCHO and*OCHOH [15].The study of Pan et al.had shown that the initial conversion rate and product formation rate of glycerol depended on the size of Pt,the average particle size of Pt affected its reaction activity and selectivity [16].In our previous work [17],the effect of different structures of Pdnclusters (n=1,2,3,4,6)on CO oxidative coupling reaction was studied,and found that Pd4/TiO2-Ovshowed significantly better catalytic activity than Pd (111),implying the number of atoms greatly influence catalytic performance.Similarly,the DMO generation by CO oxidative coupling was investigated over Pdn(n=1,4,6)clusters supported on single defect graphene(SVG),and it was found that the active order of Pdn-SVG (n=1,4,6) catalyst was Pd-SVG ≈Pd4-SVG >Pd6-SVG [18].Thus,it can be seen that the particle size of the ultrafine cluster with only a few atoms as the catalyst for CO oxidative coupling to DMO affects the catalytic performance.
The interaction of heterogeneous atoms can induce geometric and/or electronic effects to alter the surface adsorption properties and reactivity,changing an atom in a very small cluster is likely to signally alter the electronic structure of the entire cluster and its catalytic properties[19-21].The propane dehydrogenation reaction was calculated by DFT,and it was found that the monoatomic Pt/CeO2catalyst had a strong adsorption capacity to the generated alkenes,so it was not conducive to propylene desorption.But when Sn was added to CeO2,the PtSn cluster made the product easy to desorption thereby highly improved the selectivity of propylene [22].Peng et al.[23] studied the influence of Cu2+doping on CO oxidative coupling to DMO,and the small size and high dispersion of Pd nanoparticles promoted by Cu2+ions can explain the phenomenon of the excellent catalytic activity.The influence of core-shell Pd catalyst doped with the second metal Al and Ag on DMO generation was studied by DFT method,indicating that Al13@Pd42can improve the catalytic performance while reducing the amount of Pd [24].The activity and selectivity of Pd-Cu bimetallic catalyst in selective hydrogenation of acetylene were greatly enhanced by the synergistic action between metals [25].During the acetone-butanol-ethanol (ABE) condensation,the alloying effect of Cu and Pd leaded to the selectivity of the dehydrogenation reaction which occurred at the PdCu site exceed the decarbonylation reaction [26].Begum et al.[27] studied the effects of Pd2and PdM(M=Cu,Rh,Ag,Au,Pt) dimer catalysts on nitric oxide oxidation,the PdCu bimetallic catalyst showed the lowest activation energy for the rate-determining step because the active Pd-site had stronger ability to activate oxygen.
Because of the poor stability of small metal clusters,they are usually embedded on the surface of metal or carrier to stabilize their existence and prevent agglomeration [28,29].At the same time,the carrier of the supported metal catalyst can change the reaction performance by affecting the nature of the supported metal [30].Graphdiyne (GDY) is a planar two-dimensional structure with sp and sp2hybridized carbon atoms which formed by the connection of butadiyne and benzene ring,its abundant porous structure can provide space for the deposition of metal clusters,the strong metal-carbon interaction can effectively stabilize metal clusters,and its unique electronic structure is also an important reason to improve the catalytic performance of catalysts [31-33].It has been reported that GDY can be used as a material for deposition of highly dispersed Pd nanoparticles,and the Pd/GDYO catalyst prepared by chemical deposition has higher catalytic performance than other carbon materials in the reduction reaction of 4-nitrophenol [34].Similarly,the Pd1/GDY/G catalyst synthesized by Li et al.had also been proved to have significant catalytic activity and stability for the 4-NP reduction reaction,and the charge transfer of GDY in the catalytic process can effectively improve the activity [35].Xue et al.[31] had successfully loaded single atom Fe and Ni on GYD,and proved that it can be used as an efficient catalyst for HER reaction.The strong charge transfer between the active site and the carrier was considered to be the reason for the improved catalytic performance.The effects of Mn,Fe,Co and Ni monomers,dimers and trimers fixed on GDY monolayer on N2reduction were investigated,the results indicated that all dimers improved catalytic performance compared with Ru(0001) catalyst [36].The GDY supported MxM′3-x(M,M’=Ru,Os) bimetallic catalysts had obvious selectivity for the selective hydrogenation of acetylene to ethylene due to the synergy between the bimetals and the interaction between metal clusters and GDY [37].Research by Shen et al.[38] showed that the catalytic performance of Cu supported on triphenylene-cored graphdiyne for CO2electrolytic reduction was better than that of bulk Cu and other Cu-containing catalysts.In addition,metal-modified GDY is not only widely used in the field of catalysis,but also has potential and important application prospects in the fields of electronics and energy,the doping of noble metal atoms (Pd,Pt,Rh and Ir) effectively changes the electronic properties of GDY,making it of great application value in gas molecule sensors [39].And the Cr-and Mn-doped GDY have spin polarization feature which would be promising used in spintronics devices [40].The theoretical calculations have proved that light metal (Li,Na,K or Ca) modified GDY or BGDY (boron-graphdiyne)has excellent hydrogen storage performance [41,42].
In this work,we will discuss the reaction mechanism of CO oxidative coupling to produce DMO on Pdx/GDY(x=1,2,3,4) to explore the influence of metal clusters of different sizes on the reaction.And in order to obtain an efficient catalyst,doping the second metal Cu to regulate its catalytic performance on DMO generation,this work can provide a reference for the method design of low-cost and high catalytic performance Pd catalysts for CO oxidative coupling to DMO.
2.Computational details
2.1.Calculation methods
In this work,all calculations were carried out by Dmol3module in Materials Studio 8.0 [43,44].The related energy calculation and geometry structural optimization were completed when the energy was converged to 2×10-5Ha(1 Ha=2625.5 kJ mol-1),the maximum force was converged to 0.004 Ha Å-1(1 Å=1 × 10-10m),and the maximum displacement was converged to 0.005 Å.The electronic exchange and correlation functions used generalized gradient approximations (GGA) functional of Perdew,Burke and Ernzerhof (PBE) [45-47].Symmetry and spin polarization were set to no symmetry and spin unrestricted.Plane-wave electronic density functional theory with long range dispersion interaction corrections DFT-D2 from Grimme [48] was considered to balance computational efficiency and accuracy[49,50].The k-point of the Brillouin zone was set to 3×3×1 and the global orbital off was set to 4.0 Å.The doubled-numerical quality basis set with polarization functions (DNP)[51,52]was employed with the effective core potentials(ECP)scheme[53,54].The complete LST/QST method was selected to search the transition states (TS) for every elemental step during the CO oxidative coupling to DMO [55,56].In the meantime,TS confirmation was carried out to confirm the accuracy of the transition state.
The binding energy between the metal cluster and the support GDY is calculated according to the formula (1):
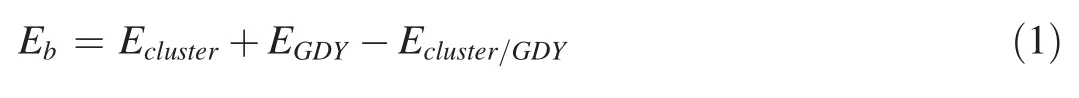
where the Ecluster/GDY,Eclusterand EGDYrepresent the energies of GDY after loading the metal cluster,the metal cluster and clean GDY,respectively.
The adsorption energy of related species in the CO oxidative coupling to DMO is calculated by the following formula(2):
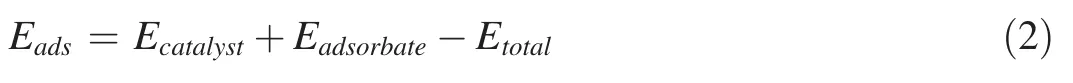
where the Etotal,Ecatalystand Eadsorbaterepresent the energies of the system after adsorption,catalyst and the adsorption species in the gas state,respectively.
The activation energy for the elementary reaction is defined as formula (3):

where ETSand ERrepresent the energies of transition state and initial reactant.The heat of reaction (ΔH) is the difference in energy between the products and the reactants in each step of the elementary reaction.
2.2.Models
GDY is a two-dimensional planar network of large conjugated carbon rings containing several alkyne bonds,and has large triangular holes with 18 C atoms (Fig.1.) [33,57].To construct the GDY structure,the 2×2×1 supercell is chosen(a=18.880 Å,b=18.880 Å)which contains 72 C atoms,the C-C bond in the benzene ring has a distance of 1.424 Å,the distances of C-C in diacetylenic bond are 1.392,1.233 and 1.336 Å.The vacuum layer above the GDY surface is 30 Å to avoid interaction between two successive layers along the z axis,the surrounding red C atoms are all fixed during the calculation.
The large triangular hole is selected as the location of metal cluster deposition because previous studies have shown that clusters are more likely to be stable at this location [58].The Pdx/GDY (x=1,2,3,4) are obtained by optimizing the structure of Pd clusters loaded on GDY,and the GDY loaded with metal clusters will undergo minor deformation.Considering the spatial compatibility of metal clusters with GDY structure and the stability of cluster itself [59,60],Pd4loaded on GDY is a three-dimensional structure.Furthermore,Pd atoms are replaced with different numbers of Cu atoms to obtain PdxCuy/GDY (x+y ≤4) structures,and the structure optimization was performed for all possible different structures,the most stable structure was selected for subsequent calculations.The most stable PdxCuy/GDY (x=1,2,3,4;x+y ≤4) structures are shown in Fig.2.
3.Results and discussion
3.1.Stability of PdxCuy/GDY(x=1,2,3,4;x+y ≤4)
The binding energies of Pdx(x=1,2,3,4) and GDY are between 241.4-407.5 kJ mol-1,indicating that Pdx(x=1,2,3,4) clusters can stably exist on GDY,which are consistent with previous studies [38,58].And the binding energies between the Pdx(x=1,2,3,4) clusters and GDY increase monotonously with the increase of Pd atoms.The triangular Pd3cluster is most similar to the triangular hole of the GDY,while the Pd4cluster loaded on GDY is a three-dimensional structure considering the suitability of space,the binding energy of Pd4loaded on GDY increases by only 13.2 kJ mol-1and the addition of a fourth atom which will separate Pd3from its vantage point near the pore plane [58].And it can be seen from Table 1 that the binding energies between the bimetal PdxCuy(x+y ≤4) clusters and GDY are between 285.8-434.4 kJ mol-1,which is enough to ensure the stable existence of PdxCuy(x+y ≤4) clusters on GDY.In addition,the addition of Cu also reduces the sinking distance of the cluster below the GDY monolayer,the bimetal clusters are elevated in the vertical direction compared to before replacement.
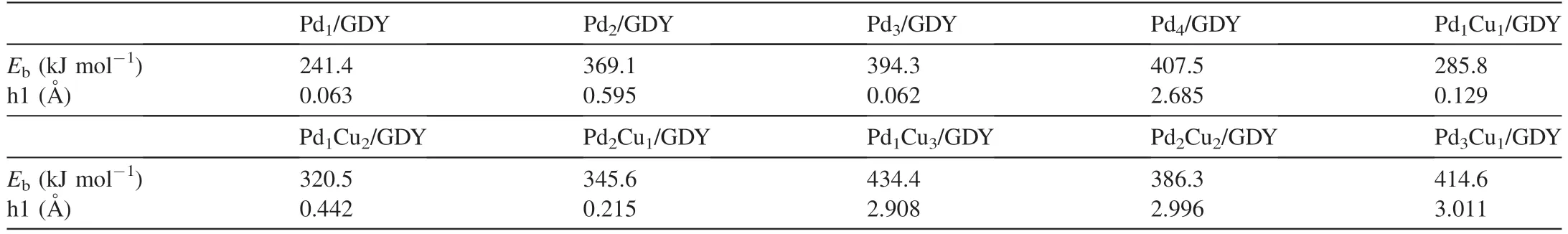
Table 1 The binding energies (Eb) of Pdx (x=1,2,3,4) and PdxCuy (x+y ≤4) on GDY.The highest distance (h1) of the cluster above the GDY monolayer.
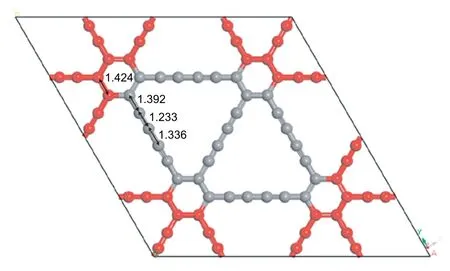
Fig.1.The GDY structure after optimizing.
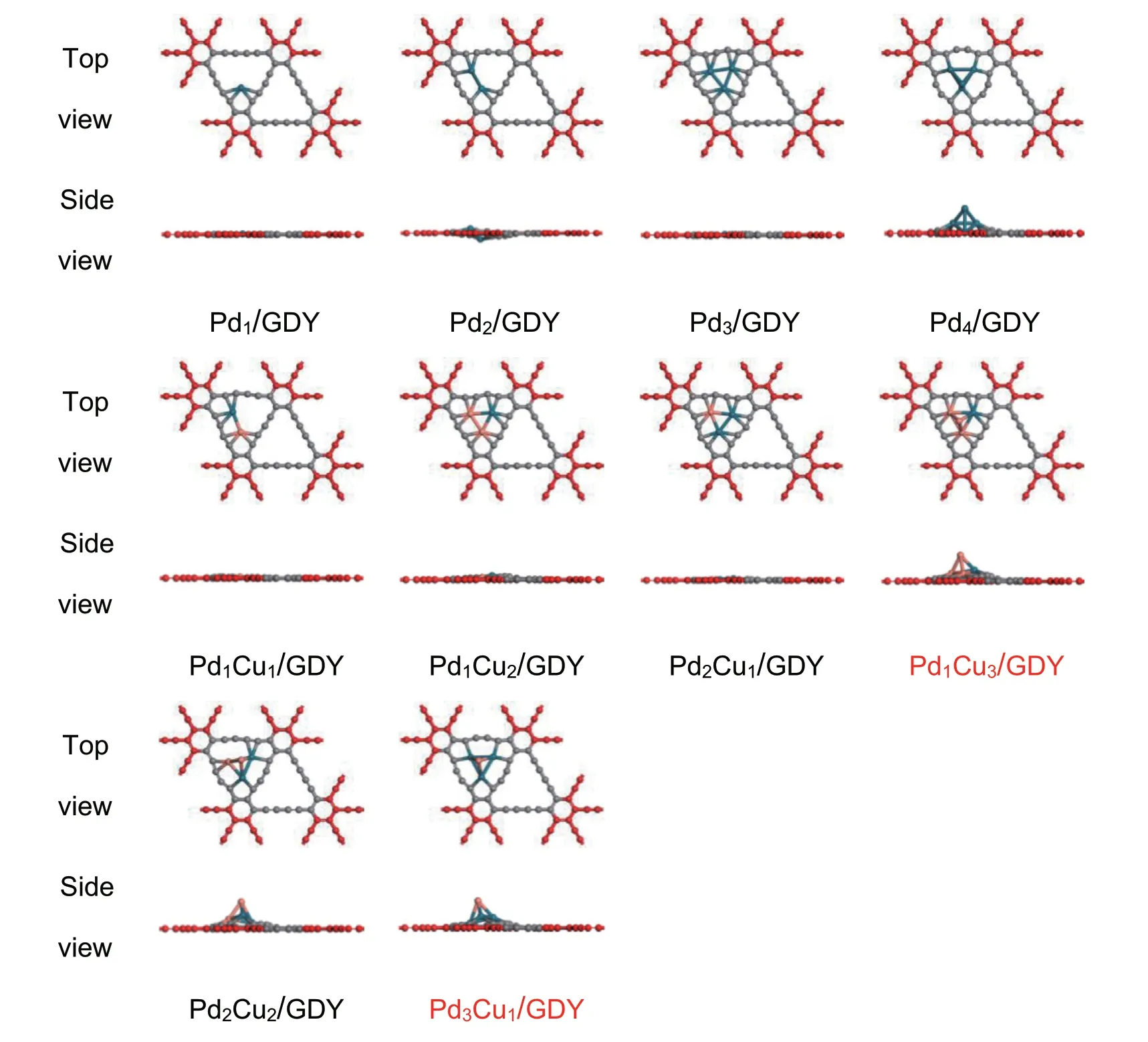
Fig.2.The most stable configurations of PdxCuy/GDY (x=1,2,3,4;x+y ≤4).
3.2.The reaction pathway of CO oxidative coupling to DMO
The DMO is synthesized using CO and methyl nitrite(MN)as the initial reactants by the gas phase method,MN is easily decomposed into NO and OCH3because of the small energy of N-O bond [5,61].Therefore,CO and OCH3are used as initial reactants in subsequent studies and the adsorption of species which may participate in the formation of DMO are considered.For CO oxidative coupling to DMO,it can be judged that there are two DMO reaction pathways(Path 1 and Path 2,Scheme 1) by analyzing the adsorption of species on the surface that may participate in the reaction.Since the OCCO intermediate cannot exist stably on PdxCuy/GDY(x=1,2,3,4;x+y ≤4),the way of generating DMO involving OCCO is not considered.Both Path 1 and Path 2 involve the formation of COOCH3,except that C-C bond is formed in different ways.For the Path 1,COOCH3reacts with CO to form OCCOOCH3intermediate.At this time,C-C bond has been formed,and then OCOCOCH3reacts with OCH3to form target product DMO.For the Path 2,two COOCH3are generated firstly,and then they are coupled as a reactant to generate DMO.In the whole reaction,the formation of byproduct dimethyl carbonate (DMC) is also considered to clarify the selectivity of the catalyst for CO oxidative coupling.
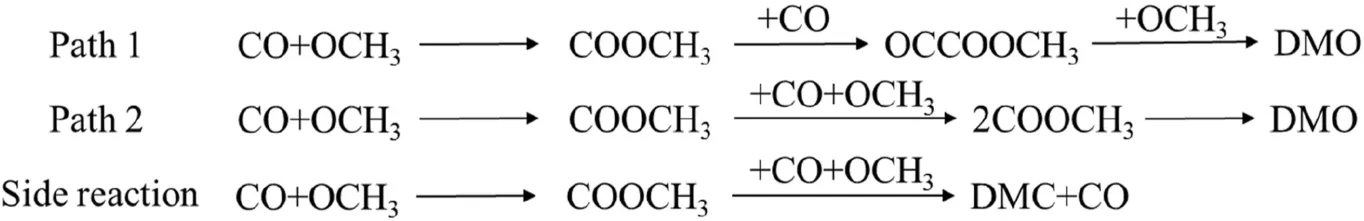
Scheme 1.Pathways of CO oxidative coupling to DMO [9,18,24].
3.3.DMO formed by oxidation coupling of CO on Pdx/GDY (x=1,2,3,4)
3.3.1.The DMO generation on Pdx/GDY (x=1,2,3,4)
For the reaction of CO oxidative coupling to DMO on Pd1/GDY,because single atom Pd is the active center of the reaction,all species participating in the reaction exist on the catalyst in the way of top adsorption,The potential energy diagram of the reaction and the cor111responding structures are shown in Fig.3.First,the initial reactant CO and OCH3are co-adsorbed on Pd1/GDY,and form a new C-O bond in COOCH3through the transition state TS1,which needs to cross 88.2 kJ mol-1reaction energy barrier and release heat 56.8 kJ mol-1.After that,the reaction is divided into two paths,the activation energies needed to generate OCCOOCH3and DMO through Path 1 are 143.0 and 107.1 kJ mol-1,respectively,and the reaction heats are 48.5 and -79.3 kJ mol-1,respectively.The activation energies required to generate the second COOCH3and the coupling of two COOCH3to form the DMO in Path 2 are 94.9 and 84.0 kJ mol-1,respectively,and the reaction heats of these two primitive reactions are-107.1 and -14.9 kJ mol-1.
Similar to Pd1/GDY,the correlation reactions of the two paths of CO oxidative coupling to generate DMO on Pd2/GDY,Pd3/GDY and Pd4/GDY are calculated,the potential energy diagram and the cor111responding structures are given at the supporting information,and the activation energy of each primitive reaction is summarized in Table 2.On Pd2/GDY,CO mainly takes part in the reaction by bridge adsorption,the activation energies required for the first COOCH3and OCCOOCH3generation in the reaction are 114.4 and 188.2 kJ mol-1,and the generated COOCH3and OCCOOCH3are adsorbed on the top position.The activation energy required for the final elementary reaction of Path 1 to generate DMO is 144.2 kJ mol-1.In Path 2,the energy barrier to be overcome for the second COOCH3generation is 110.1 kJ mol-1,and the DMO generated by COOCH3-COOCH3coupling needs to overcome 101.4 kJ mol-1.On Pd3/GDY,the bridge adsorbed CO and OCH3overcame 125.2 kJ mol-1energy barrier to form bridge adsorbed COOCH3.For CO+COOCH3→OCCOOCH3and OCCOOCH3+OCH3→DMO in Path 1,the activation energy barriers are 126.6 and 111.4 kJ mol-1,respectively.For COOCH3+CO+OCH3→2COOCH3and OCCOOCH3+OCH3→DMO in Path 2,the required activation energies are 168.2 and 144.4 kJ mol-1,respectively.On Pd4/GDY,the reaction occurs mainly around the Pd atoms at the top of the cluster,after calculating the primitive reaction at each step,it is concluded that it takes 230.4 kJ mol-1to generate DMO through Path 1,and 158.1 kJ mol-1through Path 2.
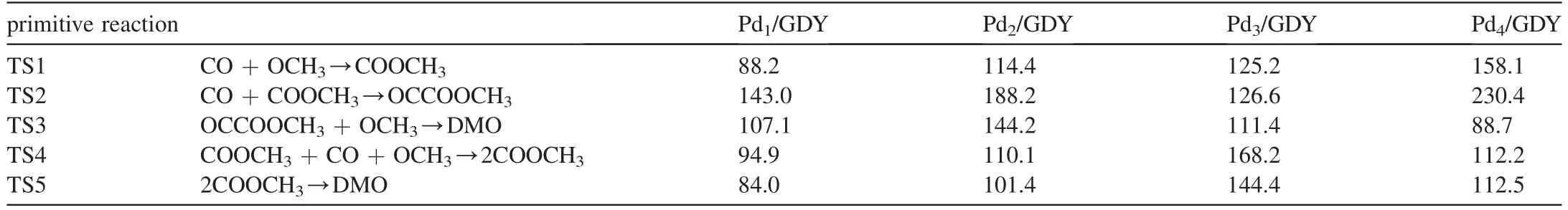
Table 2 The activation energy barrier of each primitive reaction for DMO generation on Pdx/GDY (x=1,2,3,4).The values are given in kJ mol-1.
It can be seen from the above that when x=1,2 and 4,the optimal path for DMO generation is COOCH3-COOCH3coupling path,while the optimal path is COCOOCH3path on Pd3/GDY.Similar to previous studies,when Pdnis supported on TiO2-v,the optimal path to generate DMO will change when the number of Pd atoms is different [6].When x=1 and 2,the activation energy barriers of the rate-determining steps to generate DMO are 94.9 and 114.4 kJ mol-1,which exhibit higher catalytic activity than the pure Pd (111) (120.6 kJ mol-1) [9].While the activation energy barriers of rate-determining steps for DMO generation on Pd3/GDY and Pd4/GDY are higher than Pd (111) [9].
3.3.2.The DMC generation on Pdx/GDY (x=1,2,3,4)
Further,to investigate the selectivity of CO oxidative coupling to generate DMO on Pdx/GDY (x=1,2,3,4),the generation of by-product DMC is calculated (Fig.4.).The formation of DMC starts from co-adsorption COOCH3+CO+OCH3,and then formed by the combination of COOCH3and OCH3through the transition state TS6.The energy barriers to be overcome to generate DMC on Pdx/GDY (x=1,2,3,4) are 71.2,102.2,85.4 and 244.6 kJ mol-1,respectively.Compared with the activation energies of DMO generation (94.9,114.4,126.6,158.1 kJ mol-1on Pdx/GDY (x=1,2,3,4)),it can be seen that DMC is easier to be formed on Pd1/GDY,Pd2/GDY and Pd3/GDY.Although Pd1/GDY and Pd2/GDY show high catalytic activity,the selectivity is poor and they are not ideal catalyst for DMO synthesis.It is worth mentioning that although the activation energies required to generate DMO is high on Pd4/GDY,it still shows a good selectivity for the generation of DMO.In summary,it can be seen that the Pdx/GDY (x=1,2,3,4) catalyst cannot be both highly active and selective for DMO formation,so they are not recommended for DMO production.
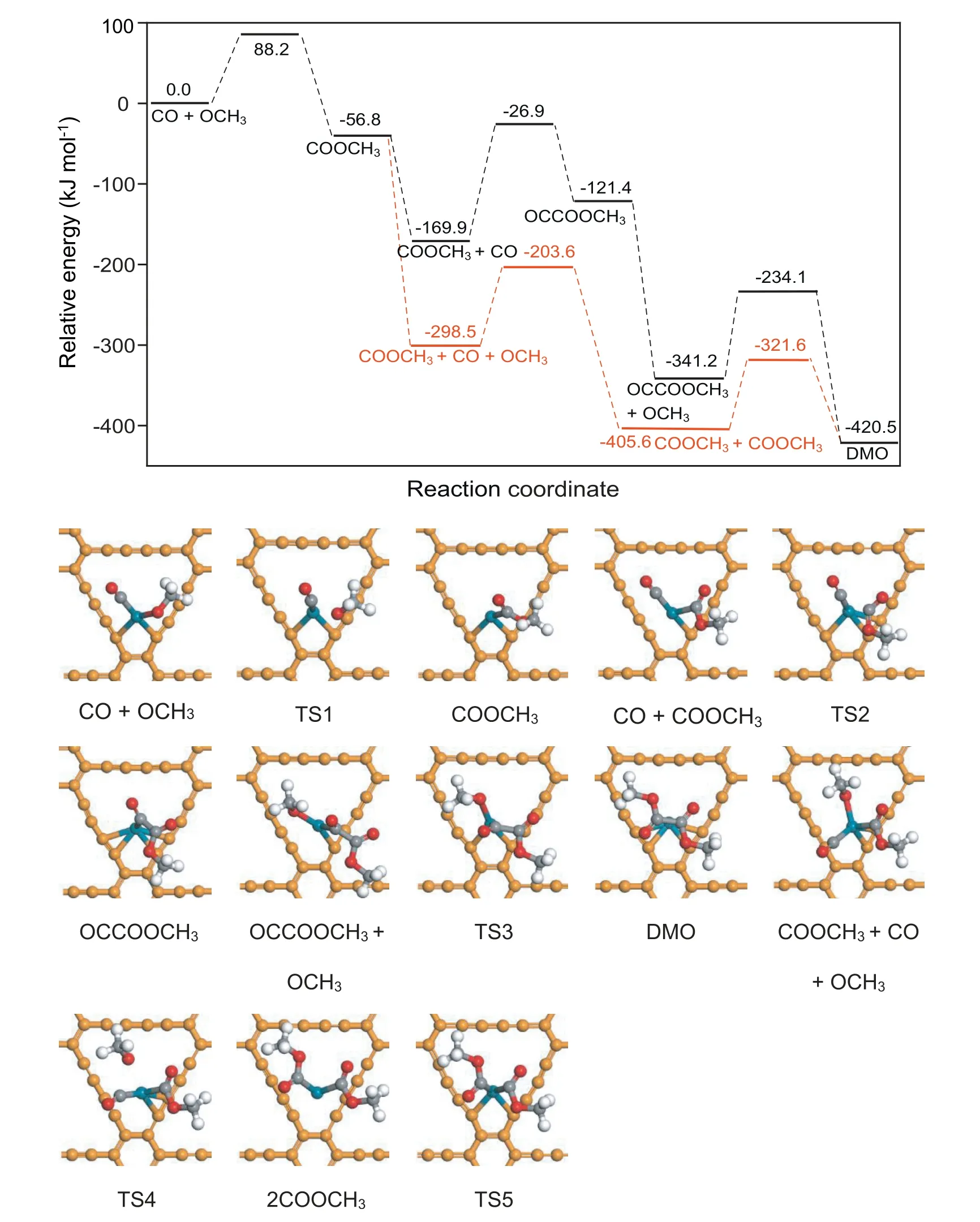
Fig.3.Potential energy diagram of the reaction of CO oxidative coupling to DMO,as well as structures of initial states,transition states,and final states on Pd1/GDY.The yellow,dark cyan,gray,red and white represent GDY,Pd,C,O and H,respectively.
3.4.Research on the DMO generation on PdxCuy/GDY(x+y ≤4)
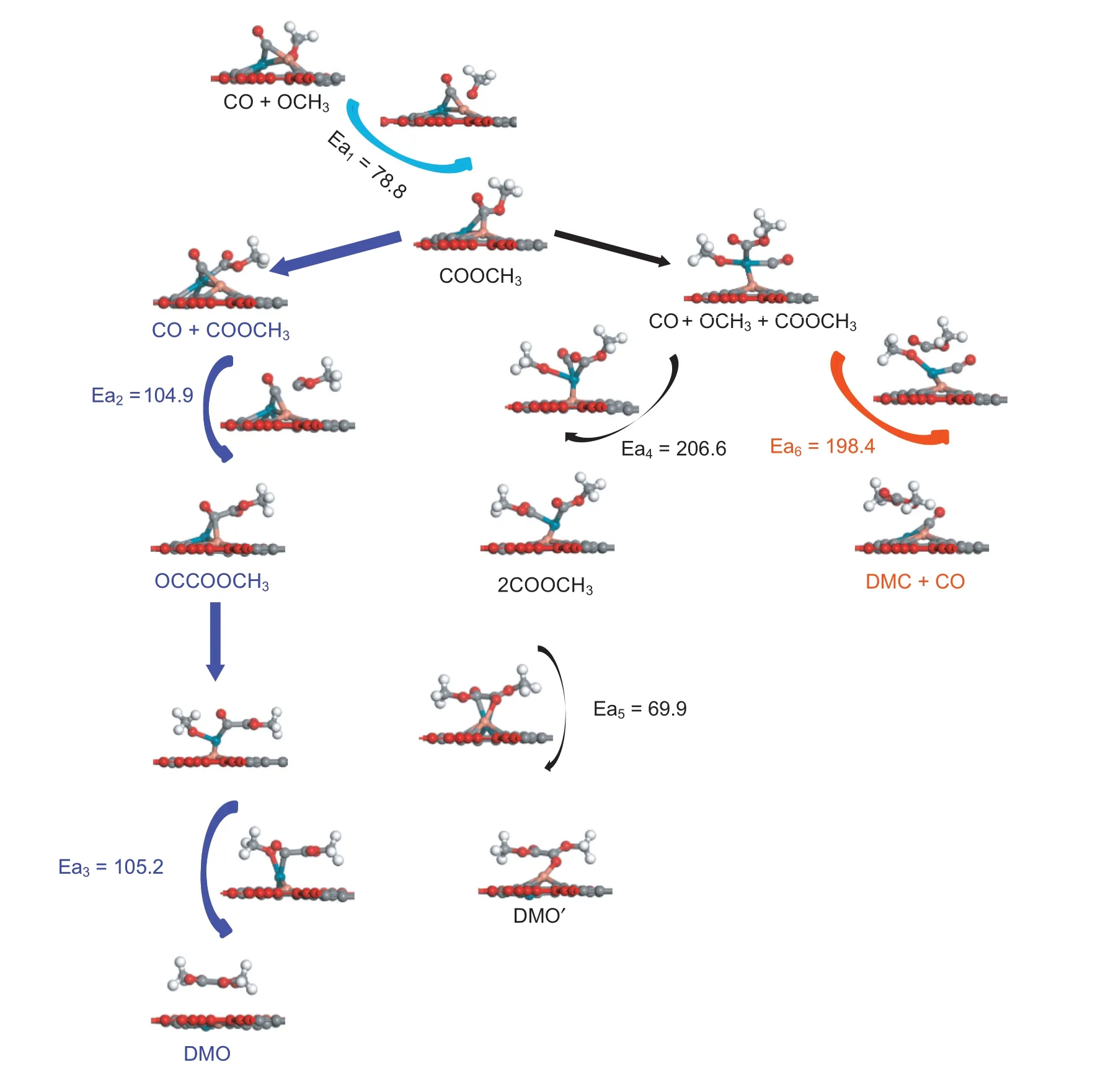
Fig.5.The activation energy and structure of each element reaction in CO oxidative coupling reaction on Pd1Cu1/GDY.
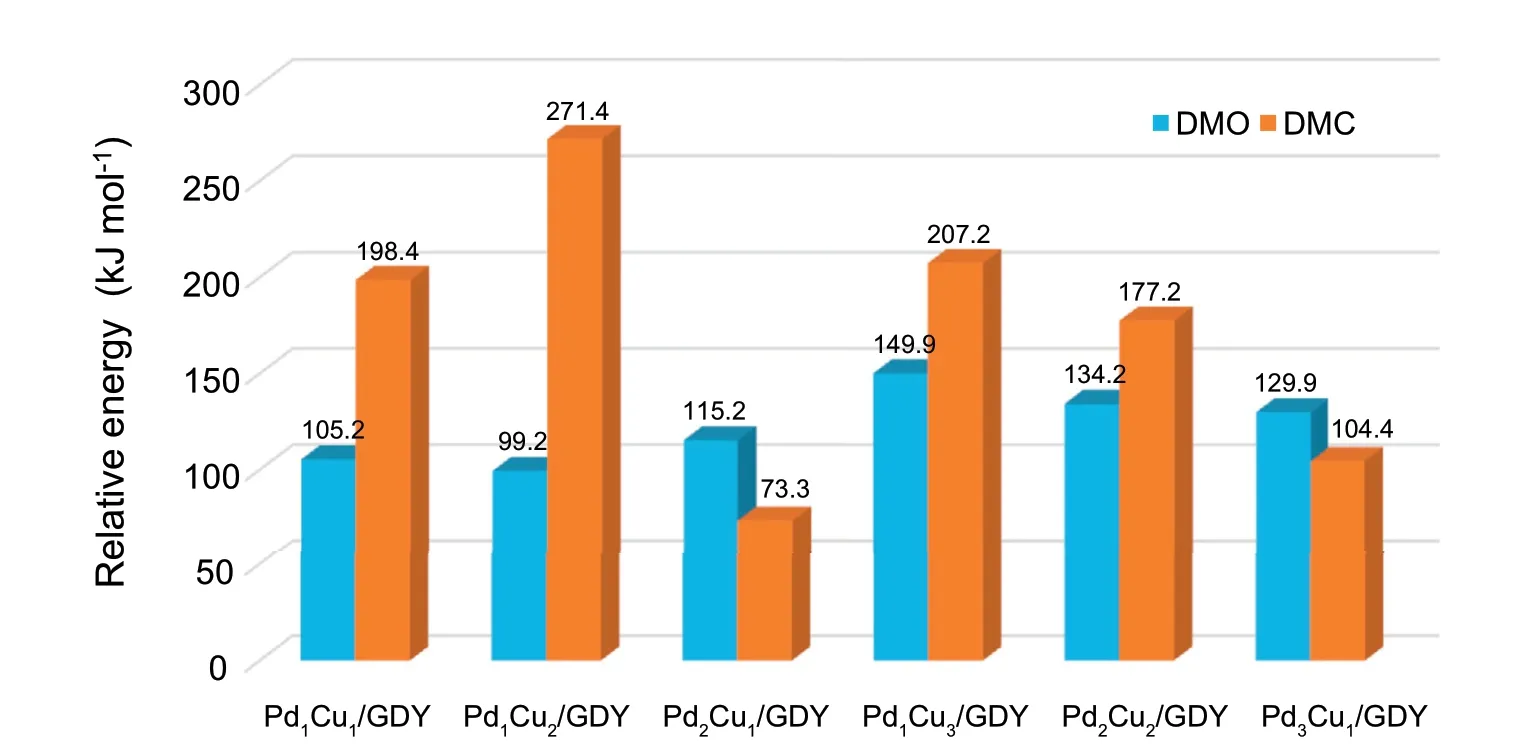
Fig.6.The activation energy barriers of the rate-determining steps for CO oxidative coupling reaction and the activation energies required for DMC generation on PdxCuy/GDY (x+y ≤4).
In order to improve the catalytic performance of Pdx/GDY(x=1,2,3,4),the second metal Cu is used for modification.For Pd2/GDY,doping Cu can only form Pd1Cu1/GDY with Cu:Pd ratio of 1:1,while for the other two catalysts,due to the different number of substituted Pd atoms,catalysts with Cu:Pd ratio of 1:2,2:1,1:3 and 3:1 can be formed,the bimetallic catalyst model is similar to that of Xing et al.[37].And then CO oxidative coupling reaction on PdxCuy/GDY (x+y ≤4)catalysts with different Pd:Cu ratios are studied,the specific potential energy diagram and the cor111responding structures are shown at the supporting information.On Pd1Cu1/GDY with the Pd:Cu ratio of 1:1,DMO is more inclined to be generated through the CO-COOCH3coupling and the rate-determining step is OCCOOCH3+OCH3→DMO whose activation energy is 105.2 kJ mol-1(Fig.5).Comparing with Pd2/GDY,it can be seen that the optimal path for CO coupling to DMO after Cu doping is changed,but the activation energy required for generating DMO is reduced,and both are lower than Pd(111).The optimal path of the CO oxidative coupling reaction on Pd1Cu2/GDY is Path 2,and Pd2Cu1/GDY is the same as those of Pd3/GDY.The rate-determining steps are 2COOCH3→DMO on Pd1Cu2/GDYand OCCOOCH3+OCH3→DMO on Pd2Cu1/GDY which the activation energies are 99.2 kJ mol-1and 115.2 kJ mol-1,respectively.Similarly compared with Pd3/GDY,the energy barrier required to generate DMO on Pd1Cu2/GDY and Pd2Cu1/GDY is sharply reduced.For Pd1Cu3/GDY,Pd2Cu2/GDY and Pd3Cu1/GDY with a three-dimensional metal cluster structure,the activation energies required to generate DMO are 149.9,134.2 and 129.9 kJ mol-1,the activation energy barrier also goes down relative to Pd4/GDY (158.1 kJ mol-1).Therefore,it can be concluded that the doping of the second metal Cu with different proportion can increase the activity of Pdx/GDY(x=1,2,3,4) to the CO oxidative coupling reaction.
Moreover,in order to better understand the influence of different proportion Cu doping on DMO generation,it is necessary to calculate DMC generation on PdxCuy/GDY(x+y ≤4) (Fig.S9.).By comparing with the activation energies of the rate-determining steps to generate DMO(Fig.6.),it can be known that DMO is more advantageous on Pd1Cu1/GDY,Pd1Cu2/GDY,Pd1Cu3/GDY and Pd2Cu2/GDY.If the activation energies difference between DMO and DMC is used as a descriptor to measure the selectivity of PdxCuy/GDY (x+y ≤4),for catalysts containing the same number of metal atoms,the greater the proportion of Cu atoms,the better the selectivity of the catalyst.As the proportion of Cu in PdxCuy/GDY(x+y ≤4) increases to greater than or equal to 1:1,the DMO becomes more favorable product for CO oxidative coupling reaction.
3.5.General discussion
Based on the above research,the reaction mechanism of Pdxclusters loaded on GDY for CO oxidative coupling reaction was obtained,and the regulation of the catalytic performance of the second metal Cu doping was explored,the effect of support,particle size and Cu doping were investigated.
3.5.1.The role of GDY as supporter
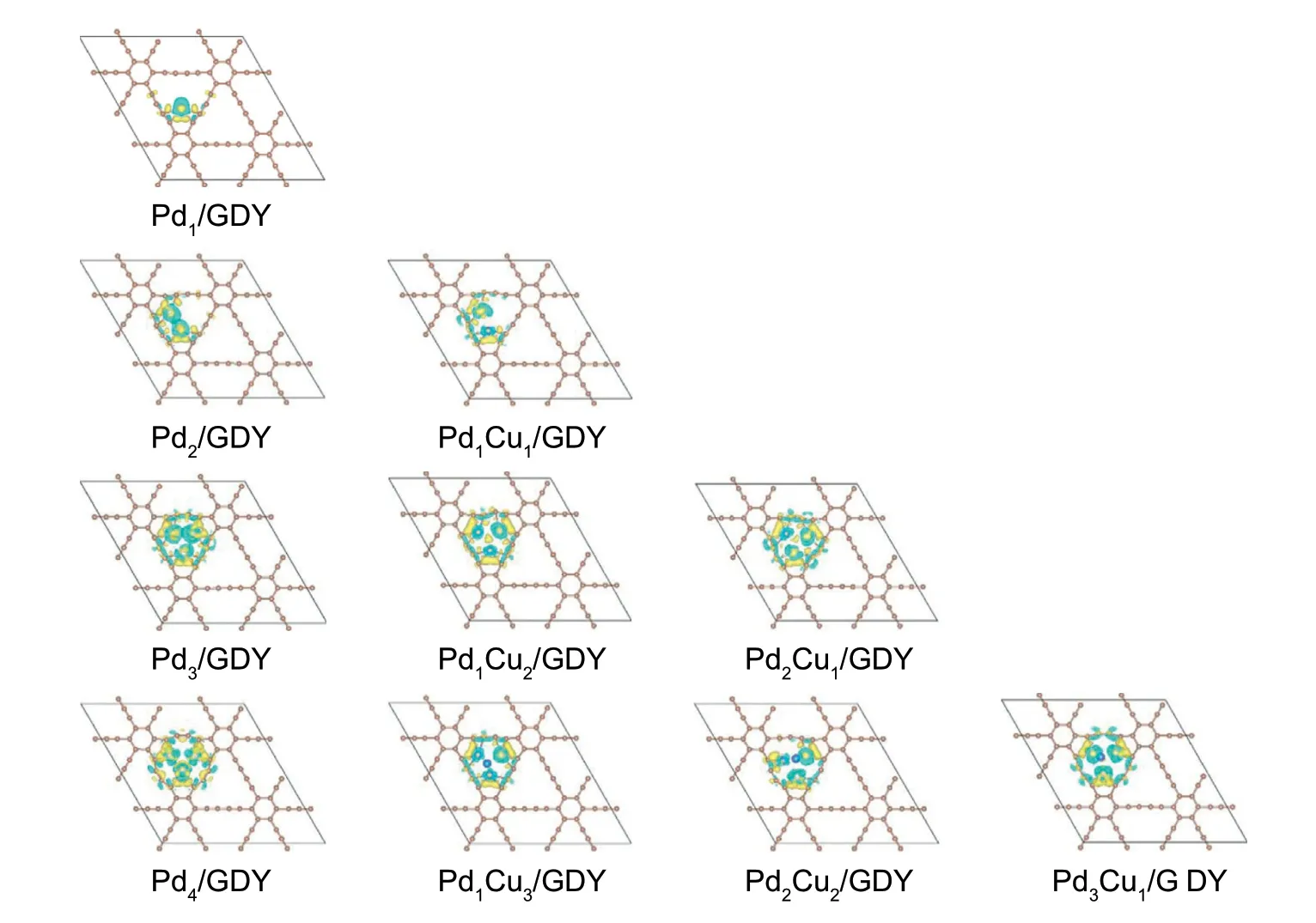
Fig.7.The charge density differences of PdxCuy/GDY(x=1,2,3,4;x+y ≤4).Green and yellow represent charge depletion and accumulation,respectively.The values of absolute isosurface is 0.005 e Å-3.
The larger binding energy between Pdx(x=1,2,3,4),PdxCuy(x+y ≤4) and GDY indicates that these metal clusters can exist stably on GDY,it also shows that GDY has an outstanding advantage as an effective carrier of targeted smaller metal catalysts.In order to effectively explore the effect of the GDY on metal clusters,the charge density differences analysis of the catalyst is carried out(Fig.7.),and the results show that there is an obvious charge exchange among Pdx(x=1,2,3,4),PdxCuy(x+y ≤4) metal clusters and GDY.With the increase in the number of atoms of Pdx(x=1,2,3),there is more charge interaction between Pdx(x=1,2,3) and GDY,which is also the reason for the increase of the binding energy.When the metal cluster becomes a tetrahedron,the redistribution of charge is significantly different from Pdx(x=1,2,3).The addition of the second metal Cu reduces the charge density of the metal clusters and has different catalytic performances for CO oxidative coupling reaction.Concretely,the electron cloud of the metal cluster on Pd1Cu1/GDY is significantly smaller than that on Pd2/GDY,which means that the charge density of the metal cluster on Pd1Cu1/GDY is smaller and the charge interaction between GDY is weak,so the binding energy with GDY changes from 369.1 to 285.8 kJ mol-1.A similar phenomenon also occurs on Pd3,the charge density of the metal clusters decrease with the addition of Cu,and the cor111responding binding energy also weakens (394.3,345.6 and 320.5 kJ mol-1on Pd3/GDY,Pd2Cu1/GDY and Pd1Cu2/GDY,respectively).When the number of atoms becomes four,the cluster becomes a threedimensional structure,and the addition of the fourth atom makes the electronic structure redistribute.Because of the electron redistribution when the number of atoms becomes four,the change of relevant binding energy by Cu substitution is obviously different from that of metal clusters with the number of atoms at two and three.In addition,as the charge density of the metal clusters decreases for the metal clusters with two and three atoms supported by GDY,the process of DMO generation will also change,which will result in different rate determination steps.For instance,the ratedetermining step for generating DMO through Path 2 is CO+OCH3→COOCH3on Pd2/GDY,while on Pd1Cu1/GDY is OCCOOCH3+OCH3→DMO through Path 1.Similarly,the favorable path to generate DMO is Path 1 on Pd3/GDY,but is Path 2 on Pd1Cu2/GDY and Pd2Cu1/GDY is still Path 1.And the rate-determining step is CO+COOCH3→OCCOOCH3on Pd3/GDY,while are 2COOCH3→DMO on Pd1Cu2/GDY and OCCOOCH3+OCH3→DMO on Pd2Cu1/GDY.Further,the comparison of the activation energy of the rate-determining step shows that the addition of Cu not only reduce the charge density,but the reaction activity can be improved because the energy barrier of the rate-determining step is significantly reduced.The activation energies of ratedetermining change from 114.4 kJ mol-1on Pd2/GDY to 105.2 kJ mol-1on Pd1Cu1/GDY,and go down from 126.6 kJ mol-1on Pd3/GDY to 115.2 kJ mol-1on Pd2Cu1/GDY and 99.2 kJ mol-1on Pd1Cu2/GDY.Many studies have also shown that the charge transfer between the GDY and the metal cluster is the reason for the large binding energy and the change of catalytic performance.In previous work,Ag38was stably presented on GDY with a large adsorption energy,and GDY promoted CO oxidative by regulating the electronic structure of metal cluster Ag38which had Ag4active site[62].In addition,Panigrahi et al.[42] discovered that the light metals (Li,Na,K,Ca,Sc,Ti) supported by GDY caused the charge redistribution of the C atoms in GDY,and charge transfer occurred between GDY and metal atoms,which markedly improved the hydrogen storage capacity.And the TM4/GDY(TM=Sc,Ti,Mn,Fe,Co,Ni and Cu)showed high HER catalytic activity also because the evident electron transfer between the TM4and GDY [63].
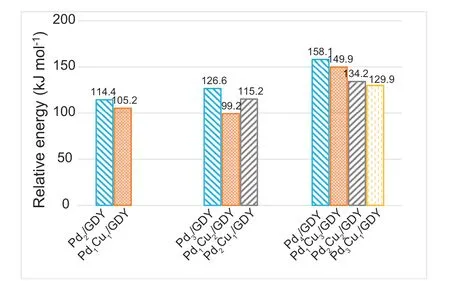
Fig.8.The activation energy barriers of the rate-determining steps for CO oxidative coupling reaction to DMO on PdxCuy/GDY (x+y ≤4).
We further calculate the Hirshfeld charge of the clean GDY and GDYof PdxCuy/GDY(x=1,2,3,4;x+y ≤4)(Table 3),and find that the total charge of clean GDY is 0.0075 e,but after the supported metal clusters,due to the strong interaction between the metal clusters and GDY,a large number of electrons are gathered on the GDY.When Pd1,Pd2,Pd3and Pd4are loaded on GDY,the total charge amount of GDY are-0.3537 e,-0.6131 e,-0.7570 e and -0.7936 e,respectively.The total charge of GDY reduces with the increase of Pd atoms when the number of Pd atoms is 1-4,the more charges on the GDY are accumulated,the smaller the value of total charge,and the greater the binding energy of metal clusters with GDY.In addition,for metal clusters with the same number of atoms,when the cluster contains Cu atoms,the total charge of GDY is higher than that of pure Pd clusters,and it is more obvious when the number of atoms is two and three,which means that Cu will weaken the charge accumulation on GDY.
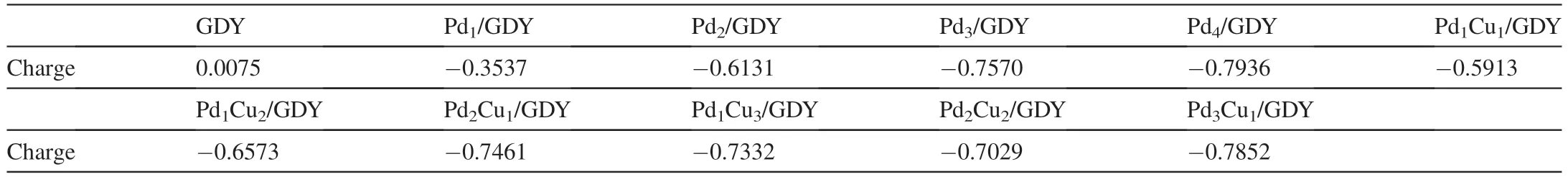
Table 3 Hirshfeld charge analysis to obtain the specific charge status of the clean GDY and GDY of PdxCuy/GDY (x=1,2,3,4;x+y ≤4).(e).
3.5.2.Effect of cluster size on CO oxidative coupling to DMO
The catalytic activity of CO oxidative coupling to DMO on Pdx/GDY (x=1,2,3,4) are analyzed.The activation energy barriers for rate-determining steps of DMO formation are 94.9and 114.4 kJ mol-1on Pd1/GDY and Pd2/GDY,which perform better catalytic activity compared to Pd (111) [9].However,Pd3/GDY and Pd4/GDY are different from Pd1/GDY and Pd2/GDY showing poor activity for the CO oxidative coupling reaction because of their larger activation energy barriers for rate-determining steps,which are 126.6 and 158.1 kJ mol-1.The selectivity of the Pdx/GDY (x=1,2,3,4) for CO oxidative coupling reaction is obtained by considering the formation of by-product DMC.Observing the optimal path for DMO generation on these four catalysts,it can find that through COOCO3-COOCH3coupling path to generate DMO is more favorable on Pdx/GDY (x=1,2,4),but more likely through CO-COOCH3coupling path on Pd3/GDY.When x=1-3,the final main product is DMC.Although the activation energy barrier to DMO is high on Pd4/GDY,the DMO is still the main product.From Fig.7,it can be found that the charge density differences of Pd4/GDY is obviously different from Pdx/GDY (x=1,2,3),and the charge density of Pd4is significantly lower than that of Pdx(x=1,2,3),which seems to be the reason for the change in catalyst selectivity.
3.5.3.Effect of doping Cu on the formation of DMO on Pdx/GDY (x=1,2,3,4)
The introduction of the second metal Cu aims to regulate the catalytic performance of Pdx/GDY(x=1,2,3,4),based on the above research,we find that adding Cu to Pd2/GDY don't weaken the catalytic activity for CO oxidation coupling reaction,the activation energy barrier of the speed determination step is reduced from 114.4 kJ mol-1on Pd2/GDY to 105.2 kJ mol-1on Pd1Cu1/GDY (Fig.8).In addition,the addition of Cu significantly reduces the activation energies required for DMO formation on Pd3/GDY and Pd4/GDY with poor CO oxidation coupling reaction activity,it goes down from 126.6 kJ mol-1on Pd3/GDY to 99.2 and 115.2 kJ mol-1on Pd1Cu2/GDY and Pd2Cu1/GDY which will show better activity compared with Pd(111) [9].And on Pd4/GDY,the activation energy required to generate DMO is 158.1 kJ mol-1,also reduces to 149.9,134.2 and 129.9 kJ mol-1on Pd1Cu3/GDY,Pd2Cu2/GDY and Pd3Cu1/GDY.
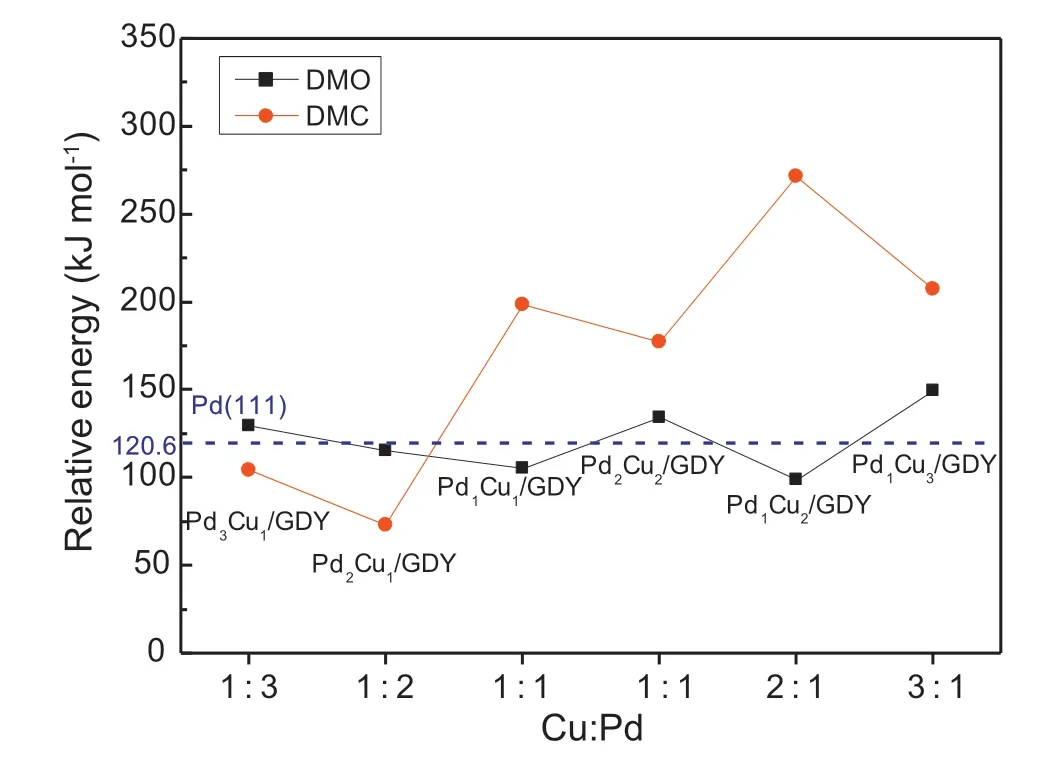
Fig.9.The activation energies barriers required for the generation of DMO and DMC on PdxCuy/GDY (x+y ≤4) with different Cu:Pd ratios.
Similar to the previous calculation process,considering all possible paths to generate DMO,the results show that DMO is generated through the CO-COOCH3coupling path on Pd1Cu1/GDY,Pd2Cu1/GDY and Pd1Cu3/GDY,and through COOCH3-COOCH3coupling path on Pd1Cu2/GDY,Pd2Cu2/GDY and Pd3Cu1/GDY.We find that the more the number of doped Cu atoms,the more likely the optimal path for DMO generation will change,the optimal path to generate DMO on Pd1Cu1/GDY is different from Pd2/GDY,on Pd1Cu2/GDY is different from Pd3/GDY,and on Pd1Cu3/GDYis different from Pd4/GDY.Moreover,through the study of the influence of doping second metal Cu on the reaction selectivity,it can be found that the proportion of Pd:Cu on the catalyst is the key factor.There are Pd1Cu1/GDY,Pd2Cu2/GDY,Pd1Cu2/GDY and Pd1Cu3/GDY exhibiting excellent selectivity for CO oxidative coupling to DMO(Fig.9.),as the proportion of Cu in PdxCuy/GDY(x+y ≤4)increases to 1:1 or more,the DMO becomes more favorable product for CO oxidative coupling reaction.The introduction of Cu changes the charge distribution of Pdx/GDY (x=1,2,3,4) (Fig.7.),the charge density of the metal clusters is significantly weakened after doping Cu atoms in Pdx/GDY(x=2,3),and the charge is redistributed on Pd1Cu3/GDY,Pd2Cu2/GDY and Pd3Cu1/GDY compared with Pd4/GDY.The changing of charge density of the metal clusters is also an important internal factor for the change of selectivity,when the charge density of metal clusters is reduced,it is more conducive to improving the selectivity of DMO.Compared with Pd(111)which needs 120.6 kJ mol-1to generate DMO [9],the activation energies required for the formation of DMO on Pd1Cu1/GDY and Pd1Cu2/GDY with 105.2 and 99.2 kJ mol-1are lower that on Pd (111).Considering the activity of CO oxidative coupling reaction and the selectivity to form DMO,Pd1Cu1/GDY and Pd1Cu2/GDY can be used for DMO generation.
4.Conclusions
The mechanism of CO oxidative coupling reaction on PdxCuy/GDY (x=1,2,3,4;x+y ≤4) is studied by DFT calculation,the influence of the Pdxcluster size on the catalytic performance and the regulation of the second metal doping on the catalyst for the DMO generation have been investigated.The results show that the size change of Pdxcluster will change the optimal path for DMO generation,and the DMO prefers to be formed by COOCH3-COOCH3coupling path on Pd1/GDY,Pd2/GDY and Pd4/GDY,while by CO-COOCH3coupling path on Pd3/GDY.The cluster size also influence the activity and selectivity,among which the catalytic activity is good but more conducive to generate byproduct DMC on Pd1/GDY and Pd2/GDY,and DMO generation on Pd3/GDY and Pd4/GDY is less active than Pd (111),Pd4/GDY shows better selectivity which may be caused by the decrease of charge density.The Pdx/GDY (x=1,2,3,4)cannot guarantee both good activity and selectivity for the formation of DMO,so introduce the second metal Cu to regulate the catalytic performance.
After the introduction of the second metal Cu,the PdCu bimetal clusters can still stably exist on the GDY,but the charge density of metal clusters are changed.The activation energy barriers of rate-determining steps for CO oxidative coupling reaction are lower compared with before the substitution,which can maintain or even improve the catalytic activity.Moreover,the selectivity of DMO can be adjusted by controlling the Cu:Pd ratio of catalyst,when the ratio of Cu:Pd in the catalyst is smaller than 1:1,it tends to generate the by-product DMC.After comprehensive consideration,Pd1Cu1/GDY and Pd1Cu2/GDY not only have smaller DMO formation barrier compared with Pd (111),but also have considerable selectivity for DMO formation.
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgements
This work is financially supported by the Key projects of National Natural Science Foundation of China (No.21736007),the National Natural Science Foundation of China(Grant Nos.21576178 and 21476155),Research Project Supported by Shanxi Scholarship Council of China(No.2016-030).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.11.011.
杂志排行
Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
