Increasing the greenness of an organic acid through deep eutectic solvation and further polymerisation
2022-08-30LitengLiXiofngLiSusuZhngHongyunYnXioqingQioHongynHeToZhuBokunTng
Liteng Li,Xiofng Li,Susu Zhng,Hongyun Yn,Xioqing Qio,Hongyn He,To Zhu,,Bokun Tng,
a College of Pharmaceutical Science,Institute of Life Science and Green Development,Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education,Hebei University,Baoding,071002,China
b CAS Key Laboratory of Green Process and Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing,010190,China
Abstract Acrylic acid(AA)is an important and widely used industrial chemical,but its high toxicity renders its use incompatible with the concept of green development.By leveraging its terminal carboxyl group and unsaturated bond,we designed and explored a new strategy to increase the greenness of AA via its eutectic melting using a quaternary ammonium salt(choline chloride)to form a deep eutectic solvent(DES),followed by polymerisation of the DES to form a polymer(poly(DES)).The greenness of AA,DES,and poly(DES)was evaluated via an in vitro test using MGC80-3 cells and an in vivo test using Kunming mice.The toxicity improved from Grade 2 (moderately toxic) for AA to Grade 1 (slightly toxic) for DESs and Grade 0 (non-toxic) for poly(DES) in the in vitro test.Moreover,the poly(DES)s showed a lower toxicity in mice than the DESs in the in vivo test.Thus,greenness enhancement was successfully achieved,with the greenness following the order AA <DES <poly(DES).Furthermore,the mechanisms underlying the change in toxicity were explored through microscopy and flow cytometry,which revealed that the DES can permeate the MGC80-3 cell membrane during the G0/G1 phase to adversely affect DNA synthesis in the S phase,but the poly(DES) cannot.Finally,the green poly(DES),which showed good adsorption properties and flexible functionality,was successfully applied as a carrier or excipient of drugs.Through the novel strategy reported herein,greenness enhancement and the broadening of the application scope of a toxic organic acid were achieved,making such acids applicable for green development.
Keywords: Greenness;Deep eutectic solvent;Polymer;Toxicity;Application
1.Introduction
Acrylic acid (AA) is used both directly as a raw industrial chemical in many fields,including paints,fibres,adhesives,and oil additives and indirectly as a synthetic monomer for building,packaging,coating,spinning,and other purposes[1,2].However,the use of AA has serious effects on the ecological environment because of its high toxicity to various biological systems [3].To mitigate these effects in terms of decreasing AA-induced pollution,various post-treatment methods,such as incineration,biological processing [4],wet air oxidation [5],and supercritical oxidation [6],have been developed and adopted by organisations and researchers;these methods follow the typical approach of ‘treatment after pollution’.However,green chemistry promotes the use of‘chemical products and processes that reduce or eliminate the use and generation of hazardous substances’ [7];the aforementioned post-treatment methods fail to meet the requirements of green synthesis.Thus,the development of a strategy that takes the reverse approach,i.e.,a strategy that enhances the greenness of AA prior to its application,is necessitated.
The key problem associated with the development of such a strategy is determining how the greenness of AA can be enhanced.To this end,we analysed the inherent characteristics of AA and utilised recently developed green-chemistry-based approaches to develop a novel greennessenhancement strategy[8].AA is an organic unsaturated fatty acid that has a terminal carboxyl group and an unsaturated double bond.These functional properties allow it to serve as the hydrogen bond donor (HBD) component of a deep eutectic solvent (DES) as well as a monomer for polymerisation.Therefore,benefitting from these dual roles played by AA,our proposed strategy aimed to enhance the greenness of AA.
DESs were first synthesised and named by Abbot et al.,in 2003 and have since been explored to form a new class of solvents consisting of a quaternary salt as a hydrogen bond acceptor (HBA) and an organic amine/alcohol/acid as an HBD [9,10].DESs comprise hydrogen bond networks between HBAs and HBDs and have a low melting point,low volatility,high thermal stability,and other excellent physicochemical properties.Their properties are similar to those of ionic liquids(ILs);thus,DESs have been referred to as a new type of ILs or as analogues of ILs[11].Because ILs have been popularly labelled as green solvents by many researchers[12],DESs,as a type of ILs,are now being referred to as green solvents [13].In addition,choline chloride (ChCl),a highly popular HBA for DESs [14],is a member of the vitamin B family and exhibits biodegradability and non-toxicity [15].Additionally,most HBDs originate from natural products such as edible sugars [16],acids [17],and polyols [18].Therefore,DESs are referred to as natural DESs by some researchers[19].The use of DESs as a new type of solvents is highly compatible with the concept of green development.Therefore,utilising AA as an HBD in a DES is an efficient method for improving its greenness,and thus,has been explored in this work.
Furthermore,polymerisation is an efficient method to enhance the greenness of some organic compounds and can also impart new properties or functionalities to them,thus facilitating their large-scale applications [20,21].Poly(IL)s have been successfully prepared and applied by many researchers,including Roggers et al.[22],Han et al.[23],Zhang et al.[24],and Yan et al.[25],among others.Several poly(-DES)s have been prepared by our group and Wang's group in recent years[26-28].Thus,to further enhance the greenness of AA,AA-based DESs could be further polymerised via the terminal double bond of AA through free radical polymerisation.The proposed strategy involves the transformation of AA to DESs and poly(DES)s in the liquid and solid phases,respectively,and evaluation of their greenness by determining their effects on biological systems.
ILs are popularly referred to as green solvents because of their selected physicochemical properties [29].However,the greenness of ILs in terms of biocompatibility,biotoxicity,and biodegradability in organisms has not been widely investigated and has been questioned by some researchers[30,31].In particular,reports related to the greenness of DESs and poly(DES)s as new IL analogues are scarce.According to green chemistry,greenness is defined on the basis of the toxicity or hazardousness of a product.Thus,in this work,we have developed a new evaluation or investigation system to determine the toxicity of the AA-based DESs or poly(DES)s in organisms by using cells and animals for in vitro and in vivo experiments,respectively.In the proposed system,the effects of the AA-based DESs and poly(DES)s on cell viability,morphology,and apoptosis ratio and the cell cycle were explored in vitro,and the acute toxicity of such DESs in mice was tested in vivo.The results of the tests conducted in this study reveal the reason for the decreased toxicity of the AA-based DESs and poly(DES)s relative to that of AA.Furthermore,the proposed strategy facilitates the expansion of the functional application scope of the AAbased DESs.
In this study,we have not only proposed a new strategy to enhance the greenness of AA but also developed a novel evaluation system to verify the reliability of the strategy and to broaden the functional application scope of AA.
2.Materials and methods
2.1.Materials and reagents
AA (99%,wt%) and ethylene glycol dimethacrylate(EGDMA,98%,wt%) were purchased from Aladdin Industrial Corporation (Shanghai,China).Benzoyl peroxide(BPO,99%,wt%) was supplied by Shanghai Macklin Biochemical Company,Limited (Shanghai,China).ChCl(98%,wt%) was acquired from Tianjin Reagent Factory(Tianjin,China).N,N-Dimethylaniline (DMA,analytical reagent) and acetonitrile (HPLC reagent) were obtained from Tianjin Kemiou Chemical Reagent Company,Limited(Tianjin,China).L-Asparaginase (L-ASP,biotech reagent)was supplied by Changzhou Qianhong Bio-Pharma Company,Limited (Changzhou,China).The acridine orange/ethidium bromide double stain kit,3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),penicillinstreptomycin mixture,Dulbecco's modified Eagle medium,0.01 mol L-1of phosphate belanced solution (PBS,pH 7.2-7.4),annexin V-fluorescein isothiocyanate/propidium iodide apoptosis detection kit,trypsin digestion solutions(0.25%,wt%),dimethyl sulfoxide (99.9%,wt%),and foetal bovine serum were purchased from Beijing Solarbio Science and Technology Company,Limited (Beijing,China).Doxorubicin (DOX,98%,wt%) was sourced from Meryer(Shanghai) Chemical Technology Company,Limited(Shanghai,China).Human breast cancer cells (MCF-7) and human gastric cancer cells(MGC80-3)were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai,China).Kunming mice were purchased from Beijing Vital River Laboratory Animal Technology Co.,Ltd.(Beijing,China).Cornstalk was collected from local farms in Baoding,China.
2.2.Preparation and characterisation
2.2.1.DESs
Mixtures of different molar ratios of AA and ChCl were heated to 95°C for 30 min with constant stirring until the AAbased DESs were formed as a homogenous liquid (Table 1).The ChCl-AA mixtures were characterised via differential scanning calorimetry (DSC,NETZSCH,Germany) (Fig.S1).The structures of ChCl,AA,and their complex,which represented the DES,were optimised using the Gaussian 09 software package at the B3LYP/6-311G(d,p) level of theory,and their optimised geometries were confirmed to be local minima or global minima by the absence of imaginary vibrational frequencies.The single-conformation two-stage restraint electrostatic potential (RESP) method was used to obtain the atomic charges by fitting the electrostatic potentials calculated at the same level.The interaction energy between ChCl and AA was refined at the B3LYP/6-311++G(d,p)level.Molecular dynamics simulations were carried out for DESs containing 500 ChCl pairs and 1500 AA molecules using Gromacs 5.1.1.For [CH]+,Cl-,and AA,the reported OPLS-AA force fields were used in our simulation.The initial simulation box was prepared using PACKMOL in a periodic boundary condition (PBC) box,and the energy minimisation was performed using the steepest descent method to avoid the possible coordinate collision of the configurations.Subsequently,the system was equilibrated for 2 ns under the NPT ensemble at 227°C via Velocity Rescaling,followed by a 4-ns simulation under the NPT ensemble at 25°C and 1 bar using the Parrinello-Rahman algorithm.The production simulation was then continued under the NPT ensemble for an additional 20 ns to collect the data for analysing the structures.For the calculation of long-range electrostatic interactions,particlemesh Ewald summation was used,and the cutoff radius for electrostatic and van der Waals interactions was 1.2 nm.All covalent bonds containing hydrogen were fixed using the LINCS algorithm.Radial distribution function and spatial distribution function calculations were performed based on the trajectories of the molecules during the final 10 ns
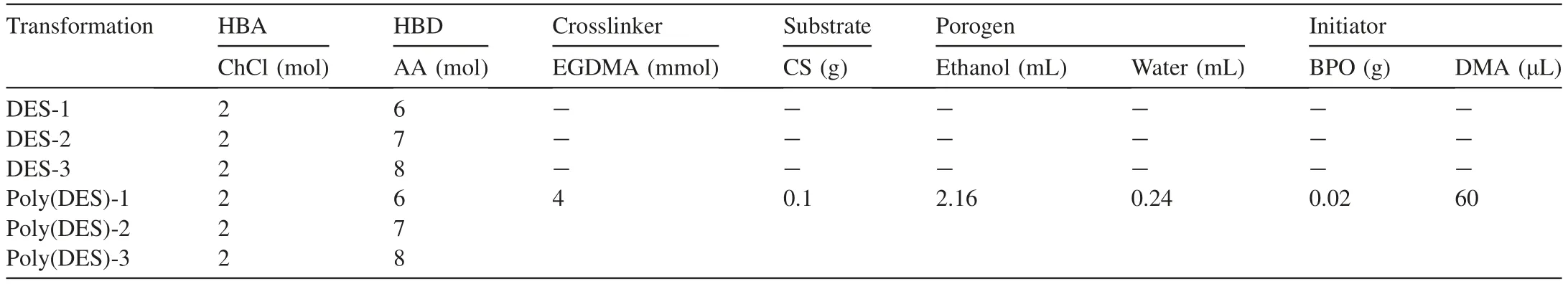
Table 1 The transformed DESs and poly(DES)s in this work.
2.2.2.Poly(DES)s
An AA-based DES (monomer),EGDMA (crosslinker),ethanol-water mixture (9:1,v/v,porogen),and BPO (half of the initiator) were mixed in a vial and subjected to ultrasonication for 5 min to completely dissolve them.Subsequently,corn stalk powder (substrate) was added to the above solution,and ultrasonication was continued for 15 min.Next,the other half of the initiator,DMA,was added,and the mixture was stirred at room temperature for 30 s using a vortex shaker.After being allowed to stand for 30 min,the product was washed several times with methanol and deionised water,dried at 60°C,and ground into powder for further use.
The AA-based poly(DES)s were characterised via field emission scanning electron microscopy(FE-SEM,JSM-7500,Japanese Electronics,Japan),Fourier transform infrared spectroscopy (FT-IR,Vertex 80 V,Bruker,USA),Brunauer-Emmett-Teller surface area and porosity analysis (BET,TRISTAR II3020,Micromeritic,USA),thermal gravimetric analysis (TGA,NETZSCH,Germany).
2.3.Toxicity assay
2.3.1.In Vitro/In vivo tests
The MGC80-3 cell line was selected to evaluate the toxicity of the AA-based DESs and poly(DES)s.The cells were cultured at 37°C in Dulbecco's modified Eagle medium with 10% (wt%) foetal bovine serum and 20 μL of penicillinstreptomycin in a humidified atmosphere containing 5% (vol%) CO2.Once the cell growth reached 90%,the cells were trypsinised and collected for subsequent experiments.
The toxicity of the samples in terms of their effect on cells was evaluated via an MTT colourimetric test using a microplate reader (Synergy HT,BioTek,USA) [32].MGC80-3 cells were seeded in a 96 well plate at a density of 2 × 104-5 × 104cells per well with 90 μL of Dulbecco's modified Eagle medium and allowed to stand for 12 h for adherence.AA and its derivatives were added to the individual wells to achieve different concentrations(1,10,20,40,60,80,100,and 200 μg mL-1),and the cells were incubated for a set time.Next,10 μL of MTT reagent per well (5 mg mL-1) of MTT reagent was added to the cells,followed by further incubation for 4 h at 37°C.Subsequently,the MTT reagent and medium were removed,100 μL of dimethyl sulfoxide was added to each well,and the optical density(OD)values of the samples were measured at 490 nm using a microplate reader.
The preliminary acute toxicity test on mice was carried out using the fixed-dose method.Eight-week-old male Kunming mice were randomly divided into three groups of four mice each.Different amounts of the AA-based DESs and poly(-DES)s were administered to the mice by gavage,and the same amount of water was administered to the control mice in the same manner.Kunming mice were then randomly divided into three groups of ten mice each,with five males and five females in each group.Based on the preliminary experiment,500 mg kg-1of the AA-based DESs and 2000 mg kg-1of the poly(DES)s were administered to the mice by gavage,and the state of the mice was observed via their survival number and weight over time.
2.3.2.Microscopic analysis
MGC80-3 cells were incubated with the AA-based DESs and poly(DES)s and then washed twice with PBS to remove the residual medium and unattached cells.Next,the cells were treated with 40 μL of the acridine orange/ethidium bromide(1:1,v/v) working solution,followed by incubation at room temperature for 10 min in the dark.The light and fluorescence observations of the cells were performed using an inverted microscope (FRD-4C,Century Kexin,China).
After incubating the MGC80-3 cells with AA-based DESs or poly(DES)s,the cells were washed twice with PBS.Next,they were treated with 1 mL of 3,3'-dioctadecyloxacarbocyanine perchlorate (DIO) (excitation wavelength:484 nm;measurement wavelength:501 nm)solution,followed by incubation at room temperature for 10 min in the dark.The samples were then washed twice with PBS,treated with 0.8 mL of 4′,6-diamidino-2-phenylindole (DAPI) (excitation wavelength: 358 nm;measurement wavelength: 461 nm) solution was added and incubated in the dark for 10 min at 25°C.Finally,the residual stain was removed using PBS,and the stained cells were observed using high-resolution and twophoton laser confocal microscopy(LSM880,Zeiss,Germany).
2.3.3.Flow cytometry analysis
The effects of the AA-based DESs or poly(DES)s on the apoptosis and cell cycle of the MGC80-3 cells were assessed using flow cytometry.After the culturing of the MGC80-3 cells,they were stained using the Annexin V-FITC/PI Apoptosis Detection Kit.Briefly,the MGC80-3 cells were digested with trypsin and collected in a centrifuge tube after being washed with PBS.They were then centrifuged at 1200 r min-1for 4 min and then suspended in PBS to achieve a density of 1 × 106cells mL-1.Next,100 μL of the cell suspension and 5 μL of Annexin V-FITC were added to a centrifuge tube,shaken gently,and allowed to stand at room temperature for 10 min in the dark.Subsequently,5 μL of PI was added to the mixture,followed by incubation for 5 min.Finally,500 μL of PBS was added to the mixtures;the mixtures were analysed using flow cytometry.
Additionally,the cell cycles of MGC80-3 cells treated with the AA-based DESs and poly(DES)s were studied using flow cytometry.After the culturing of the MGC80-3 cells,they were stained using the DNA Content Quantitation Assay Kit.Briefly,when the MGC80-3 cells achieved a density of 1 × 106cells mL-1in solution,1 mL of the cell suspension was centrifuged,followed by the addition of 75% (wt%)ethanol;this mixture was allowed to stand for 6 h.Subsequently,100 μL of RNase A solution was added to the mixture;the mixture was then placed in a water bath at 37°C for 30 min.Finally,the mixtures were treated with 400 μL of PI staining solution at 4°C and allowed to stand for 30 min in the dark before analysis using flow cytometry.
2.4.Drug loading
Three anticancer drugs,namely,DOX,cisplatin,and LASP,were loaded on the AA-based poly(DES)s,and the drug loading amount and efficacy were then evaluated.First,0.01 g poly(DES) was added to 1.0 mL of DOX (200 μg mL-1),cisplatin(200 μg mL-1),and L-ASP(400 μg mL-1)solutions,which were then allowed to stand for 3.0 h.Then,the solutions were centrifuged at 12,000 r min-1for 3 min,and the supernatants were analysed using high-performance liquid chromatography (HPLC) with the mobile phase (Table S1).MGC80-3 cells were cultured with the drug-loaded AA-based poly(DES).After incubation,the OD values of the samples were measured using a microplate reader.
3.Results and discussion
3.1.Strategy implementation
3.1.1.DES transformation
In the first step of the strategy,AA,which acted as the HBD based on its carboxyl groups,and ChCl,which is the most popular HBA in DESs,were transformed into DESs using a heating method(Fig.1a).Although the molar ratio of HBD to HBA has been reported to influence the physicochemical properties of DESs in many works [33],the influence of this ratio on their greenness in terms of their effects on organisms has only been reported in a few studies.Thus,different ratios of AA to ChCl in the DESs were investigated and explored in this work (Table 1).AA-based DESs were easily obtained in the transformation process,but the interactions between the HBA and HBD at the molecular level have not yet been explored [34].Based on the developments in computational chemistry,theoretical calculations were applied to explore the interactions in the DESs.
AA was found to have two optimised structures,AA' and AAʺ,with energies (G) of 0.00 kcal mol-1and 0.28 kcal mol-1,respectively (Fig.2a).After deep eutectic solvation,the ΔG values of ChCl-AA’ DES and ChCl-AAʺ DES were -93.76 kcal mol-1and -96.50 kcal mol-1,respectively,which indicated that the obtained DESs have strong interactions,and are more stable than the individual components.The Cl atoms of ChCl formed intermolecular hydrogen bonds with the atoms of AA in the DESs,causing the bond numbers and lengths of Cl bonds with the other atoms of ChCl to decrease.In addition,the H/O atoms of ChCl also formed hydrogen bonds with the O/H of AA,leading to the presence of many hydrogen bonds with lengths shorter than 3.0 Å.Further analysis reveals that among these hydrogen bonds,the interaction between Cl and AA is stronger than that between CH and AA and that hydrogen bond formation between the Cl and H atoms in the hydroxyl group of AA is highly favoured (Fig.2b).The distribution of Cl and CH around AA can be seen in a more intuitive fashion in the spatial distribution function (Fig.2c) with purple and blue bound contour surfaces,respectively.

Fig.1.Program of AA transformation into (a) DES and (b) poly(DES),and (c) the green evaluation system.
For obtaining the lowest melting points of ChCl-AA mixtures at a deep eutectic status,the melting points of ChCl-AA mixtures with different molar ratios were characterised via DSC.As shown in Fig.S1,the melting points of ChCl-AA mixtures have been at -5°C without obvious differences among the investigated molar ratios.In fact,when the molar ratio of ChCl-AA had been less than 2:4,the mixtures were in a solid but a liquid phase at a room temperature of~20°C.In the following,the three most of AA mole fraction (2:6,2:7,and 2:8) mixtures had been investigated for increasing the greenness of AA with a more amount.
3.1.2.Poly(DES) transformation
In addition to the transformation of AA into DESs in the liquid phase,the transformation of AA-based DESs into solidphase polymers was also carried out.The AA-based DESs,which contained an unsaturated double bond,were utilised as monomers for free radical polymerisation,and BPO-DMA was selected as the oxidation-reduction initiator.Next,EGDMA was added as a crosslinker to form a network during the polymerisation process;thus,the AA-based DESs not only polymerised with other DESs but also reacted with EGDMA to form covalent bonds.Corn stalk,a green and popular biomass with many hydroxyl groups,was chosen as a substrate on which the polymeric layer was introduced to enable inexpensive and large-scale transformation.Using these constituents,the AA-based DESs were successfully transformed into solid-phase poly(DES)s (Table 1 and Fig.1b).Although the materials were analysed in the greenness tests,since the preparation processes and reagents used to obtain all the AA-based poly(DES)s were similar,poly(DES)-1 was chosen as a typical example for poly(DES) characterisation;its morphology,composition,thermal stability,and surface properties were characterised using SEM,FT-IR,BET,and TGA,respectively.
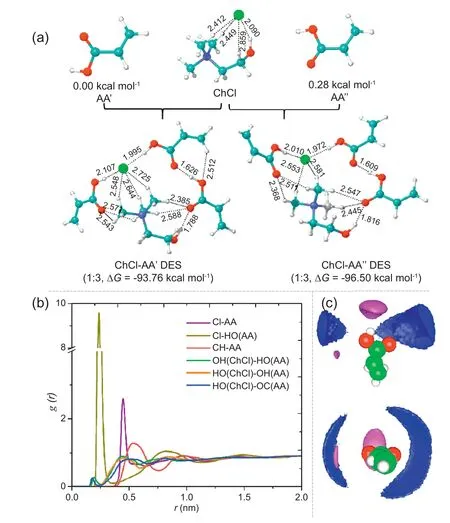
Fig.2.Computer calculation of DESs in (a) genernry and structure,(b) radial distribution functions,and (c) 3D probability distribution.

Fig.3.SEM for substrate corn stalk (a) and poly(DES)-1 with zoom out (b)/in (c),and FT-IR (d),TGA (e) and BET (f) for poly(DES)-1.
In the SEM images,the thin and planar corn stalk membranes correspond to the substrate,as shown in Fig.3a.The AA-based poly(DES)s introduced on the substrates formed fluffy and porous honeycomb layers as can be seen in the overall view at the micron scale,and exhibited many pores in the localised view with magnification at the nanoscale(Fig.3b and c).The FT-IR spectra of the poly(DES)s,corn stalk,and DESs contained markedly different peaks in some wavenumber regions,as shown in Fig.3d.In the poly(DES)spectra,the weakened peak at~3500 cm-1is related to the stretching vibration of the carboxyl -OH groups.The peak at~3000 cm-1is related to the stretching vibration of C-H bonds in the saturated alkyl chains resulting from the reaction of the double bond of AA during polymerisation;for this reason,the spectra of the corn stalk and DESs show a related peak.Some typical peaks,such as those of the C=O stretching vibration at 1700 cm-1,C-N bending vibration at~1400 cm-1,and C-OH stretching vibration at~1100 cm-1,appeared at almost the same position in the spectra of the AAbased DESs and poly(DES)s;however,these peaks were weaker in the poly(DES) spectra than in the DES spectra because of the AA polymerisation effect.The TGA plot demonstrates the stability of the poly(DES)s,which exhibited a weight loss of less than 20% at temperatures of~300°C,making them effective for applications at room temperature(Fig.3e).The BET results indicate a surface area of 49.3 m2g-1,pore volumes of 0.055-0.064 cm3g-1,and pore diameters of 58.0-60.7 Å (Fig.3f),which are in good agreement with the SEM images and further elucidate their structural properties.
3.2.Strategy verification
3.2.1.In vitro in cells
To be considered green in the ecosystem,the effects of AAbased DESs and poly(DES)s on organisms must finally be analysed;in this work,cells,as the basic unit of organisms,were first adopted.MGC80-3 is among the most popular cell lines in research and has been widely applied to assay the toxicity of many compounds [35].Thus,the toxicities of AA and its derivatives were investigated in MGC80-3 cells.
The relative growth rate (RGR) is a basic parameter that reflects the toxicity of a material on cells[36]and is calculated using the following equation [37]:
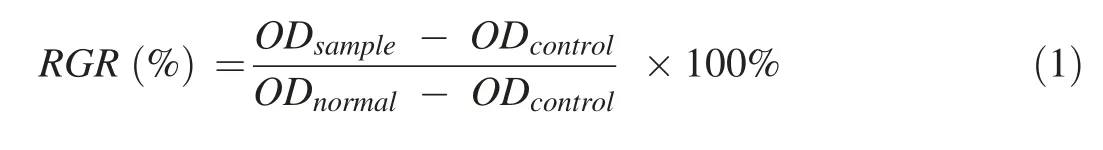
where ODsampleis the absorbance of cells incubated with the relevant material,ODcontrolis the absorbance of the blank medium without cells,and ODnormalis the absorbance of the blank medium with cells.
Using the RGR value and the standard GB/T16886.5-2003[37,38],the toxicity grade of a material to a cell type is classified as follows:
Grade 0,non-toxic: RGR >90%.
Grade 1,slightly toxic: RGR=60-90%.
Grade 2,moderately toxic: RGR=30-59%.
Grade 3,severely toxic: RGR ≤30%.
The synergetic effects between the HBA and HBD in DESs can result in the DESs having not only different chemical and physical properties but also different biological characteristics compared to those of their components [39,40].To determine the synergetic effect in the AA-based DESs,the toxicity of AA,ChCl,and the DESs were investigated and compared under the same conditions using MGC80-3 cells,as shown in Fig.4a.The RGR values of the MGC80-3 cells incubated with AA,ChCl,and the DESs showed a general decreasing trend as the concentration of the compounds was increased from 1 to 200 μg mL-1.The RGR values of the cells treated with AA decreased the most,from 95% to 35%;those associated with ChCl decreased from 95% to 70%,and those of the cells treated with DESs decreased from 95% to 50%.These results were in agreement with the statement of the physician Paracelsus regarding the toxicity of materials: ‘Solely the dose determines that a thing is not a poison’ [41].However,comparison of the RGR values for a given dose of AA and the AAbased DESs clearly reveals that AA shows a higher Grade of toxicity than the AA-based DESs.Over the investigated concentration range,the Grade of toxicity of AA increased from 0 to nearly 3 with increasing concentrations,while the highest Grade of toxicity observed for the DESs was 0 or 1 in most cases(except at the 200 μg mL-1concentration,for which the Grade of toxicity was 2).These results show that the incorporation in the DESs decreases the toxicity of AA,which corresponds to an increase in its greenness.Both Redovnikovi′c et al.[42] and Hayyan et al.[43] reported similar results and explained that acid causes the pH of the cell environment to decrease below the optimal pH,damaging the cell membrane and proteins,and thus leading to a high level of toxicity.However,the toxicity of AA is suppressed by ChCl,highlighting the tuneability of the obtained DESs.
Additionally,the molar ratio of HBA to HBD in a DES is an important factor in determining their synergetic effects on the properties of the DES [44].To obtain an in-depth understanding of the changes in the toxicity of the AA-based DESs,their RGR and Grade values were assayed using different ratios of ChCl to AA,namely,2:6,2:7,and 2:8.As shown in Fig.4b,the RGR and Grade values associated with the three DESs showed few differences and remained fairly consistent for a given concentration and time.The results clearly show a strong synergetic effect between ChCl and AA in terms of the greenness for the DESs with different molar ratios of HBA to HBD;it was deduced that a strong bonding network between ChCl and AA in the DESs effectively changed the microenvironment of the MGC80-3 cells [42].Although ChCl effectively exhibited a strong synergetic effect on the toxicity of the AA in the DESs at the different HBA:HBD molar ratios,the RGR and Grade associated with the DESs still varied with the DES concentration (1-200 μg mL-1) and treatment time(24-72 h).With increasing concentration or time,the RGR values associated with the DESs decreased and the toxicity Grade increased;the same dose-and time-related effects have been reported for other ILs [42,45].
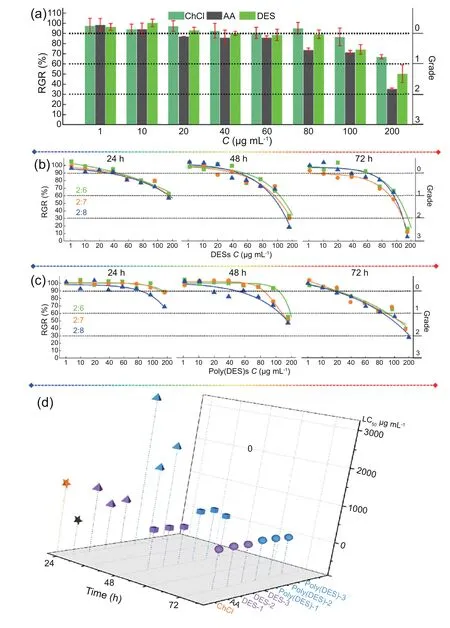
Fig.4.GRG/Grade data of ChCl,AA,and DES with different concentrations (a);GRG/Grade data of different DESs with HBA to HBD molar ratio (b);GRG/Grade data of different poly(DES)s (c);LC50 data of ChCl,AA,DESs,and poly(DES)s.
Furthermore,the toxicity of the obtained poly(DES)s with different molar ratios of HBA to HBD (ChCl to AA) were investigated in detail over a range of concentrations and times(Fig.4c).Although the general RGR/Grade trends of the poly(DES)s were similar to those of the DESs in a vertical analysis,the RGR/Grade values associated with the poly(DES)s showed a lesser change than those of the DESs with the same concentration or time change.For example,at 24 h and concentrations of 1-200 μg mL-1,the RGR values associated with the DESs or the poly(DES)s mostly decreased from 100% to 60%.The associated toxicity Grade of the DESs mostly increased from 0 to 1,while that of the poly(DES)s remained at 0.Similarly,when compared to other cor111responding time durations and concentration ranges,the RGR values associated with the poly(DES)s were generally more than 30% higher than those of the DESs,and the Grade value is generally less than one.These results clearly demonstrate that the greenness of the AA-based poly(DES)s is greater than that of the DESs and that their greenness follows the order: poly(DES)s >DESs >AA.Similarly,both commercial and laboratory-synthesised polymers are less toxic to cell lines in vitro than their cor111responding unpolymerised monomers in other reports[46].The present data are compared with the greenness data for other biomaterials in Table S2;the greenness of the present DESs/poly(DES)s is higher than that of other materials,even at high concentrations.Thus,they can fully meet the demand for new green materials.
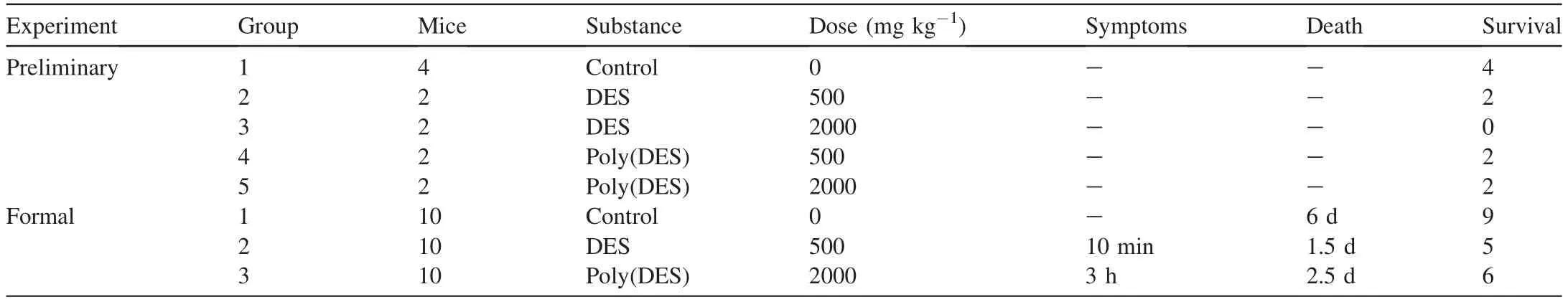
Table 2 The green assessment of the transformed DESs and poly(DES)s in vivo test of mice.
Furthermore,the median lethal concentration(LC50)is also an important parameter to assess the toxicity of a substance in biological systems;this value was obtained from the concentration-RGR plots(Table S3 and Fig.4d)[47].In the 3D LC50figure,it can clearly be seen that AA has the lowest LC50among the investigated substances at 24 h and that the LC50values follow the order poly(DES)s >DESs >ChCl >AA.Furthermore,with increasing time,the LC50values maintained the following tendency poly(DES)s >DESs.The results of the above investigations demonstrated that the DESs/poly(DES)s exhibited enhanced greenness compared to unmodified AA.
3.2.2.In vivo in mice
In addition to evaluating the changes in the toxicity of the DESs/poly(DES)s in cell lines in vitro,the evaluation of these new materials in organisms in vivo was also reported to be essential for their application [48].Thus,in this work,Kunming mice were used as experimental animals to assess the acute toxicity of the DESs/poly(DES)s (Table 2 and Fig.5).
No toxicology information for the DESs/poly(DES)s is available in other references;hence,a preliminary experiment was carried out before the formal experiment.To select an appropriate dose of the DES/poly(DES),a low dose of 500 mg kg-1and a high dose of 2000 mg kg-1were administered to the mice.In the results,none of the mice in Groups 1 to 5,except those in Group 3(2000 mg kg-1dose),died or showed obvious symptoms,which demonstrated that the poly(DES) is less toxic than the DES.Hence,the DES was administered at the low-dose level and poly(DES)was used at the high-dose level in the formal experiments for the detailed assessment of symptoms,body weight,death time,and other parameters of the mice.

Fig.5.Number of survivors (a),survival status (b),and weight change (c) of mice during the green evaluation of the DES/poly(DES) in vivo test.
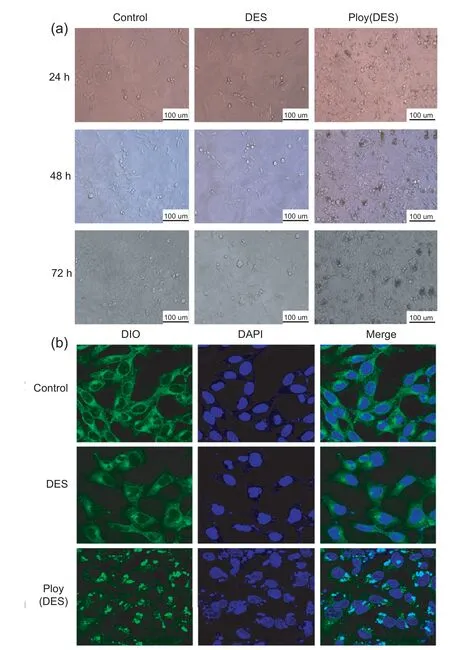
Fig.6.Morphologies of MGC80-3 cells treated with DES and poly(DES) based on inverted light (a) and confocal (b) microscopy.
The formal experiment was continued for 28 days,and the number of mouse deaths in each group is shown in Fig.5a.Group 1 was the control sample in the formal test.In this group,only one male mouse died on day 6 due to fighting among the mice,and the survival rates of the female and male mice in the control group were typical.In Group 2,in which the mice were administered DES,the greatest number of deaths (5) was observed,along with the earliest death at 1.5 days.Group 3 also showed a high number of deaths (4)starting at 2.5 days (Fig.5b and Table 2).In addition,the mice in Group 2 showed varying symptoms as soon as 10 min after DES administration,and the mice in Group 3 showed symptoms starting at 3 h.These symptoms included convulsions,listlessness,and sluggishness and had disappeared three weeks later;the related photographs are shown in Fig.5b.Furthermore,the increase in body weight in Group 1 was significantly faster than that of the mice from Group 2 and Group 3,and the bodyweight of mice in Group 2 and Group 3 decreased with increasing time (Fig.5c).
Based on the in vivo animal experiments,both the DES and poly(DES) showed increased toxicity compared to the control sample.The poly(DES) showed lesser toxicity than the DES,which was consistent with the results of the in vitro experiments.In the following section,we deeply explore and discuss the mechanisms underlying this difference.
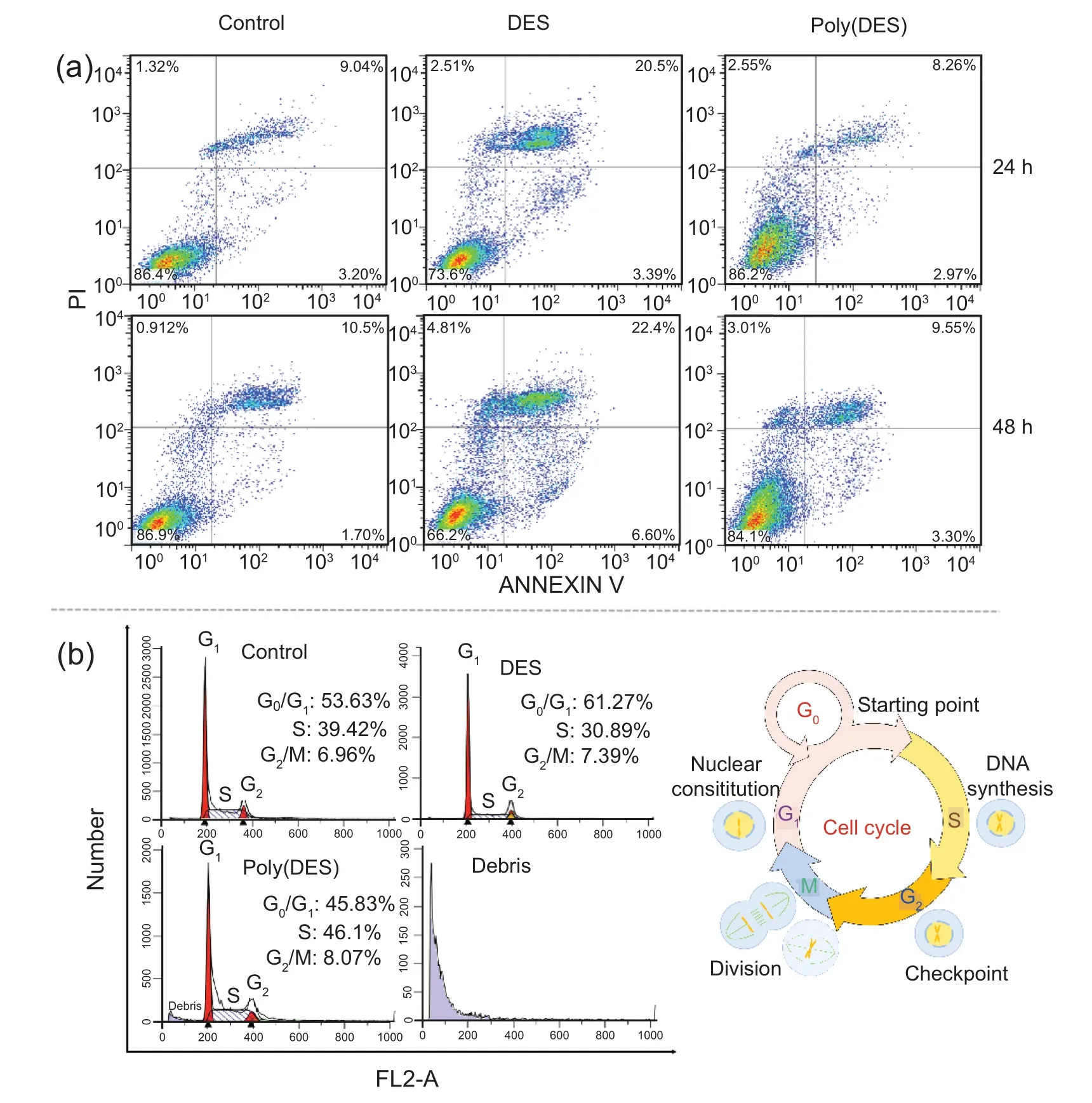
Fig.7.Flow cytometry statistics of MGC80-3 cells in induction methods (a) and cycle area (b).
3.3.Strategy mechanism
3.3.1.Microscopy analysis
To understand the mechanisms responsible for the different toxicities of materials in cells,two main techniques microscopy and flow cytometry,have been used [49].First,an inverted microscope was used to observe the changes in the morphology of MGC80-3 cells treated with DES and the poly(DES)for different time durations(Fig.6a).Compared to those of the control group,neither the DES-nor poly(DES)-treated cells showed obvious morphological changes at 24 h,but the number of cells in the DES-treated group decreased slightly.After 48 h of incubation,some of the cell membranes in cells from the DES group had disappeared,and the number of cells was further reduced.After 72 h,the DES-treated cells in DES showed extensive membrane disappearance,but the number of cells in the poly(DES) group had only decreased slightly;these cells clearly showed compatibility with the substance.The numbers and general morphologies of the cells confirmed that the poly(DES)showed a lesser toxicity than the DES.
To explore the differences between the changes caused to the cells induced by the poly(DES)and DES in detail,the cell membranes and nuclei were stained using the popular sensors DIO and DAPI,respectively,for observation using high-resolution and two-photon laser confocal microscopy,respectively (Fig.6b).The membranes and nuclei of the cells in the control group were intact.However,the membranes were obviously blurred and the nuclei were collapsed in case of the DES-treated cells due to the infiltration and permeation of the liquid DES;this is similar to the usual behaviour of liquids in cells [50].In addition,other works have suggested that DES increases the permeability of the lipid membrane of cells through hydrogen bonding or electrostatic interactions [51].By contrast,the poly(DES),as a solid with a large size,could not permeate the membrane and existed in the environment surrounding the cells;thus,the cells showed greater compatibility with the poly(DES) than with the DES.

Fig.8.(a) Poly(DES) loading with DOX in a tablet,(b) chromatographic analysis poly(DES) loading with three medicines,and (c) RGR data of three medicine with DES/poly(DES).
The morphological observations clearly showed that the DES can easily permeate the cell membrane and further damage the nucleus,but the poly(DES) did not exhibit these behaviours in the cells and showed greater cytocompatibility.Thus,the poly(DES)shows lesser toxicity than the DES in the MGC80-3 cells.
3.3.2.Flow cytometry analysis
Flow cytometry is another important technique for investigating the mechanism whereby a substance affects cells,as it allows the analysis of cell death induction and cell cycle arrest[52].Thus,MGC80-3 cell death due to apoptosis and necrosis in the MGC80-3 cells was measured at 24 h and 48 h in the presence of DES and poly(DES)using the Annexin V-FITC/PI Apoptosis Detection Kit and by flow cytometry,respectively.
A cell death analysis plot is divided into four quadrants:the upper-left (UL) quadrant,which represents necrotic cells,the lower-left (LL) quadrant,which represents normal cells,the upper-right(UR)quadrant,which represents the cells showing late apoptosis,and the lower-right (LR) quadrant,which represents the cells showing early apoptosis [53].The cell death analysis results for the present work are shown in Fig.7a and Table S4.In all cases,the percentage of necrotic cells in the UL quadrant was less than 5%.In the DES-treated group,the percentage of normal cells was close to 70%.The percentage of normal cells in the poly(DES) group was greater than 80%,which was close to the value for the control group.All these results indicate that the obtained DES/poly(DES)showed good greenness.A careful comparison reveals that the percentages of necrotic and apoptotic cells(UL,UR,and LR)were greater in the DES group than in the poly(DES) group,which indicated greater attachment of DES on cell surfaces or greater internalisation of DES by the cells[54].In detail,the greater proportion of cells showing early apoptosis (LR) proves that more phosphatidylserine was exchanged from the inner to the outer side of the plasma membrane of MGC80-3 cells and more DNA damage occurred in the DES group than in the poly(DES)group[55];additionally,the effects of poly(DES) on the MGC80-3 cells were less adverse than those of DES.
Furthermore,we hoped to determine the phase of the MGC80-3 cell cycle in which the specific stimuli leading to apoptosis arose in the DES/poly(DES)-treated cells (Fig.7b).In the G0/G1phase of the cell cycle in MGC80-3 cells,the DES,based on its permeability,led to the introduction of more substances for cell cycling than in the control group and the poly(DES) group,which resulted in an obvious increase in the percentage of cells in contact with the DES(61.72%) compared to that in case of the control group(53.63%),while the percent of cells in contact with the poly(DES) decreased (45.83%).On the other hand,because the DES led to the introduction of many substances to the cells in G0/G1phase,it induced highly adverse effects on DNA synthesis in the S phase,which caused the percent of cells in the S phase in the DES group to decrease markedly to 30.89% versus 39.42% in the control group,while the percent of cells in the S phase in the poly(DES) group increased to 46.1%.Surprisingly,a debris peak appeared in the cell cycle in the poly(DES) group,which was proved to be caused by the pure poly(DES) molecules,which acted as debris.The DES (7.39%) and poly(DES) (8.07%) did not cause a notable accumulation of cells in the G2/M phase compared to the case for the control group(6.96%).Thus,we clearly determined that the DES first permeated the cell membrane in the G0/G1phase and then adversely affected the DNA synthesis in S phase,leading to apoptosis,while the large and impermeable poly(DES) showed the opposite effects in these phases.
3.4.Strategy extension
Based on the superior structural and greenness properties of the poly(DES),it was further applied in drug loading.Three important cancer drugs,namely,the small organic molecule DOX,large molecule L-ASP,and metal complex cisplatin,were selected for loading onto the poly(DES),and their loading was studied using HPLC.It was found that 94% of DOX,80% of L-ASP,and 20% of cisplatin were adsorbed by the poly(DES).The poly(DES) can quickly decolourise a DOX solution and be formed into a tablet (Fig.8a),which demonstrates the strong loading capacity of poly(DES) for small organic molecules (Fig.8b).Furthermore,the drugloaded poly(DES) had similar effects on the MGC80-3 cells compared to the drug themselves (Fig.8c).Hence,the poly(DES) not only reduces the toxicity of AA but can also be utilised as a medicine carrier.
4.Conclusions
A novel strategy for the transformation of AA via deep eutectic solvation and subsequent polymerisation has been explored and successfully implemented.After the transformation,a greenness evaluation system based on in vitro and in vivo testing was first applied,and the greenness of the materials was verified to increase,showing the following order:AA <DES <poly(DES).Via microscopy and flow cytometry analyses,we determined that the DESs show a greater toxicity than the poly(DES)s because they can easily permeate the cell membrane and adversely affect DNA synthesis,leading to reduced numbers of normal cells while the poly(DES)s cannot.Based on the advantageous green and structural properties of the poly(DES),it was successfully utilised for DOX loading.Considering these results,this strategy should serve as a rational method to increase the greenness of many acids similar to AA prior to their application.
Conflict of interest
All authors declare that No conflict of interest exists.
Acknowledgments
This study was supported by National Natural Science Foundation of China (22178081),Interdisciplinary Research Program of Natural Science of Hebei University (No.DXK202116),Functional Pharmaceutical Chromatographic Materials Innovation Team (605020521006),and High-level Talents Introduction Program of Hebei University.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2021.02.006.
杂志排行
Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
