A correlation of the hydrogen evolution reaction activity to the number of defects formed by the decomposition of doped phosphorus species in carbon nanotubes
2022-08-14AIJieLIUZiwuSUNMaomaoLIULingWANGQuande
AI Jie, LIU Zi-wu, SUN Mao-mao, LIU Ling, WANG Quan-de
(Jiangsu Key Laboratory of Coal-based Greenhouse Gas Control and Utilization, Jiangsu Province, School of Chemical Engineering and Jiangsu Province Engineering Laboratory of High Efficient Energy Storage Technology and Equipments, China University of Mining and Technology, Xuzhou 221008, China)
Abstract:Phosphorus-doped carbon materials are one of the novel carbon catalysts for the hydrogen evolution reaction (HER)that have attracted considerable attention in recent years. However, the role of C―P species played in the HER activity is still not clear. Phosphorus-doped carbon nanotubes were prepared by chemical vapor deposition and annealed at 900, 1 000 and 1 200 °C to remove all or part of the phosporus, resulting in four samples with different amounts of substitutional-, pyridine- and pyrrole-like P species. The correlations between the HER activity and the contents of the three species were investigated. Results showed that the content of substitutional P decreased with annealing temperature and none was retained at 1 200 °C. The HER activity increased with annealing temperature and the sample annealed at 1 200 °C had the highest HER activity in an acid medium with an overpotential of 0.266 V at a current density of 10 mA cm−2. Density functional theory calculations showed that the pentagon- and nine-membered ring defects formed by the elimination of substitutional P mainly contributed to the HER activity.
Key words: Phosphorus-doped carbon nanotube;C―P species;Defect;Hydrogen evolution reaction;Water splitting
1 Introduction
Since the doping of phosphorus into carbon skeleton was proved to be able to boost the electrocatalytic activity of carbon materials towards the oxygen reduction reaction[1-3], various phosphorus-doped carbon materials with different morphological characters were developed and displayed remarkable performances in ion batteries[4-6], supercapacitors[7-9]and water splitting[10-13]. However, for these emerging striking phosphorus-doped carbon materials, up to now,their active P species are still ambiguous mainly due to the coexistence of many different P species such as graphite-like P (C3―P), pyridine- and pyrrole-like P (C2―P), C3―P=O, C—O—P= O(OH)2, C―P=O(OH)2, which impede their further practical application greatly. Our previous work[13]demonstrated that the defects resulted from the decomposition of C3―P=O group and the C―O―P species contributed mainly to the performance improvement of hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). The absence of C―P species such as C2―P and C3―P from the reported phosphorus-doped materials made their effects on HER performance unknown.
We prepared four phosphorus-doped carbon nanotubes (P-CNTs) with different P species to investigate the effects of graphite-, pyridine- and pyrrole-like three P species on the HER activity of phosphorusdoped carbon materials. Results indicated that the phosphorus-doped carbon nanotubes prepared at 1 200 °C with an overpotential of 0.266 V at the current density of 10 mA cm−2exhibited a much high HER activity in an acid medium. Density functional theory (DFT) calculations further revealed that compared with the defects from the pyrolysis of pyridineand pyrrole-like P species, the pentagon- and ninemember ring defects resulted from the decomposition of graphite-P species were the main active sites for the HER, providing a new perspective for the rational development of high-efficiency HER catalysts.
2 Experimental
P-CNTs were synthesized by chemical vapor deposition with triphenylphosphine (TPP) as a phosphorus source, toluene as a carbon source, ferrocene and Fe-Mo/Al2O3as catalysts. In a typical procedure,TPP, toluene and ferrocene were mixed at a mass ratio of 15∶100∶7.5. The tubular furnace was heated to 750 °C after a clean quartz boat with 0.1 g of Fe-Mo/Al2O3catalysts was put into the high temperature area of the quartz tube[2]. The product in the quartz boat was collected and denoted as P-CNTs1. Finally,P-CNTs1 was calcined at 900, 1 000 and 1 200 °C under vacuum for 2 h to produce samples named as PCNTs2, P-CNTs3 and P-CNTs4, respectively.
3 Results and discussion
The SEM and TEM images (Fig. 1) of P-CNTs1,P-CNTs2, P-CNTs3 and P-CNTs4 indicated that all of the four samples had a nanotube morphology. From the HRTEM images, it could be found that the carbon lattice fringes in P-CNTs1 were much more regular than those in P-CNTs4, indicating the presence of more edge defects in P-CNTs4.
The two dominant diffraction peaks near 23° and 43° in the XRD spectra of 4 samples (Fig. S1a) could be attributed to the (002) and (100) crystal planes of graphitic carbon, respectively, confirming that the prepared samples were mainly composed of CNTs. Raman spectra of four samples in Fig. 2 could be deconvoluted intoD,G,IandD" peaks. TheDandGbands at ca. 1 350 and 1 580 cm−1reflect the degree of lattice defects and theE2gvibration of carbon atoms, respectively.Iband at ca.1 220 cm−1corresponds to the disorder in the graphitic lattice, such as sp2-sp3bonds and polyenes, andD" band at ca. 1 500 cm−1is generally related to the amorphous carbon[14-16]. TheID/IGvalues of four samples increased from 0.47 to 1.78 as the synthesis temperature increased (Fig. 2), indicating that the defect degree of the materials increased with the synthesized temperature increase.
The XPS spectra in Fig. S1b show that P-CNTs1,P-CNTs2 and P-CNTs3 were mainly composed of C,O and P, and the P-CNTs4 only had C and O elements (Table 1). The four peaks at 284.8, 285.9,286.9, and 289.5 eV deconvoluted from C 1s spectra(Fig. 3) could be attributed to sp2, sp3, C―O and π-π*,respectively[17]. The sp3/sp2carbon ratios increased with the synthesis temperature (Table 2) . Meanwhile,four peaks at 130.6, 132.3, 133.1 and 134 eV in P2p spectra (Fig. 4) could be ascribed to C―P, C3―P=O,C―P―O and C―O―P, respectively[18].
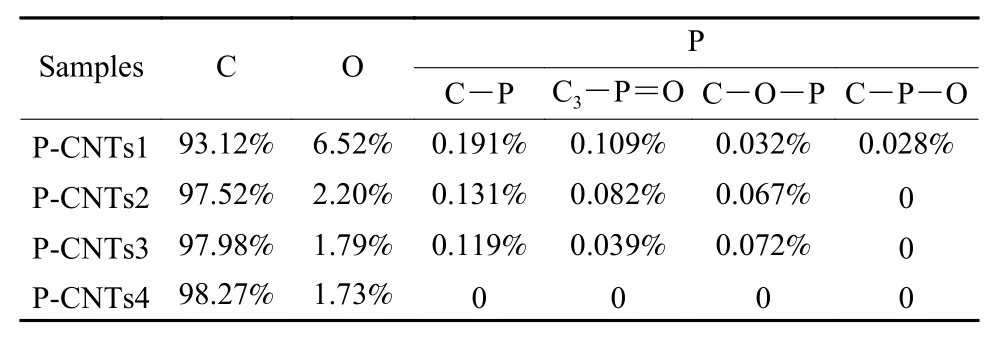
Table 1 Elemental contents in 4 samples from the XPS.
Fig. 5a and 5b show the HER electrocatalytic activities of four samples in an acidic medium, which indicated a gradual increase of their HER activities with the synthesis temperature. The P-CNTs4 as a HER catalyst had an overpotential of 0.266 V at a current density of 10 mA cm−2, which was the best among them and superior to most of reported carbonbased metal-free electrocatalysts[13]. Fig. 5c indicated that the tafel slope (89.1 mV dec−1) of P-CNTs4 was the smallest, suggesting the fastest HER kinetics of PCNTs4 among the four samples. Meanwhile, Fig. 5d showed that the HER activities of four samples decreased with the increase of the C―P content, suggesting that C―P species could not promote HER directly. Interestingly, their HER activities increased with the increase ofID/IGvalues, implying that the breaking of C―P bonds in them would yield defects that improved the HER activity (Fig. 5d, Fig. S2). The Nyquist diagrams in Fig. 5e show that the P-CNTs4 had the smallest semicircle diameter, the least charge transfer resistance and the fastest catalytic kinetics.Meanwhile, Fig. 6 and 7 indicated that the HER activity of the four samples had a positive correlation with their electrochemically active surface area (ECSA).And the P-CNTs4 with the best HER activity exhibited an excellent stability as well (Fig. 5f), showing a good application prospect in future water electrolysis.

Table 2 The contents of sp2 and sp3 carbons, C=O, C―O, π-π* and the ratios of sp2/sp3 carbon from the C 1s spectra.
Since the breaking of C―P bonds would yield defects and bring about the improvement of the HER activity, as confirmed by turnover frequency (TOF)values at 0.3 V overpotential and the decrease of C―P contents (Table S3), we employed DFT to calculate the charge and spin densities of carbon atoms around graphite-P, pyridine-P and pyrrole-P to further ascertain what defects would form and which carbon pyrrole-P, revealing that these two defects were mainly the HER active sites. To further explore active carbon atoms in pentagon- and nine-membered ring defects, the H+adsorption energy was also calculated by the DFT. Table 4 demonstrated that the H+adsorption energies on C3, C4 and C5 in the pentagon defect and those on C1, C9, C10 and C11 in the ninemembered ring defect were obviously larger than those of carbon atoms in the blank CNT network, imatoms would serve as HER active sites. Interestingly,results in Fig. 8 and Fig. 9 showed that with the breaking of C―P bonds in graphite-P in the CNT network,pentagon- and nine-membered rings defects would form. And the decomposition of pyridine-P and pyrrole-P would form armchair and pentagon zigzag defects (Fig. S3 and S4). Table 3, Table S1 and S2 indicated that the breaking of graphite-P bond would bring more changes in charge and spin densities of carbon atoms in pentagon- and nine-membered ring defects as compared with that of pyridine-P and plying that these seven carbon atoms in the two defects were the main HER active sites. Based on this finding, the possible HER routes were proposed in Fig. 10.
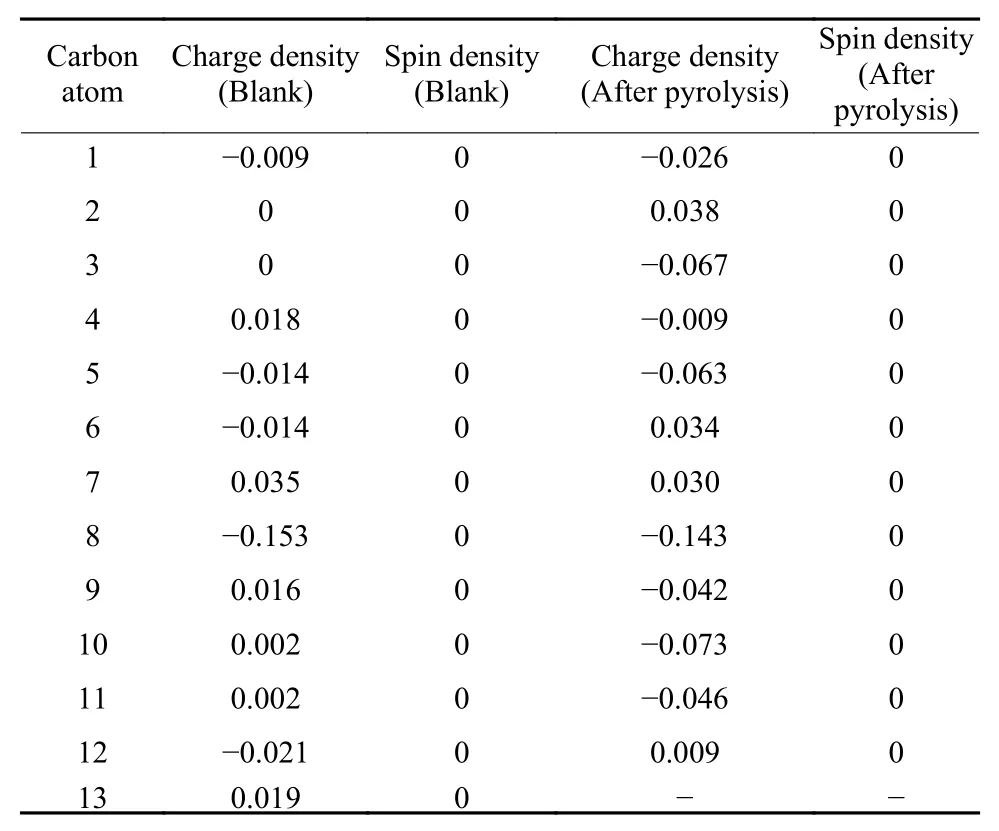
Table 3 The charge and spin densities (a.u.) of carbon atoms in the new formed defects after the decomposition of the C3―P graphite-like structure and those of the corresponding carbon atoms in the blank.
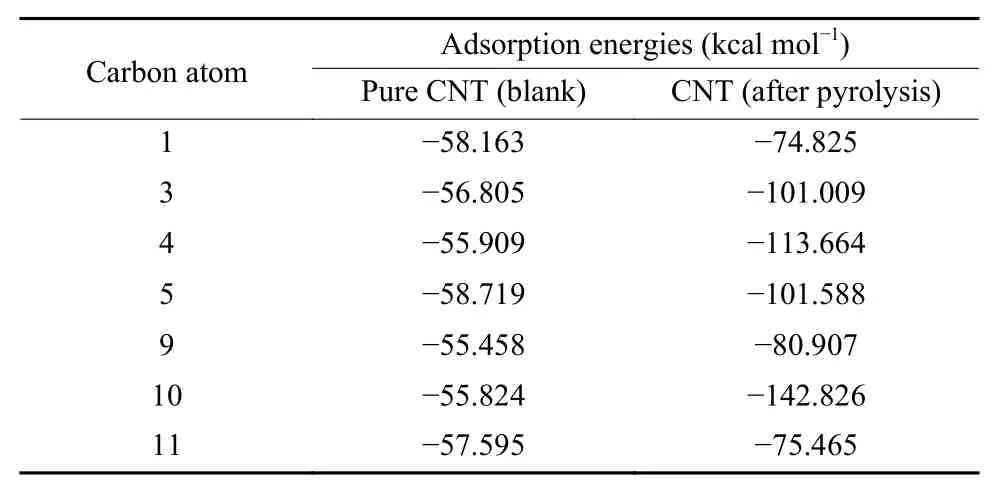
Table 4 The H+ adsorption energies (kcal mol−1) on carbon atoms in the new formed defects from the decomposition of the C3―P graphite-like structure and those of corresponding carbon atoms in the blank.
4 Conclusions
We have systematically explored the influence of C―P species on the HER performance of P-CNTs through the experimental investigation and theoretical calculation. Results indicated that the P-CNT4 had an overpotential of 0.266 V at the current density of 10 mA cm−2, which exhibited a much high HER activity in an acid medium, and the decomposition of graphite-P species would form defects that boosted the HER of P-CNTs. DFT calculation further revealed that about seven carbon atoms in the pentagon- and ninemembered ring defects were mainly the HER active sites. This work provides a deep insight into the relationship between the type of P species and the HER performance.
Acknowledgement
This work was supported by Jiangsu Key Laboratory of Coal-based Greenhouse Gas Control and Utilization (2020ZDZZ04A, 2019B002).
杂志排行
新型炭材料的其它文章
- Guide for Authors
- Electrochemical sensing of phenacetin on electrochemically reduced graphene oxide modified glassy carbon electrode
- A sustainable strategy to prepare porous carbons with tailored pores from shrimp shell for use as supercapacitor electrode materials
- The synthesis of porous carbons from a lignin-rich residue for high-performance supercapacitors
- 《新型炭材料》征稿简则
- CoN4 active sites in a graphene matrix for the highly efficient electrocatalysis of CO2 reduction
