CoN4 active sites in a graphene matrix for the highly efficient electrocatalysis of CO2 reduction
2022-08-14ZHANGHuinianWANGHuiqiJIASupingCHANGQingLINingLIYingSHIXiaolinLIZiyuanHUShengliang
ZHANG Hui-nian, WANG Hui-qi, JIA Su-ping, CHANG Qing, LI Ning, LI Ying, SHI Xiao-lin, LI Zi-yuan, HU Sheng-liang
(School of Energy and Power Engineering, North University of China, Taiyuan 030051, China)
Abstract:Developing highly selective, economical and stable catalysts for the electrochemical conversion of CO2 into value-added carbon products to mitigate both CO2 emission and the energy crisis is challenging. We report an efficient and robust electrocatalyst for the CO2 reduction reaction (CO2RR) by embedding CoN4 active sites in a graphene matrix. These highly dispersed CoN4 sites show an extraordinary CO2RR activity, with a high CO Faradaic efficiency of nearly 95% at −0.76 V (vs. RHE) and remarkable durability. The corresponding overpotential is 0.65 V. Our finding could pave the way for the design at the atomic scale of highly efficient electrocatalysts for the CO2RR.
Key words: CO2 electroreduction;Cobalt;Single-atom catalysts;Electrochemistry;Graphene
1 Introduction
Direct aqueous electroreduction of COinto valuable chemicals and fuels powered by electricity from renewable energy is a desirable technology to realize environmental and energy sustainability.However, this desired technique faces highly challenging in practical implementation due to the chemical inertness of COand multiple distribution of products.Moreover, inevitable competition hydrogen evolution reaction (HER) can concomitantly occur with COreduction in aqueous media. Converting COinto CO by electrolyzing is currently one of the most practical targets owing to the kinetical favor of CO production compared with other COreduction products.Moreover, CO is the key intermediate for the formation of oxygenates and hydrocarbons. In this regard,robust, economical and efficient COreduction electrocatalysts with good durability and high activity must be developed to highly favor CO production over the competing HER.
To address these issues, various kinds of noblemetals electrocatalysts, such as Pd, Ag, Au,,have been screened for the CO-to-CO conversion.However, these catalysts still suffer from the sensitivity to poisoning and high cost, which hinder their practical application. The strategy to mitigate these problems is to develop noble-metal-free electrocatalyst for converting COto CO. N-doped porous carbon supported single non-noble metal electrocatalyst has been widely explored to convert COto CO at a high efficient in recent years. The special electronic structure and maximized atomic efficiency of these single metal active sites make them can be used as a promising electrocatalyst for CORR. The singleatom Co sites have been successfully introduced to graphene matrix by ball milling of cobalt phthalocyanine (CoPc) and graphene, and the relevant performance of CORR has not been reported due to the high cost. In order to scale up CORR process for practical implementation, developing of high-performance and economical electrocatalyst is the cornerstone. However, for the purpose of enhancing the performance for CORR, developing a robust single-atom active electrocatalyst with cost-effective still remains a great challenge.
Herein, we report an effective approach for uniformly dispersed CoNcenter with Co sites anchored on the matrix of graphene (CoN/G) for efficient CORR. In brief, the CoN/G composite was prepared by high-energy ball milling of graphene nanosheets and cobalt tetrasulfonated phthalocyanine (CoTsPc).The electrocatalytic activity of CORR with cobalt macrocycles was investigated in detail. However,CoTsPc macrocycle molecules are dissolved in aqueous electrolyte as CORR catalyst. It is difficult to make gas diffusion electrodes in aqueous electrolyte and separate and recycle the catalyst. The CoTsPc has a similar structure to CoPc with much cheaper price. The resultant CoN/G exhibits high CO Faradaic efficiency of ~95% at −0.76 V (vs. RHE) and the corresponding overpotential of 0.65 V. We expect that our findings will pave an avenue for designing novel single-atom electrocatalysts for efficient CORR.
2 Experimental
2.1 Chemicals and materials
Cobalt tetrasulfonated phthalocyanine (99.5%,Aladdin), KHCO(99.7%, Sigma-Aldrich), Nafion solution (5%) and Nafion 117 membrane were supplied by Alfa Aesar. Graphite paper (99.95%) was from Qingdao Huarun Graphite Co. Ltd. Carbon paper was from Toray Industries Inc, Toray TGP-H-060.All reagents were used without further purification.
2.2 Material synthesis
2.2.1 Synthesis of CoN/G
The graphene flake (G) was prepared by an electrochemical method. The CoN/G synthesis was similar to the previous report. Briefly, 2.8 g of graphene and 1.2 g of CoTsPc was ball milled for 20 h with the stainless steel balls of 120 g and the diameter of 0.9−1.4 cm. After forming CoN/G, the residual CoTsPc was washed out with deionized water, and the CoN/G was separated by centrifugation.
2.2.2 Synthesis of N/G
100 mg of graphene and 800 mg of melamine were first mixed in 50 mL ethanol and sonication for 90 min. Ethanol was removed by the rotary evaporation. The as-obtained homogeneous solid mixture was dried in vacuum at 80 °C for 12 h. Finally, the Ndoped graphene (N/G) was obtained through the carbonization of the mixture at 800 °C for 2 h under flow of Ar. The heating rate was 5 °C minand then cooled naturally.
2.3 Characterization
2.3.1 Material characterizations
Scanning transmission electron microscopy(STEM) images were recorded using a JEM ARM200F equipped with a cold field emission gun and double aberration correctors in Institute of Physics Chinese Academy of Sciences in Beijing. Transmission electron microscopy (TEM) measurement was carried out using a JEM-2100F electron microscope. X-ray photoelectron spectroscopy (XPS) were obtained with a Thermo ESCALAB 250 spectrometer.X-ray diffraction (XRD) was recorded using a D8 ADVANCE A25 system configured with the Cuα 0.154 06 nm radiation. Raman spectra was carried out on a Jobin-Yvon equipped with 532 nm laser line and HR-800 Raman system. UV-Visible spectra were taken on Shimadzu UV-3600 spectrophotometer.SEM images and EDS maps were acquired on JSM-7001F field-emission system. FT-IR spectra were obtained using a Bruke Vertex 70 V FT-IR spectrometer.The Co K-edge absorption spectra (EXAFS) was acquired on the XAFCA beamline at the Singapore Synchrotron Light Source (SSLS), using a Si (111) double crystal monochromator to record data.
2.3.2 Preparation of working electrode
Catalyst inks were prepared by dispersing 10 mg of catalyst in a mixture of 10 μL of Nafion solution(5%) and 1.0 mL of water-ethanol mixture with the volume ratio of 1∶2. All the catalyst inks were sonicated for 1 h to ensure uniform mixing. The working electrode was fabricated by casting a certain amount of catalyst inks onto a 1 × 1 cmporous conductive carbon paper with the loading amount of 2 ± 0.2 mg.
2.3.3 Electrochemical measurements
CORR performance was performed on an electrochemical workstation (CHI760E). The COreduction reaction occurred in a gas tight H cell configuration, which was separated by Nafion 117 membrane.The carbon paper modified with the catalyst was used as the working electrode of 1 cm. A saturated calomel reference electrode (SCE) and a Pt sheet counter electrode were used. In this work, the SCE electrode was adjusted to the reversible hydrogen electrode (RHE) by the formula:=+ 0.24 V +0.0591 × pH. The COelectrocatalytic conversion was investigated in 30 mL of CO(99.999 9%) saturated with 0.1 mol Lof KHCOsolution. The pH of this solution was 6.8. The KHCOelectrolyte was sparged with COfor 60 min prior to the measurement. The scan rate of linear sweep voltammetry (LSV) is 20 mV s. FE are the average values of three GC measurements and the GC measurement repeats every 15 min.
2.3.4 Catalytic product analysis
Gas products were quantified on an online GC equipped with flame ionization detector and thermal conductivity detector. The GC was calibrated using calibration gas, which was commercially available from Dalian Special Gases Co. Ltd.
The liquid products were quantified through an NMR spectroscope with water suppression. After electrolysis, 0.5 mL of KHCOelectrolyte was mixed with 0.1 mL of DO in an NMR tube and dimethyl sulfoxide (DMSO, 0.05 μL) as an internal standard.
The calculation of Faradaic efficiency (FE) for gaseous products using the following equation:
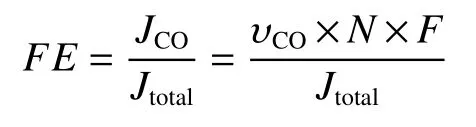
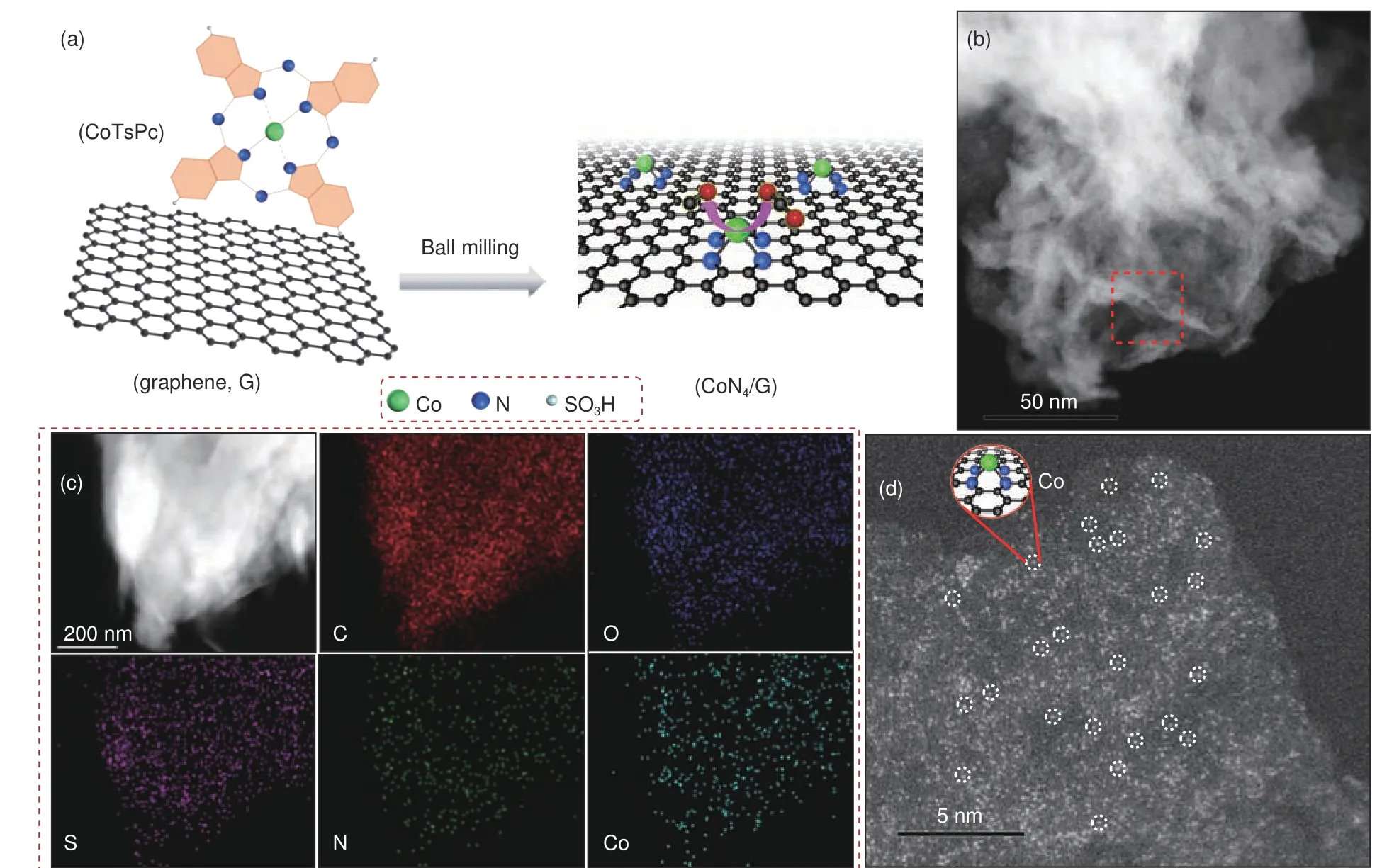
Fig. 1 (a) Illustration of the synthesis route towards CoN4/G catalyst, (b) STEM image of CoN4/G, (c) SEM image and the corresponding EDS mapping of CoN4/G, (d) HAADF-STEM image of CoN4/G.
where,is faradaic efficiency for CO production;(mA cm) is total current density;(mA cm)is the partial current density for CO production;is volume concentration of CO (GC data); F is Faradaic constant, F = 96 485 C mol;is the number of electron transferred for product formation.
3 Results and discussion
In this work, atomically dispersed CoNon graphene matrix, denoted as CoN/G, was synthesized via high-energy ball milling of graphene nanosheets and CoTsPc for 20 h followed by deionized water washing to remove residual CoTsPc with the highly water solubility (Fig. 1a and Fig. S1b). Furthermore, in Fig. S1b, the blue was not observed for CoN/G, in contrast to a mixed G/CoTsPc sample containing the same amount of CoTsPc by hand lapping.This result confirms that the CoTsPc cannot adsorbed on the graphene by mild physical-mixing method. The as-obtained CoN/G hybrid material was firstly studied by transmission electron microscopy (TEM),showing the same “sheet-like” structure of graphene(Fig. 1b). The EDS maps of CoN/G show that C, N, S and Co are uniform distributed in the graphene network (Fig. 1c). To investigate the atomic structure of Co-Nspecies, the HAADF-STEM imaging was employed. Fig. 1d displays that most bright spots were uniformly dispersed on graphene, which were ascribed to Co atoms.
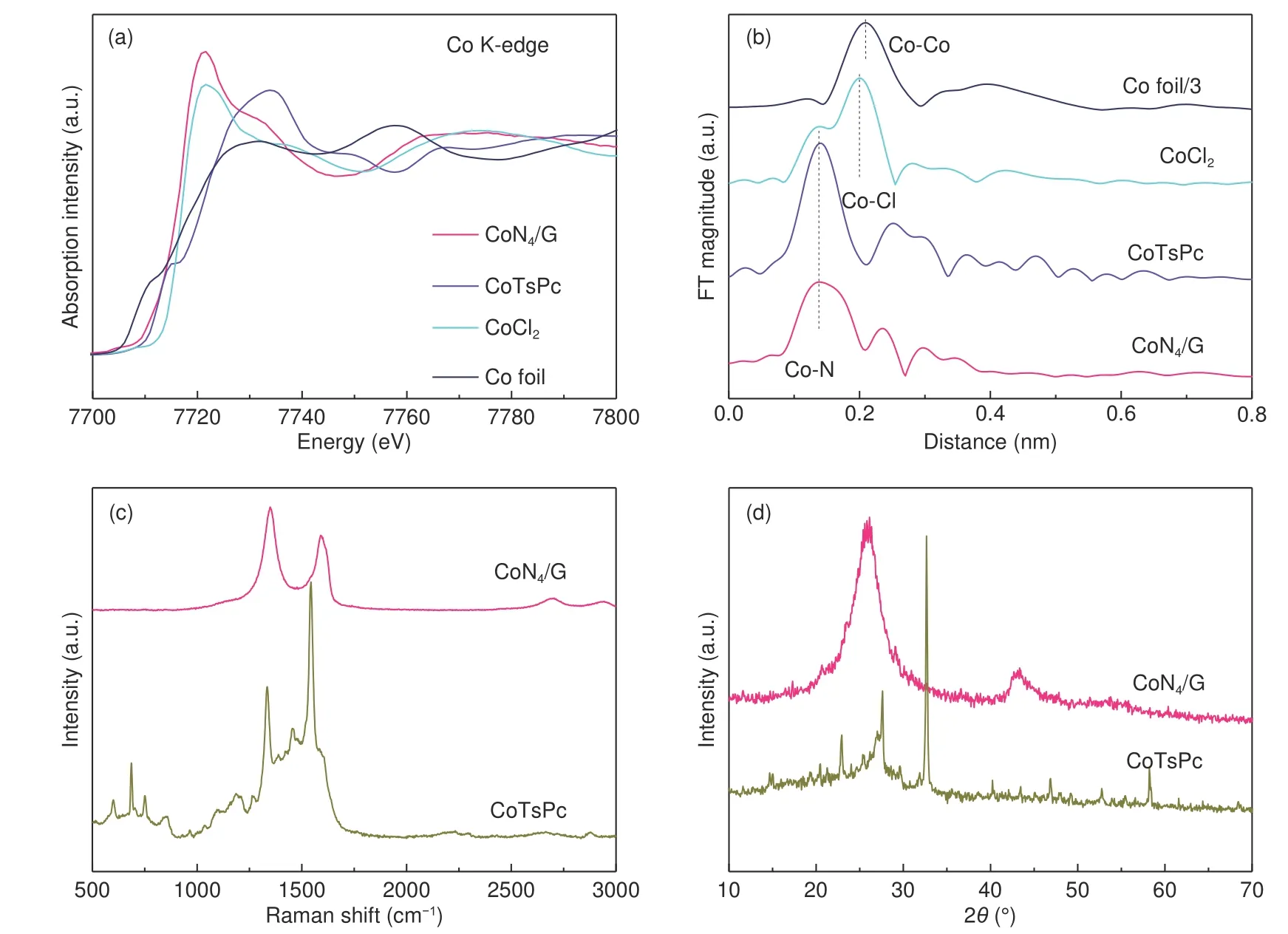
Fig. 2 (a) Co K-edge XANES spectra of CoN4/G catalyst in comparison with Co foil, CoCl2 and CoTsPc and (b) Fourier transformed (FT) of EXAFS.(c) Raman spectra and (d) XRD patterns of CoN4/G sample in comparison with CoTsPc.
X-ray absorption near-edge structure (XANES)was employed to further analyze the local coordination structures and chemical environments of Co atoms. As exhibited in Fig. 2a, the Co K edge XANES spectrum for CoTsPc sample showed noticeable spectroscopic signature at approximately 7 716 eV, indicating that CoNin CoTsPc is a planar geometry. By contrast, it was not observed for CoN/G catalyst, suggesting its nonplanar structure. When the CoN/G catalyst was exposed to air after ball milling, the Co-Nsites may be modified by hydroxy groups. Additionally, the fourier-transform (FT) curve shows Co-N coordination structure at about 0.137 nm in CoN/G,which is similar to the coordination of Co in the CoTsPc. No other obvious peak for the Co-Co coordination was detected (Fig. 2b). According to the XANES spectra, the local CoNstructure is well retained. The Raman spectroscopy indicates that the breathing mode (B1g) near 550-1 000 cmof the characteristic macrocycle has become very weak in CoN/G hybrid catalyst, indicating that the macrocycle structure of CoTsPc may have been destroyed during the ball milling process (Fig. 2c and S5). Furthermore,XRD also confirm the original CoTsPc crystal structure has been destroyed since the characteristic diffraction peaks of CoTsPc in CoN/G disappeared(Fig. 2d). The XRD pattern of CoN/G presents only two distinct diffraction peaks at approximately 26.15 and 43.31, corresponding well with the (002)and (101) planes of graphitic carbon (Fig. 2d).The UV-Vis spectra of CoTsPc showed a characteristic absorption peak at approximately 597 nm.However, in the case of CoN/G catalyst, the absorption peak was disappeared (Fig. S1a), which could be attributed to the reduced aromatic regions caused by the ball milling. These characterizations indicated that the crystalline structure of CoTsPc has been destroyed with the residues of CoNspecies incorporated in graphene through the ball milling. The surface atomic compositions of CoN/G were acquired by XPS survey spectrum. The survey spectrum indicates the presence of N for 5.36%, C for 63.27%,O for 7.07%, S for 2.64% and Co for 1.66% in CoN/G(Fig. 3a and S2). Considering the limited detection depth of XPS test, Co atoms in CoN/G were mostly incorporated in carbon layers, the elment contents of CoN/Gwere measured by ICP measurement. The Co content in CoN/G was determined to be 1.61%.While the surface C, N, O, S and Co content in CoTsPc is ca. 40.71%, 16.25%, 29.22%, 6.18% and 7.64%, respectively (Fig. S2). The Co 2p spectrum(Fig. 3b) exhibits peaks at 796.5 and 781.3 eV, which matches well with the Co 2porbitals and 2porbitals of a Cospecies, further indicating the valence state of Co-Nspecies was not reduced by ball milling. The exhibited two distinct categories of N species including pyrrolic Nat 401.3 eV combined with Co atom and pyridinic Nat 399.9 eV bonded with carbon atom of the outer macrocycle (Fig. 3c).The intensity ratio of Nto Nin CoN/G is significantly reduced compared with CoTsPc. This results indicates that ball milling leads to the destruction of part of the pyridinic Nspecies, whereas, pyrrolic Nspecies are well maintained in the CoN/G sample. Based on the XPS results, we estimated that the Co atoms are embedded in the matrix of graphene and bonded with four pyrrolic Natoms, which was consistent with the previous observation for MN/GN. The S 2p spectrum is shown in Fig. 3d, in which the peaks at 168.5 and 169.1 eV for the sulfate species (SO)can be observed in the CoTsPc. While signals from C―S―C groups at 163-165 eV were absent in the CoN/G, indicating that the S atoms were not embedded in graphene lattice after ball milling. The characteristic peak at 1 027 cmcontributed to the―SOH group were absent in the CoN/G (Fig. S3).This also clearly indicated the ―SOH and the outside macrocyclic structure of CoTsPc has been broken during ball milling. According to mechano-chemical process by ball milling, graphitic C―C bonds at the edge in graphene could be homolytic and reacted with―SO―C― during ball milling (Fig. S4). Meanwhile, ―C―SO―C― groups are predominately located at the edge of the graphene. FT-IR spectrum of CoN/G shows a unique sharp peak at 1 562 cm,which was associated with S―O stretching exclusively from ―C―SO―C―. Based on the above results, atomically dispersed Co-Nspecies have been successfully embedded in the matrix of graphene through ball milling of CoTsPc and graphene.
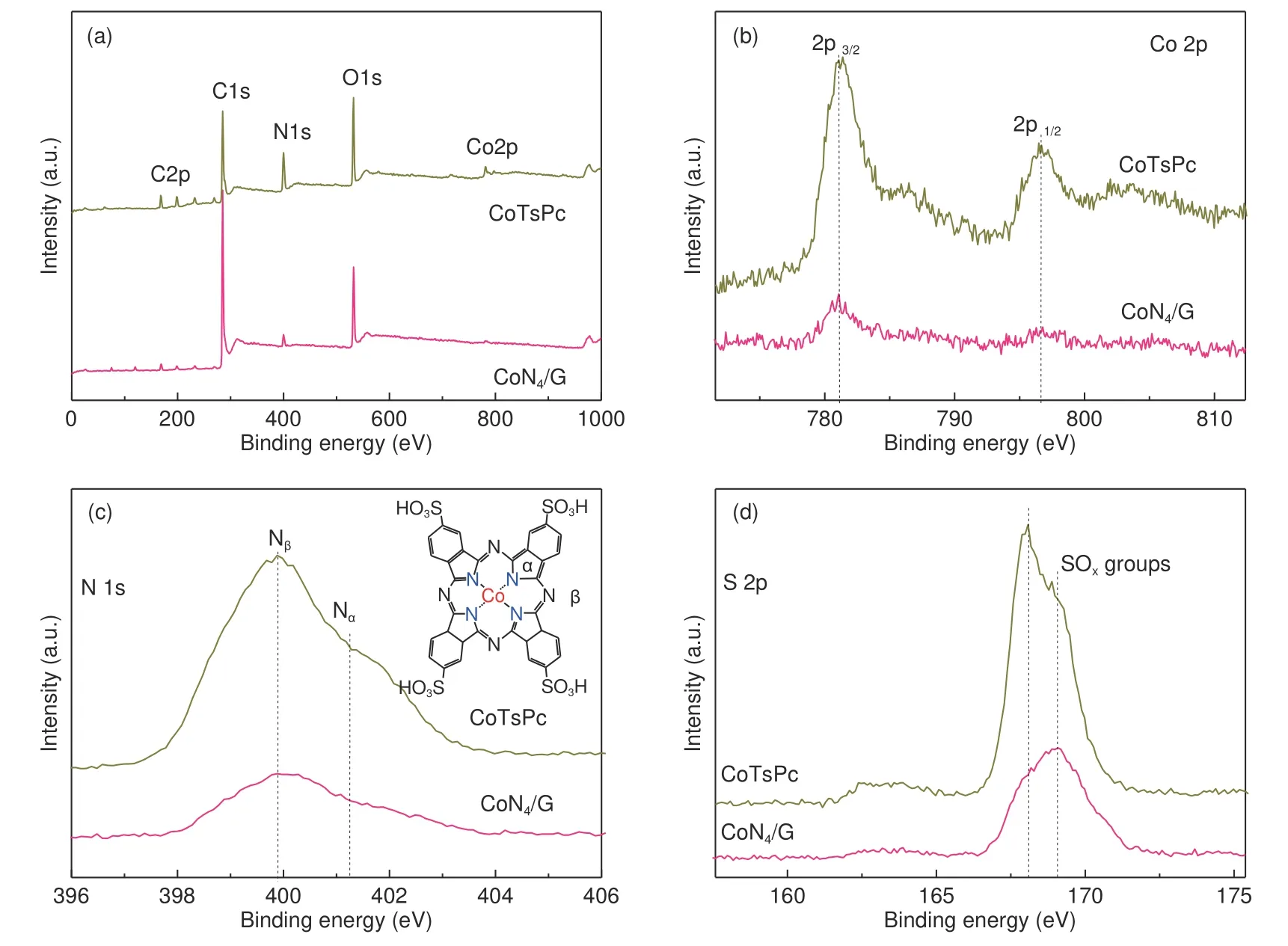
Fig. 3 XPS characterization of CoN4/G sample in comparison with CoTsPc: (a) XPS full spectra, (b) Co 2p XPS spectra,(c) N 1s XPS ( insert of CoTsP structure), and (d) S 2p XPS spectra.
The electrocatalytic performance of COconversion of the CoN/G catalyst was evaluated in a twocompartment gas-tight electrochemical cell. The linear sweep voltammetry (LSV) measurements for CoN/G catalyst were conducted in Ar- and CO- saturated 0.1 mol LKHCOelectrolyte. As shown in CO-saturated KHCOsolution (Fig. 4a), the CoN/G catalyst ball milled for 20 h exhibited a low onset potential of −0.41 V (vs. RHE), corresponding 0.30 V in onset overpotential with the standard equilibrium potential of CO/CO of −0.11 V at pH 6.8 (vs. RHE)).Meanwhile, the resulting total geometric current density in Ar-saturated solution was far less than those obtained in CO-saturated electrolyte. The change in current density alone cannot provide conclusive evidence for COreduction. This could be ascribed to the interconnevtion of COreduction and HER. The pH will lower upon COdissolving in KHCO, which will in turn further enhance the HER. Thus, the occurrence of COreduction was further confirmed by analyzing the reduction products.
We employed gas chromatography (GC) to analyze the gaseous products from CORR. Under working potentials between −0.26 and −0.96 V (vs. RHE),CO is observed as the dominant product. No liquid product is detected byH NMR spectroscopy in CO-saturated electrolyte. While in Ar-saturated electrolyte, no CO is detected. The corresponding FE for CO was measured for CoN/G. With the goal of investigating the role of CoNsites in CORR, N/G was prepared (Fig. 4b). N/G was synthesized via pyrolysis the mixture of graphene and melamine. The Raman spectra and XRD pattern of N/G was shown in Fig. S6.The CoN/G catalyst presented a higher selectivity for CO formation in comparison with N/G. Specifically,at −0.76 V (vs. RHE) corresponding 0.65 V in overpotential, the CoN/G could reach a maximum FE of~95%, which is comparable to the best single Co atom electrocatalyst reported to date and better than most of the reported N-doped carbon matrix (Fig. S7 and Table S1). Meanwhile, the FEs of CoN/G gradually decreases as the potential changes to more negative value, probably due to the dominant competitive HER. This may indicate that the CoN/G catalyst was limited in the mass transport of COinto the single-atom Co sites. This observation agrees well with the LSV results. However, the maximum FE of N/G for CO was only 25%. By optimizing the Co content and ball milling time, the CoN/G with 1.61% Co ball milled for 20 h was optimized (Fig. S8 and Fig. S10). Fig. 4c showed the CO partial current density () of CoN/G and N/G,which are normalized by the geometrical surface area.The CoN/G exhibited a higherthan that of N/G at each potential, and theof CoN/G reaches up to 19 mA cmat −0.96 V, which was 16 times more than that of N/G at the same potential. The CO production rate of CoN/G catalyst increased acutely with increased applied potential, and it was significantly superior to N/G catalyst for all the potentials tested(Fig. 4d). The COreduction activity of CoN/G including cobalt element significantly exceeds that of N/G without cobalt, indicating the CoNstructure plays a vital role in enhancing COconversion. Although the N contents of N/G and CoN/G are 6.67%and 5.36% respectively (Fig. S2), the as-prepared N/G sample exhibits poor electrochemical activity for COreduction compared with CoN/G catalyst (Fig. 4b-d),excluding the major contribution of nitrogen dopant enhanced the electrocatalytic performance. Thus, the higher catalytic activity of CORR for CoN/G was predominately attributed to the CoNdoping. The stability of CoN/G catalyst during long-term operation for CORR was further investigated (Fig. 4f). The current density maintained steadily at ~10.5 mA cmand less than 2% decay over 15 h at −0.76 V (vs.RHE). The corresponding FEs of electrochemical CO― to ―CO conversion maintained constantly(~94%) throughout the test, unambiguously indicating remarkable cycling stability of CoN/G catalyst.After the electrolysis of CoN/G catalyst, the single Co atoms still remain atomically dispersed on graphene matrix (Fig. S9).
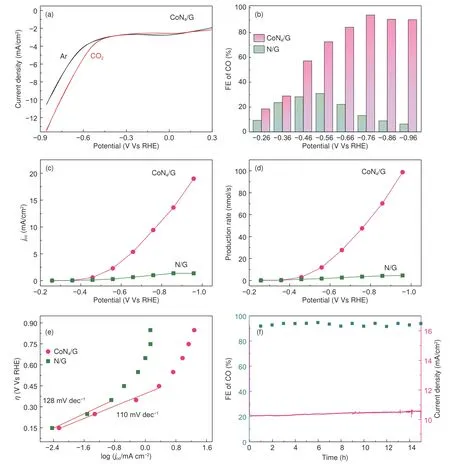
Fig. 4 CO2RR catalytic performance of as-synthesized catalysts. (a) 20 mV s−1 LSV scans for the CoN4/G catalyst in KHCO3 solution. Comparison of electrocatalytic activity of CoN4/G and N/G: (b) FE of CO, (c) jCO and (d) production rates of CO at varoius applied potentials. (e) Tafel plot.(f) Long-term stability for CoN4/G catalyst at −0.76 V (vs. RHE) for 15 h.
In addition, Tafel analysis was undertaken to further uncover the kinetics of CORR on CoN/G catalyst. The Tafel slope of CoN/G was 110 mV dec, which is smaller than that of N/G with 128 mV dec, indicating more favorable kinetics for CO format (Fig. 4e).. The Tafel slope of activation process was about 118 mV dec. The Tafel slope of CoN/G was close to the theoretical value, indicating the similar reaction mechanism. Threfore, the ratelimiting step for CoN/G was the initial COactivation process.
4 Conclusion
In conclusion, the atomically dispersed CoNsites have been embedded in the matrix of graphene by a one-step ball milling method, which exhibits an outstanding catalytic performance for CORR. The asprepared CoN/G catalyst affords a high CO FE of about 95% at −0.76 V( vs. RHE), making it comparable to the reported single Co atom electrocatalys. Our results provide a new avenue to design novel SACs for a wide range of electrocatalysis, energy conversion and storage related applications.
Acknowledgement
The authors thank for the financial support from the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi(2019L0597, 2019L0557, 2020L0279), the National Natural Science Foundation of China (21703209), the Shanxi Province Science Foundation for Youths(201901D211225), the Talent Introduction Fund of Shanxi Province (18001913), the starting fund for scientific research of North University of China(11012316), the project supported by Science Foundation of North University of China (XJJ201819,XJJ201913) and the Key Research and Development(R&D) Projects of Shanxi Province(201803D121037).
杂志排行
新型炭材料的其它文章
- Guide for Authors
- A correlation of the hydrogen evolution reaction activity to the number of defects formed by the decomposition of doped phosphorus species in carbon nanotubes
- Electrochemical sensing of phenacetin on electrochemically reduced graphene oxide modified glassy carbon electrode
- A sustainable strategy to prepare porous carbons with tailored pores from shrimp shell for use as supercapacitor electrode materials
- The synthesis of porous carbons from a lignin-rich residue for high-performance supercapacitors
- 《新型炭材料》征稿简则
