High performance of TiO2/CuxO photoelectrodes for regenerative solar energy storage in a vanadium photoelectrochemical cell
2022-08-30HarinYooDoohwanLeeJungHyeunKim
Harin Yoo,Doohwan Lee,Jung Hyeun Kim
Department of Chemical Engineering,University of Seoul,163 Siripdaero,Dongdaemun-gu,Seoul,02504,South Korea
Abstract Photocatalysts for harvesting solar energy to either electricity or chemical fuels have attracted much attention recently,but they have big obstacles such as wide bandgaps and rapid charge recombinations to overcome for final applications.In this study,we investigates a useful method to utilize vanadium redox pairs,which are commonly applied for vanadium redox flow batteries,to diminish charge recombinations and thus to enhance photocurrent response in regenerative solar energy storage.The results reveal significant improvements in photocurrent density by forming cuprous and cupric oxides in TiO2/CuxO electrodes under solar AM 1.5 illuminations using the vanadium photoelectrochemical storage cell at 0.025 mol L-1 of vanadium redox species in the acid electrolytes.In addition,the stabilized photocurrent density of the copper content optimized TiO2/CuxO electrodes is almost tripled from the TiO2 only electrode because the charge recombinations can be mitigated with the content optimized TiO2/CuxO electrodes.Therefore,the optimized TiO2/CuxO electrode results in the highest charge storing performance in the catholyte chamber,and the roles of vanadium redox species are also clearly demonstrated.
Keywords: Photocatalyst;Photoelectrochemical;Copper oxide;Charge recombination;Redox pairs
1.Introduction
Harvesting natural sunlight energy has been a great way of substituting limited fossil fuels for the future sustainable energies because it is considered as clean,unlimited,and pollution free resources for human beings.Amongst the harvesting technologies such as solar cell,photocatalyst,solar thermal,and photoelectrochemical(PEC)methods,a PEC cell is considered as a practical means of transforming solar energy into electric energy or chemical fuels.Vanadium batteries are a groundbreaking research subject in terms of high safety,durability and high capacity [1].
For the general PEC cell,a semiconductor photoelectrode is used in converting photons to charge carriers which are then separated at the electrode-electrolyte interface under light illumination.In various photocatalytic energy conversion process,the separated charges can be used in various targeted applications such as hydrogen generation by water splitting[2-6] and charge storage in redox flow battery [7-12].These approaches offered the prospect of creating a clean and sustainable fuels from natural unlimited resources even with a low utilization efficiency.
In order to improve the overall efficiencies in harvesting solar energy using various photocatalytic materials,many attempts have been tried to increase photocatalytic hydrogen production by employing heterostructure components[13-15],metallic doping [16-18],sacrificial reagents [19-21],and etc.Amongst the various photocatalyst materials,TiO2has been considered as one of the most promising candidates for hydrogen productions.It has many advantageous properties such as strong photocatalytic activity,photocorrosion resistance,low cost,and non-toxicity comparing with other semiconductors [22-24].Nonetheless,its large bandgap(3.2 eV)and the fast recombination possibility are the inherent disadvantage for the practical applications in solar energy conversion.Therefore,many approaches such as non-metal doping[25,26],metal doping[27,28],z-scheme addition [29-31],and sensitization [32,33] were attempted.In addition,engineering the appropriate conduction band potentials is expected to facilitate the charge transfers for effective charge separations [34,35].
In this study,we propose to use TiO2/CuxO electrodes in conjunction with vanadium redox couples in a vanadium PEC cell for improving the photocurrent density in solar energy storing mechanisms.Copper was chosen as an additional component for the TiO2base materials because it is easy to synthesize on TiO2layer with cuprous and cupric oxide forms of narrow band gaps.The cuprous and cupric oxides form a Zscheme structure for highly efficient charge transfers under light illumination conditions.The vanadium redox couples are commonly employed in a vanadium redox-flow battery,a well utilized energy storage system for commercial applications.This vanadium PEC storage cell possesses various intrinsic advantages [36-38]: fast electrochemical kinetics,high charge-discharge efficiency,low cost,and low safety issues.The photoelectrochemical performances of the TiO2only and TiO2/CuxO electrodes were investigated under solar AM 1.5 illuminations.It was found that the TiO2/CuxO electrodes exhibit the highest photo-response at a certain amount of copper addition,and it is probably due to the facilitated charge transfers in the copper content optimized TiO2/CuxO electrodes as well as the fast reaction kinetics of the vanadium redox pairs.X-ray photoelectron spectroscopy and UV-visible spectroscopy were used to characterize the TiO2/CuxO electrodes after thermal sintering at 500°C for 30 min.
2.Experimental
2.1.Electrode preparation
In order to fabricate electrodes on fluorine doped tin oxide(FTO,resistance Pilkington USA,18-20 Ω) substrates,the FTO substrates were first cleaned by sonication with ethanol(Sigma-Aldrich,99.9%)and acetone (Sigma-Aldrich,99.9%).Then,the substrates were washed deionized(DI)water,before being blow-dried and then further dried in an oven at 60°C for 1 h.For the first step in manufacturing bilayer electrodes,the TiO2only films were prepared on FTO substrates by a doctor blade method.The paste including 4.3 g of α-terpineol (laboratory grade,Fisher Scientific USA) and 1.0 g Degussa P25 TiO2(VP AEROPERL®by Evonik)was mixed under constant stirring at 60°C for 1 h to obtain a uniform TiO2.Then,the paste was deposited on the pre-washed FTO glasses by a doctor blade method.The electrodes were thermally treated in a furnace at 500°C for 1 h.The second layer was spin-coated with a solution including 0.54 g copper acetate (Sigma-Aldrich,99%),20 mL α-terpineol,and 20 mL diethanolamine(Sigma-Aldrich,99%).The number of coatings was varied up to 4 times to fabricate samples including different weight percent of the second layer.The weight percent was measured as 1.9,4.0,5.6,and 6.4 wt%,and these samples were marked as TC1.9,TC4.0,TC5.6,and TC6.4,respectively.Finally,the bilayer electrodes were thermally sintered in a furnace at 500°C for 30 min to form TiO2/CuxO films,and the active electrode area was 1 cm2.The rest of the area on the FTO was covered with epoxy resin to prevent possible side reactions between the electrolytes and the FTO surfaces under applied voltages.The SEM images of the TiO2only and TiO2/CuxO electrodes surfaces,cross-sectional view and elemental distributions of the TC5.6 sample are shown in Fig.S1 (supplementary material).In addition,the crystalline structures of the samples were analyzed using X-ray diffraction patterns in Fig.S2,but diffraction peaks related to the copper oxides were hardly detected due to the relatively small amounts of copper precursor used in fabrications of TiO2/CuxO electrodes.
2.2.Electrolytes
Two types of electrolytes,0.025 mol L-1vanadium(IV,VO2+)in 3 mol L-1H2SO4,and 0.025 mol L-1vanadium(III,V3+) in 3 mol L-1H2SO4were used in the experiments.The first electrolytes were synthesized by dissolving H2SO4(95%,Samchun USA)in DI water with vanadium(IV)sulfate oxide hydrate (VOSO4·xH2O) (97%,Merck USA).The prepared vanadium (IV)-sulfuric acid electrolyte solution appeared blue.The 0.025 mol L-1vanadium (III) electrolyte was obtained by electrochemically reducing the prepared vanadium (IV) H2SO4solution in an electrochemical cell at a constant current density of 30 mA cm-2using a battery power supply (WBCS3000M1L,WonAtech).N2gas was purged to prevent air oxidation of vanadium (III) species during the reduction.The final concentration and oxidation state of the vanadium species in the electrolytes were confirmed by UVVis spectroscopy measurement.The obtained vanadium (III)-sulfuric acid solution appeared green color.In order to give a better understanding for our experimental system,real photograph of the photoelectrochemical cell with vanadium redox pairs was taken and shown in Fig.S3 (supplementary material).
2.3.Characterization and PEC measurements
Surface analyses of the TiO2/CuxO films were conducted using an X-ray photoelectron spectroscopy (XPS,PHI-Quantera-II,Ulvac-PHI) with monochromatic Al Kα radiation(1486.6 eV) to analyze the binding energies of the samples.Carbon contamination component located at the binding energy of 284.5 eV (C 1s peak) was used as a reference peak.The PEC properties of the electrode system were examined in a three-electrode electrochemical cell.In the system,the bilayer photoelectrode was used as the working electrode(WE),and a platinum mesh and Ag/AgCl electrode were served as the counter electrode (CE) and the reference electrodes (RE),respectively.Linear sweep voltammetry (LSV)was conducted using a potentiostat (Iviumstat,A07079) in a full-cell configuration (two chambers filled with VO2+and V3+electrolytes,separated by a Nafion 117 membrane),and Fig.1 shows a schematic illustration of the cell system for better understanding.The voltage scan ranged from -0.3 to+0.3 V with 0.01 V interval of dark and light illumination.Chronoamperometry was performed at +0.3 V where a high current-to-voltage response was observed in the LSV data.Light was irradiated for 5 min and then turned off for another 5 min,and the whole experiment was performed for 50 min.The charge-discharge cycle tests were performed at fixed current conditions (charging: +1 mA cm-2,discharging:-1 mA cm-2).The light illumination conditions were the same as for the chronoamperometry.The electrochemical impedance spectroscopy was also conducted using the potentiostat at 0.01 V amplitude between 0.01 Hz and 106Hz.A solar simulator (PED-L11,Peccel technologies,150 W Xe lamp with an AM 1.5G filter) was used to irradiate the standard solar power (1 kW m-2) by mimicking the natural sunlight.The standard light intensity was calibrated by adjusting the photocurrent of a standard solar cell(PEC cell,BS-520)to ensure the standard solar condition.
3.Result and discussion
3.1.XPS analysis
Surface analyses of the TiO2/CuxO films thermally sintered at 500°C for 30 min using a bilayered TC6.4 sample were performed by XPS measurements,and Fig.2 shows the surface atomic states.Fig.2a shows the survey scan including several peaks for elemental titanium,copper,and oxygen atoms,and their expanded scans are shown (Fig.2b-d).Fig.2b shows the Ti 2p3/2and 2p1/2states at 458.3 eV and 464.3 eV with a spin orbit separation of 6.0 eV assigned to Ti 2p of TiO2,as reported elsewhere [39,40].The Cu 2p peak shown in Fig.2c can be deconvoluted into three binding energy peaks(933.7 eV,934.1 eV,and 935.1 eV)which represent the lattice copper and their binding states with oxygen[29,41].The oxygen bonds were dominantly formed as cuprous and cupric oxide phases in the photocatalysts.Fig.2d shows the asymmetric O 1s peak deconvoluted into several binding energy peaks.The strongest peak at 528.9 eV corresponds to the lattice oxygen bonded to Ti atoms in TiO2[42],and the other peaks are assigned to oxygens bonded to copper atoms.Therefore,XPS analysis clearly demonstrated that cuprous and cupric oxides were introduced in the TiO2/CuxO composite films,and the effects of the oxidized phases in the TiO2/CuxO films can be further examined on their PEC performances.

Fig.1.Schematic illustration of the photoelectrochemical cell with vanadium redox pairs for energy storage mechanism.
3.2.UV-Vis spectroscopy
UV-Vis diffuse reflectance spectroscopy results are shown in Fig.3a for the TiO2only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples(TC1.9,TC4.0,TC5.6,and TC6.4) under light illuminations.For the TiO2only electrode,the absorption edge lies in near 390 nm as known to absorb mostly UV light [43].Compared to the TiO2only electrode,the TiO2/CuxO electrodes demonstrate improved light absorption tail by increasing the CuxO contents through visible wavelength regions,which is mainly attributed to the smaller band gaps [29,44]: 1.7eV of CuO and 2.2 eV of Cu2O introduced during the thermal sintering step.However,the absorption edge remains almost in the same wavelength region because the TiO2material was occupied as a major component in the TiO2/CuxO electrodes.In Fig.3b,all the band gaps of the TiO2/CuxO samples were calculated using Tauc plots,and it showed smaller band gaps with increasing copper oxide contents due to the smaller band gaps of the CuO and Cu2O components.
3.3.PEC performance evaluation
To check the photocurrent responses of the TiO2only and TiO2/CuxO electrodes under the light illuminations,LSV was conducted for five electrode samples in a full-cell with the electrolytes of the vanadium redox pairs dissolved in 3 mol L-1H2SO4.Fig.4 shows the current density results of the several electrodes between -0.3 V and +0.3 V at a scan rate of 10 mV s-1.The photocurrent response from the TiO2only electrode revealed slight increments under light illumination.However,the TiO2/CuxO electrodes exhibited much higher improvements in photocurrent responses at higher voltages,and it is clearly related to the faster reaction rates in the following reactions:
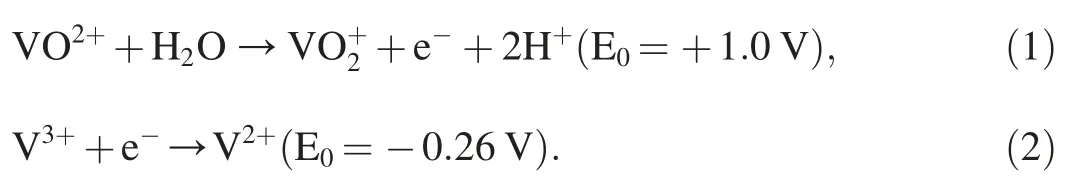

Fig.2.XPS spectra of the TiO2/CuxO electrode thermally sintered at 500°C for 30 min using a bilayered TC6.4 sample:survey scan(a),Ti 2p(b),Cu 2p(c),and O 1s (d).
Among the various electrodes,the electrode made from the TC5.6 sample showed the highest improvement in photocurrent density with a drastic increase at high voltages under light illumination.In terms the optimized photocurrent density from the electrode made the TC5.6 sample,it could be strongly related to the relative amounts of cupric and cuprous crystal phases.As Yoo et al.reported [29],the heterostructure between Cu2O and CuO crystals plays a significant role in preventing charge recombination through a direct Z-scheme system.In their study,the electrons excited from the CuO component can recombine with the remaining holes in Cu2O VB.Thus,the excited electrons in Cu2O CB can be efficiently transferred without recombination.Thus,the fraction of the Cu2O crystals in CuxO phases is critical to achieve the most efficient Z-scheme process,and it is believed that the electrode made from the TC5.6 sample would have the highest Cu2O fraction in CuxO phases.Unfortunately,it was hard to quantitatively confirm that the relative amount of Cu2O and CuO crystal states of our samples using XRD measurements due to the small amounts of copper used in manufacturing the TiO2/CuxO electrodes.Nonetheless,this improved PEC response is a clear evidence of retarded charge recombinations and enhanced PEC reaction rates.The TiO2has a more positive valance band (VB) than CuxO phases,and thus the photogenerated holes likely migrate to the VB of CuxO and then the photoelectrode surface under light illumination.To the contrary,photo-excited electrons prefer to migrate from the conduction band (CB) of CuxO phases to that of TiO2and to the anode because of the more negative CB potential of the TiO2.Therefore,photo-generated charges would be well separated rather than recombined for highly enhanced photocurrent density.
The variations of the photocurrent density were measured from the TiO2only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples (TC1.9,TC4.0,TC5.6,and TC6.4) under light on &off conditions at+0.3 V applied voltage,as shown in Fig.5.Generally,the storage of photo-generated electrons in TiO2CB (and migrated from CuxO CB) is achievable in the catholyte chamber (shown in Fig.1) during the UV-Vis light illumination at fixed external bias.In Fig.5a,initial fast redox reactions using the vanadium redox couples are capable of enhancing photo-electrochemical performance by quickly scavenging holes at the interfaces between electrodes and electrolyte and thus retarding charge recombinations [37,45].After the initial stabilizing period about a few tens of seconds,the photocurrent density revealed slight increasing tendency during light illumination periods probably because the photoexcited electrons transferred to cathode were efficiently used for V3+reduction,as shown in Eq.(2).In addition,the highest photocurrent density was also observed from the TiO2/CuxO electrodes thermally sintered using the bilayered TC5.6 samples,as demonstrated in Fig.4 with the LSV results.Fig.5b reveals the variations of stabilized photocurrent density of the TiO2only and TiO2/CuxO electrodes for five repeated runs(5 min light irradiations: on &off).The photocurrent density showed almost the same levels for the repeated runs during the light irradiation periods.However,it decreased steadily during light off periods without reaching to 0 mA cm-2completely.This residual current,commonly called non-Faradaic current[46],is possibly caused by the relatively thick TiO2layers where the photo-excited electrons during light irradiations remained alive to flow in the vanadium photoelectrochemical cell under dark conditions.
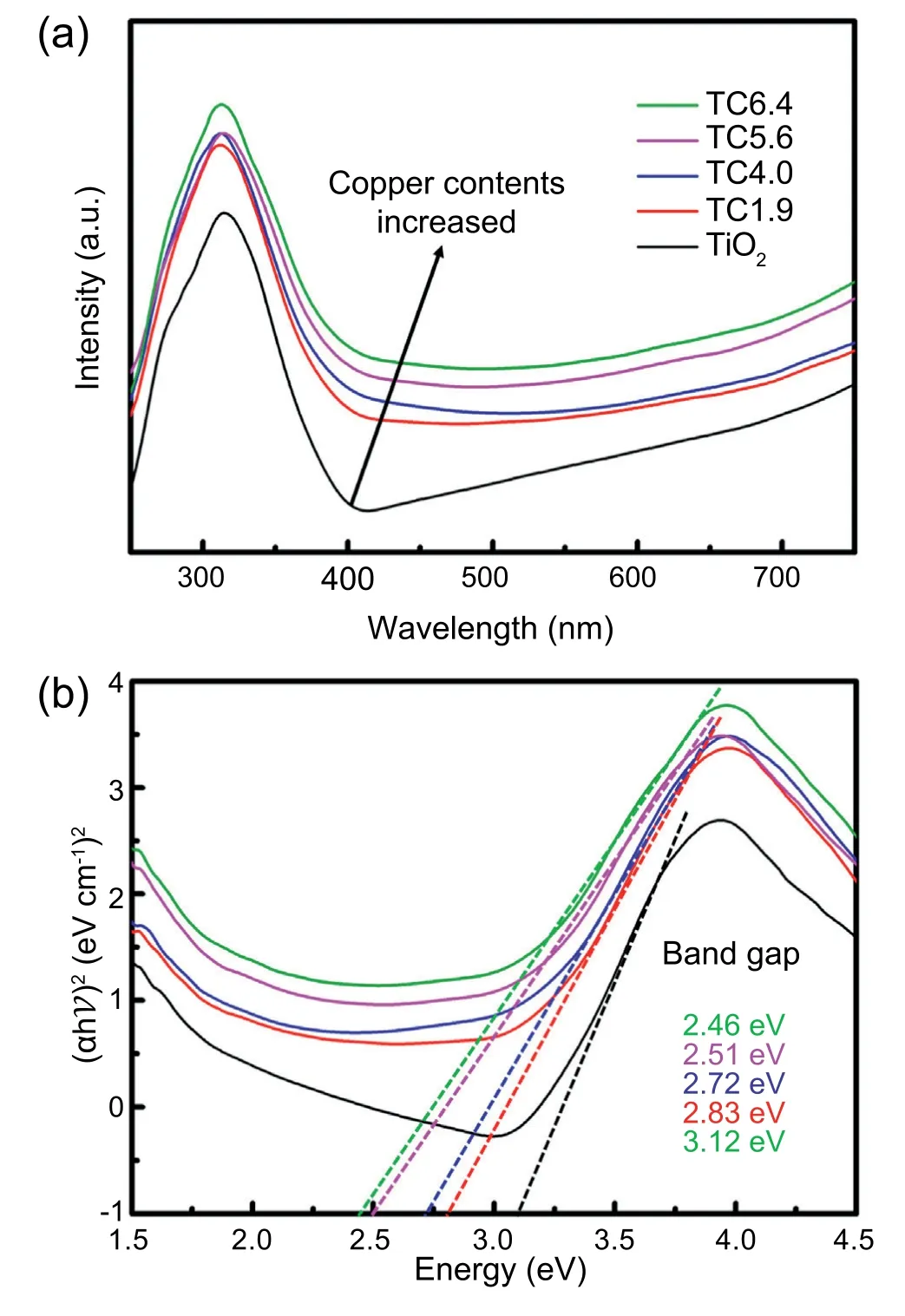
Fig.3.UV-Vis diffuse reflectance spectra (a) and Tauc plots (b) of the TiO2 only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples (TC1.9,TC4.0,TC5.6,and TC6.4) under light illuminations.
Fig.6 shows the potential variations during the chargedischarge cycle tests to check the cell stability under the light on &off conditions with the TiO2/CuxO electrode thermally sintered at 500°C for 30 min using the TC5.6 bilayered sample.For charging and discharging tests,+1 mA cm-2and-1 mA cm-2were applied for constant current conditions.During the charging periods,the potential showed a clear difference between the light on and off conditions because the number of the photo-generated charges in the TiO2/CuxO electrode were much higher under the light on condition than the light off case,and the potential revealed stable generations for 5 repeated runs.However,the potential reached the lowest value (green line) with the light on for charging and off for discharging conditions because much efficient discharging condition could be achieved under light off condition with no photocurrent disturbance.To the contrary,the potential revealed the highest level with the light on situation for charging and discharging because the discharging potential is hindered by the charge carriers generated from the photoelectrode under light irradiation.In case of light off condition for both charging and discharging,the potential showed intermediate level between (always light on) and (light on for charging and off for discharging)due to no additional charges stored in catholyte chamber and due to no hindrance by photogenerated electrons.In addition,Fig.S4 (supplementary material) shows UV-Vis spectroscopy results for the changes of the valences of vanadium ions before and after charging and discharging tests in anolyte and catholyte chambers,respectively.Charging and discharging steps clearly influence on the valence states of the vanadium ions in anolyte and catholyte chambers due to the ionic recombinations of vanadium ions with photo-generated electrons and holes.In anolyte chamber,VO2+is dominant ions before charging,but later it changes intoafter charging due to absorbing holes.Instead,in catholyte chamber,V3+ions are dominant before charging,and they are also changed to V2+by combining with electrons.Therefore,characteristic peak intensities from those ion states were clearly varied by charging and discharging processes.

Fig.4.Linear sweep voltametry of the TiO2 only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples (TC1.9,TC4.0,TC5.6,and TC6.4) under light illuminations.
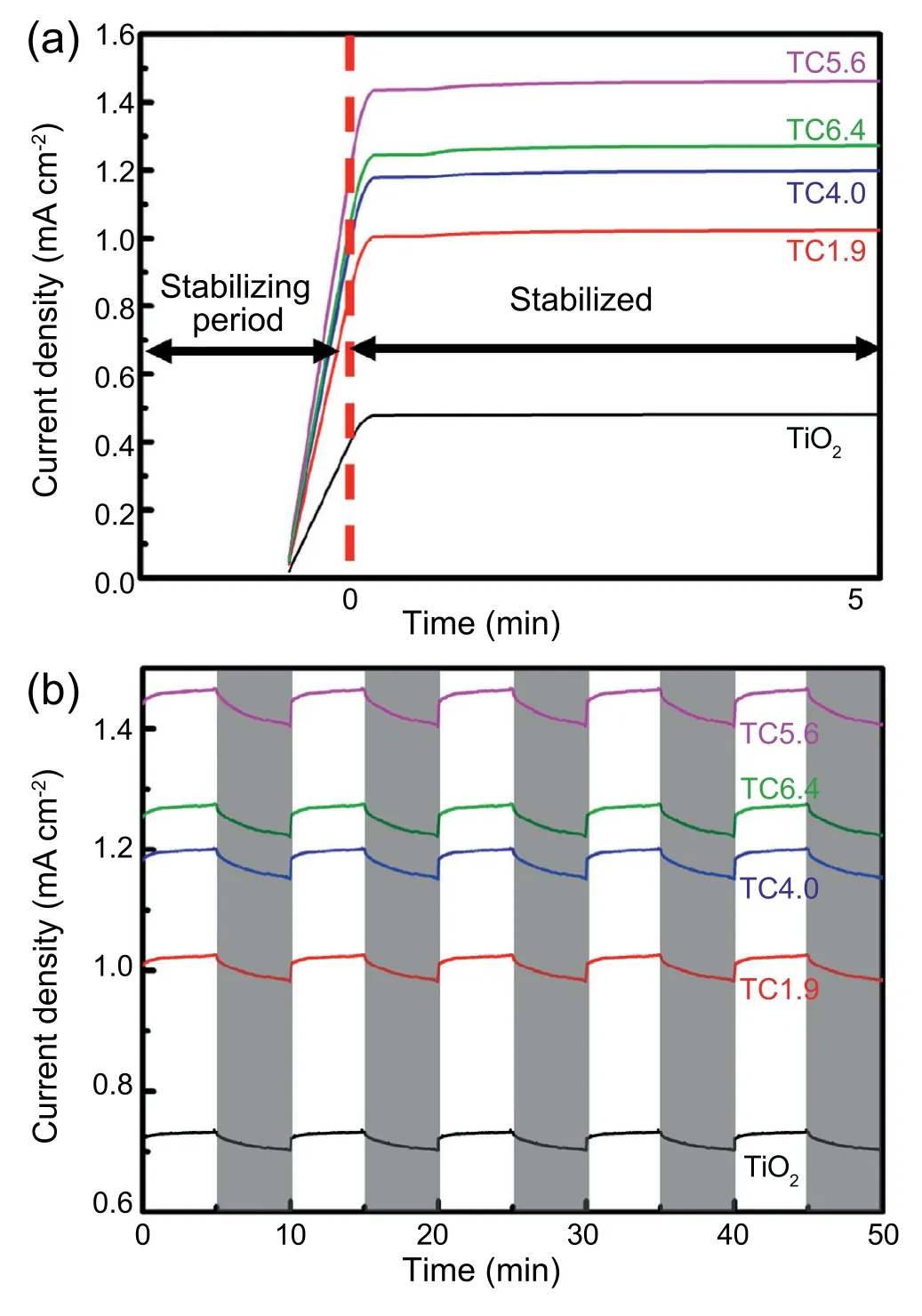
Fig.5.Changes of photocurrent density of the TiO2 only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples(TC1.9,TC4.0,TC5.6,and TC6.4)under light illuminations at+0.3 Vapplied voltage: (a) including initial stabilizing periods,(b) after stabilized.Dark regions represent changes of current density during no light illuminations.
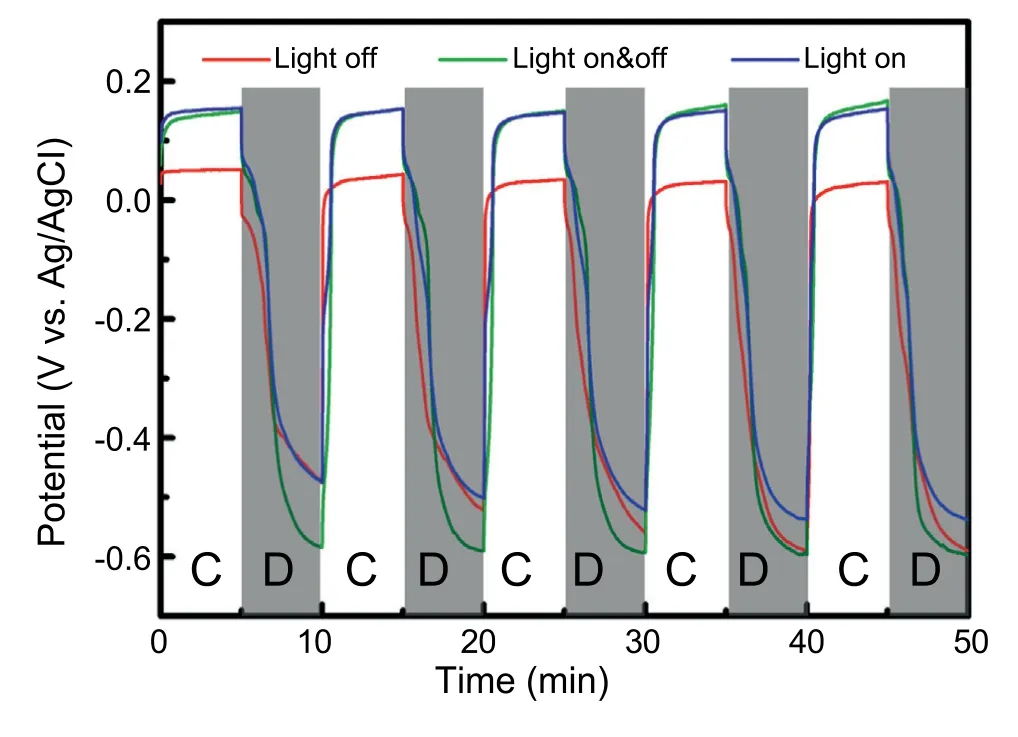
Fig.6.Potential variations during the charge &discharge cycle tests of the TiO2/CuxO electrode thermally sintered at 500°C for 30 min using the TC5.6 bilayered sample to check cell stability under light on &off conditions.Measurements were conducted under constant current conditions of+1 mA cm-2&-1 mA cm-2 for charging&discharging,respectively.C and D mean charging and discharging periods.
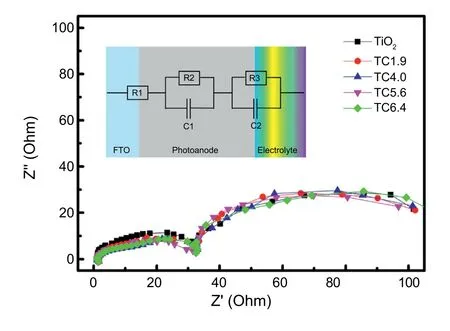
Fig.7.Impedance measurements of the TiO2 only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples (TC1.9,TC4.0,TC5.6,and TC6.4) under light illuminations.
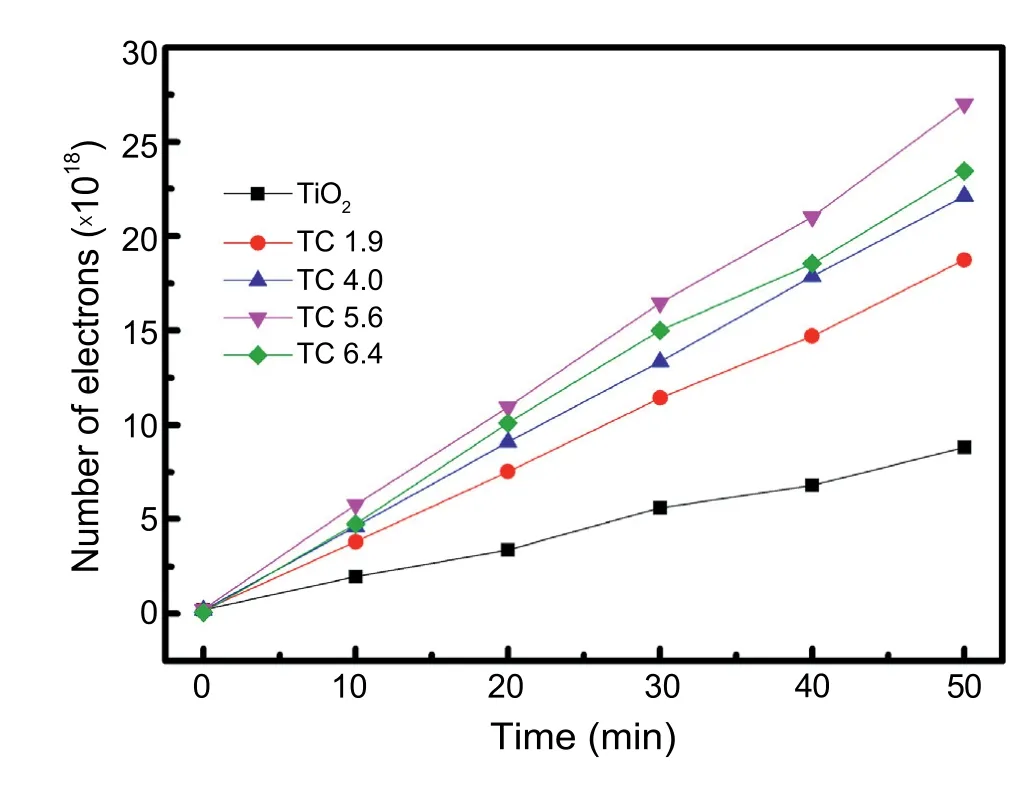
Fig.8.Number of electrons stored in catholyte chamber from the TiO2 only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples (TC1.9,TC4.0,TC5.6,and TC6.4) under light illuminations.
The Nyquist plots were obtained from the impedance measurements of the TiO2only and TiO2/CuxO electrodes thermally sintered at 500°C for 30 min using bilayered samples (TC1.9,TC4.0,TC5.6,and TC6.4) under light illuminations,and they are demonstrated in Fig.7 with equivalent circuits for the photoanodes.The Nyquist plots consist of two distinct semicircles.The smaller semicircle corresponds to the charge transfer resistance at the interfaces (R3) between electrolyte and the photo-electrode in the anolyte chamber,and the larger one represents the resistance(R2)against charge transports inside the photocatalyst layers [47,48].The R1 can be attributed to the resistance from the FTO substrate itself.In all the electrode samples,the relative amounts of CuxO components were very small,and thus the larger semicircles showed negligible differences between samples.It is possibly attributed to the similar charge transfer resistances through the electrode layers regardless of the amounts of copper oxide components.However,the smaller semicircle showed a little difference in Z′′values between the TiO2only and TiO2/CuxO electrodes,and it is believed that the TiO2/CuxO electrodes have lower charge transfer resistance at the interfaces compared with the TiO2only case.This reduced resistance at the surfaces of the TiO2/CuxO electrodes is probably due to the increments of electron diffusion possibility in the surficial cuprous and cupric oxides.In addition,the smallest semicircle with the lowest resistance was observed from the TC5.6 electrode,and it coincides with the highest photocurrent density observed from the TC5.6 electrode in LSV results(shown in Fig.4).
Finally,Fig.8 shows the number of electrons stored in the catholyte chamber for the TiO2only and TiO2/CuxO electrodes at +0.3 V applied voltage under the light irradiation conditions.The number of stored electrons were calculated based on the stabilized photocurrent densities as shown in Fig.5.As expected,the photo-electrode made from the TC5.6 sample revealed the highest number of electrons stored in the catholyte chamber under light illuminations.It also demonstrated that the optimized composition for the enhanced charge generations from the TiO2/CuxO electrodes was achieved from the bilayered TC5.6 samples.As a result,this improved number of electrons is beneficial for storing charges in catholyte chamber as V2+species by absorbing the transported electrons,as shown in Eq.(2).
4.Conclusions
A vanadium photoelectrochemical storage cell has been investigated by applying additional copper oxide layers in fabricating TiO2/CuxO electrodes as photoanode materials.The photocurrent density has been significantly enhanced in the vanadium redox pairs especially in the copper content optimized TiO2/CuxO electrodes.It is believed that the vanadium redox pairs play a crucial role in the regenerative solar energy storage due to their fast redox reaction kinetics.The hybrid TiO2/CuxO electrodes exhibited higher photocurrent response than the TiO2only electrode,and this improvement is attributed to the highly facilitated electron transfers in the conduction bands from cuprous oxide to TiO2due to their band potential energies.It was also found that an optimum content of cuprous oxide in the hybrid TiO2/CuxO electrodes could be a key to enhance charge transfers by suppressing charge recombinations.More importantly,the copper content optimized TiO2/CuxO electrode was demonstrated to play a key role in an effective electron storage in a vanadium photoelectrochemical storage cell under sunlight irradiation.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Science and ICT (NRF-2018R1D1A1A09082239).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.11.012.
杂志排行
Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
