A sustainable strategy to prepare porous carbons with tailored pores from shrimp shell for use as supercapacitor electrode materials
2022-08-14GAOFengXIEYaqiaoZANGYunhaoZHOUGangQUJiangyingWUMingbo
GAO Feng, XIE Ya-qiao, ZANG Yun-hao, ZHOU Gang, QU Jiang-ying,*, WU Ming-bo
(1. School of Environment and Civil Engineering, Dongguan University of Technology, Dongguan 523808, China;2. College of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian 116029, China;3. Institute of New Energy, State Key Laboratory of Heavy Oil, China University of Petroleum (East China), Qingdao 266580, China)
Abstract:The highly efficient synthesis of nitrogen-doped carbons with different pore structures is reported using shrimp shell as the carbon and nitrogen source, and its CaCO3 component as the hard template and activator. The CaCO3 content of shrimp shells can be easily changed by changing the leaching time to remove it. CaCO3 acts as the activator and template to tailor the pore sizes of the carbons. CO2 from the decomposition of CaCO3 also plays an activating role. Their specific surface areas, pore volumes, ratios of micropore volume to total pore volume can be adjusted in the ranges 117.6-1 137 m2 g−1, 0.14-0.64 cm3 g−1, and 0-73.4%, respectively.When used as the electrodes of a supercapacitor, the porous carbon obtained with a leaching time of 92 min has a high capacitance of 328 F g−1 at 0.05 A g−1 in a 6 mol L−1 KOH electrolyte and 619.2 F g−1 at 0.05 A g−1 in a 1 mol L−1 H2SO4 electrolyte. Its corresponding energy density at a power density of 1 470.9 W kg−1 is 26.0 Wh kg−1. This study provides a low cost method for fabricating porous carbons from biomass with a high added value.
Key words: Strategy of functional assembly;Self-activation;Pore structures tailoring;Porous carbon;Supercapacitor
1 Introduction
The environment-friendly energy storage devices have attracted much attention because of the increasing global warming and depletion of oil reserves[1,2].Supercapacitors are considered to be potential energy storage devices with short charging time, long cycle life and high power density[3-5], which include the electrostatic double layer capacitors (EDLCs) and the pseudo-capacitors. Porous carbons with the advantages of the high specific surface area and the moderate cost are currently a good choice for supercapacitor electrodes[6-9]. The researchers have demonstrated that the capacitance of the carbon electrodes depends on their porous structures and surface properties[9-12].Although the developments of carbon-based supercapacitors are encouraging, constructing different scaled pores of carbons with the sustainable synthetic strategies need to be studied in detail.
To date, well-defined porous carbons are accessible by activation methods and/or template methods with the different precursors. The most commonly used method for the synthesis of porous carbons is the activation method, which combines with the high temperature calcination of the mixtures of the carbon precursor and the activator such as KOH, ZnCl2,H3PO4[13,14]. For example, Zhu et al.[15]prepared a microporous carbon with a specific surface area of up to 1 776.9 m2g−1by KOH activation using a micro-structured skunk as the carbon source, which showed a high specific capacitance of 213.4 F g−1in 1 mol L−1H2SO4even at a current density of 20 A g−1. Generally, the acid/base activation technique suffers from the disadvantage such as the environmental pollution and the apparatus corrosion. The template method provides another way for the fabrication of tunable porous carbons with the nanostructured zeolite, ZnO,Al2O3, Mg(OH)2or mesoporous SiO2as the hard templates impregnated with the appropriate carbon precursors[16-19], which often involves dipping, curing, calcining and template removal processes. As a result, the porous carbon can be obtained with well-defined topologies and pore dimensions that depend on the kind and morphology of hard templates. The disadvantages of such synthesis include the fussy and time-con-suming procedure, the massive used hazardous substances, and the tedious removal of the hard templates.
Alternatively, a sustainable and scalable strategy for the preparation of porous carbon has been proposed. For example, Yang et al.[20]reported NaCl-template synthesis of carbon with rich mesopores and macropores, where NaCl as the soluble salt is less expensive than common templates used, and it is easy to remove by water washing because of its high solubility. But the resultant carbon exhibited a low specific surface area and its porous structure was difficult to precisely regulate. Guo et al.[21]reported carbon spheres synthesized by CaCO3template-induced activation of dopamine, which exhibited a large specific surface area of up to 1 984 m2g−1and 7.57% nitrogen doping level. Instead of commercial templates, White et al.[22]pioneered to synthesize mesoporous carbons with the surface area of 177-328 m2g−1using the intrinsic CaCO3in the prawn shell as the self-template.Inspired by his work, our group has well developed above method to synthesize adjustable porous carbons using shrimp shell as the precursor. For instance,the self-template method combined with H3PO4activation of shrimp shell produced a N, P, O doped porous carbon with a surface area of 774 m2g−1[23],while KOH activation resulted in a surface area of 2 032 m2g−1[24]. Above two kinds of samples showed the good performances as the electrode materials for the supercapacitors, the specific capacitance of which reached 206 and 328 F g−1in 6 mol L−1KOH electrolyte at 0.1 A g−1, respectively. Such a self-template combined with activation approach for the synthesis of micro/mesoporous carbons also involved the additional activation agents. Therefore, it also raised the cost of production, and caused the corrosion of equipment and serious environmental pollution. Furthermore, the reported work only focused on natural CaCO3as the template for the synthesis of porous carbon. However, CaCO3can be calcined at high temperature to produce CaO, the role of which in regulating porous structure of carbon need to be investigated in detail. Recently, we reported the synthesis of porous carbon directly using oyster shells with a CaCO3content of 96.1% as both activation agent and the template, and a soft pitch as the carbon precursor[10]. It was found that CaO produced by the thermal decomposition of CaCO3could be used as the self-activation agent, which helped to produce a series of porous carbons with the surface area of 612-1 258 m2g−1by tailoring the ratio of oyster shells and soft pitch. When used as the electrode of a lithium ion battery, a typical porous carbon exhibited a reversible capacity of 1 251 mAh g−1. Such work provided an environment friendly method for the synthesis of porous carbon for the energy storage to fulfill the high-value added use of oyster shell. Because of the high content of CaCO3in the oyster shells, additional carbon source was necessary to produce porous carbons. Based on the reported work, the synthesis of porous carbons with tailored pore sizes using one typical biomass as the trinity of carbon source, hard template and activator remains a challenge.
Herein, we select the waste shrimp shells as the only source to synthesize porous carbons with the tailored pore sizes. The effect of the pore structures on the performance of supercapacitors is also investigated. It is known that shrimp shell contains about 40%nitrogen polysaccharide chitin and protein, and about 60% inorganic CaCO3. The former can be used as the nitrogen/carbon source for the synthesis of nitrogendoped carbons, which will contribute to pseudocapacitance for the use as the supercapacitor electrode.The latter can be used as both the template and the activator for in-situ tailoring the pore sizes of the carbons for the EDLCs. The major challenge in this case is how to fabricate a porous carbon with tailored pore size by a sustainable route without the assistance of additional activator.
2 Experimental
2.1 Synthesis of shrimp shell derived N-doped porous carbons
Bohai shrimp shell was used as the source. The N-doped porous carbons were synthesized by selective demineralization, followed by self-activation at high temperature. Firstly, 7.08 g dried shell was dispersed in 50 mL of a 20% acetic acid (HAc) solution for different times to selective removal of its intrinsic CaCO3. The samples were afterwards washed with deionized H2O until neutral pH and dried at 80 °C.Secondly, the dried samples were calcinated at 900 °C for 3 h with a heating rate of 5 °C min−1under nitrogen flow. Finally, the obtained samples were adequately dissolved in a 20% HAc at room temperature until CaO residue derived from CaCO3was completely removed. Then samples were completely reclaimed by filtration and rinsed with deionized water until the solution became neutral and dried overnight at 80 °C. A series of samples were obtained and designated as C-X CaCO3(X is the percentage of remaining CaCO3in the samples). For comparison, C-100%CaCO3was synthesized by a similar procedure without the demineralization step.
2.2 Characterization
The morphologies and microstructures of the asacquired products were inspected using field emission scanning electron microscopy (SEM, Hitachi Ltd SU8010), X-ray photoelectron spectroscopy (XPS,Thermo VG Scientific Sigma Probe Spectrometer)and thermogravimetric analysis (TG). The Brunauer-Emmett-Teller (BET) surface area of the carbon samples was determined by physisorption of N2at 77 K using a Micromeritics ASAP 2020 analyzer.
2.3 Electrochemical measurements
The supercapacitors were assembled according to the literatures[25,26]. The electrochemical properties of the samples were estimated by a CHI660C electrochemical work station in a three-electrode cell.Hg/HgO and Hg/Hg2SO4electrodes were used as the reference electrodes in 6 mol L−1KOH and 1 mol L−1H2SO4aqueous electrolytes, respectively. In both case, platinum foil was employed as the counter. The mass ratio of the active materials, carbon black, and polytetrafluoroethylene (PTFE) in the working electrode was 75∶20∶5. The active mass was averagely 1.5 mg per electrode. Before testing, the prepared electrodes were soaked overnight in the electrolyte.The CV measurements were performed in a potential window between −0.1 and −0.9 V in 6 mol L−1KOH and −0.7-0.3 V in 1 mol L−1H2SO4. The electrochemical impedance spectroscopy (EIS) measurements were recorded on a CHI 660C electrochemical work station in a frequency range from 100 kHz to 0.01 Hz.The long-term cycling performance of the C-25%CaCO3electrode was measured by the consecutive galvanostatic charge-discharge in 6 mol L−1KOH and 1 mol L−1H2SO4solution at current density of 1 A g−1on a Land CT2001A cycler at room temperature. The assembly of the supercapacitor with two symmetrical work electrodes was tested at different cell voltages. The electrolyte was 6 mol L−1KOH with nickel foam as a current collector and 1 mol L−1H2SO4solution with titanium mesh as a current collector.
The specific capacitances were calculated as the following equations:
whereCsis the specific capacitance (F g−1),Iis the galvanostatic discharge current (A), Δtis the discharging time (s), ΔVis the potential window during discharge, andmis the mass of the active materials in the working electrode (g).
whereEandPare the energy density (Wh kg−1) and power density (W kg−1), respectively.Csis the specific capacitance (F g−1) of the two electrodes and ΔVis the working voltage for devices (V).
3 Results and discussion
Fig. 1 shows the whole green strategy of self-activation of shrimp shell for N-doped porous carbons with tailored pores for the supercapacitors. Herein, the intrinsic CaCO3can act as the mesoporous template,and the CaO activator derived from the pyrolysis of CaCO3can tailor micropores of the obtained carbons.The content of CaCO3in shrimp shell plays an important role, which can be regulated to be 0, 25% and 50%after 150, 92 and 30 min by leaching with 20% HAC,respectively. For comparison, pristine shrimp shell without any HAC treatment was used. All shrimp shell samples with different CaCO3contents were carbonized at 900 °C in N2atmosphere followed by the removal of CaO using a HAC solution. Ca(AC)2as the by-product could be used as the calcium supplement. The resultant carbon samples with tailored pores were used as supercapacitor electrodes. Different from other strategies including soft/hard-template and activation methods for the synthesis of N-doped porous carbons[16,17], the feature of this work is that shrimp shell are the only source for the synthesis of porous carbons without any additional activator, template and carbon source involved.
Fig. 2 shows temperature-dependent decomposition of shrimp shell in air by TGA and the demineralization process of shrimp shell with 20% HAC for different times. The mass change of shrimp shell from 10 to 900 °C was tested at a heating rate of 10 °C min−1,as shown in Fig. 2a. The loss of water below 300 °C is approximately 5.7%. The significant mass loss occurred in the range of 300-430 °C is attributed to the decomposition of organic compounds. The loss occurred in the range of 430-600 °C is believed to be the further decomposition of organic compounds, the carbon combustion and the decomposition of CaCO3to form CaO[27,28]. The weight remains stable until 800 °C,indicating the complete decomposition of CaCO3and the formation of CaO with a content of 34.5%. It can be calculated that the CaCO3content in the shell is about 61.6%. The shrimp shell with different contents of CaCO3in the range of 0, 25%, 50%, 100% can be tailored by different leaching times using 20% HAC(Fig. 2b), which can be used as the template and activator for the synthesis of porous carbons.
Fig. 3 shows the SEM images of raw shrimp shell, shrimp shell after complete removal of CaCO3and C-25% CaCO3samples. The raw shrimp shell exhibits a stacked layer morphology as shown in Fig. 3a.Fig. 3b demonstrates that the product also remains the layered structure even after the complete removal of CaCO3, indicating that organic skeleton has been strongly built in the sheet structure. As expected,layered sheets of C-25% CaCO3sample are preserved after high-temperature calcination for the removal of most oxygen and nitrogen of the precursor as shown in Fig. 3c. Such structure is beneficial to ion transport and capacitance increase of the samples. During the transition of shrimp shell into porous carbons, CaO derived from CaCO3always exits in the carbon skeleton to produce the porous structure, which will be discussed by N2adsorption-desorption isotherms.
The N2adsorption-desorption isotherms and corresponding pore size distributions are further used to investigate the porosity of the obtained samples, as shown in Fig. 4. The detailed porous properties of the carbons derived from shrimp shell are also listed in Table 1. It is found that the content of CaCO3in the precursor plays an important role on the porous structures of the resultant samples. When CaCO3in shrimp shell is completely removed by HAC, the obtained carbon named as C-0% CaCO3exhibits characteristics of a type IV isotherm, indicating C-0% CaCO3possesses a mesoporous structure with a specific surface area (SBET) of 117.6 m2g−1. The mesopores are derived from the piled pores of the carbon particles and the residual pores after the removal of CaCO3.When the content of CaCO3is retained to 25% in shrimp shell, the obtained C-25% CaCO3displays a type I adsorption isotherm with a highSBETof 1 371 m2g−1, where microporous and mesoporous specific surface areas (SMic) and (SMec) are 1 114 and 257.8 m2g−1, respectively. It should be noted thatSMicis about 12 times higher than that of C-0% CaCO3.Such an obvious increase ofSMicfor C-25% CaCO3is attributed to the activation roles of alkaline CaO and CO2derived from the pyrolysis of CaCO3at 900 °C,which has been reported in our previous work[10].When the content of CaCO3is further retained to 50%in the shrimp shell, theSBETof resultant C-50%CaCO3decreases to 678.2 m2g−1, whereSMicdecreases sharply to 154.1 m2g−1and SMesincreases to about 2 times than that of C-25% CaCO3. The results further imply that micropores collapse accompanied by formation of mesopores in C-50% CaCO3due to the excess activation by CaO and CO2at 900 °C.When pristine shrimp shell is used as the precursor,the resultant C-100% CaCO3shows further decreased bothSMic(50.3 m2g−1) andSMes(339.9 m2g−1), which indicates that intrinsic CaCO3is the activator of the resultant mesoporous carbon. Particularly, an obvious increase in 0.9-1.0p/p0indicates the presence of macropores for C-100% CaCO3. Above results demonstrate that the original micropores and mesopores in C-25% CaCO3are further enlarged to mesopores and macropores in C-50% CaCO3and C-100% CaCO3, respectively. The similar tendency of the obtained samples is also observed by the pore size distributions as shown in Fig. 4b. The pore sizes of the obtained carbons follow the order: C-25% CaCO3< C-50% CaCO3< C-100% CaCO3. The gradual enlarged pores with the increased content of CaCO3indicate the activation role of CaO and CO2. The ratios ofSMic/SBETare 0, 81.5%, 22.7%, 12.9% for C-0%CaCO3, C-25% CaCO3, C-50% CaCO3and C-100%CaCO3, respectively. Furthermore, the ratios ofVMic/VTotalwith different CaCO3contents also exhibit the similar tendency. The results indicate that CaCO3derived from shrimp shell in the carbon precursor promotes the creation of micropores with the low content of CaCO3(< 25%), mesopores and macropores with increased contents of CaCO3(> 25%) for samples C-50% CaCO3and C-100% CaCO3. Herein, CaO, as the new kind of activator derived from shrimp shell is very useful for the creation of porous carbon with various pore size, which is very different from the widely used corrosive activators such as KOH, H3PO4and NaOH[29-31].
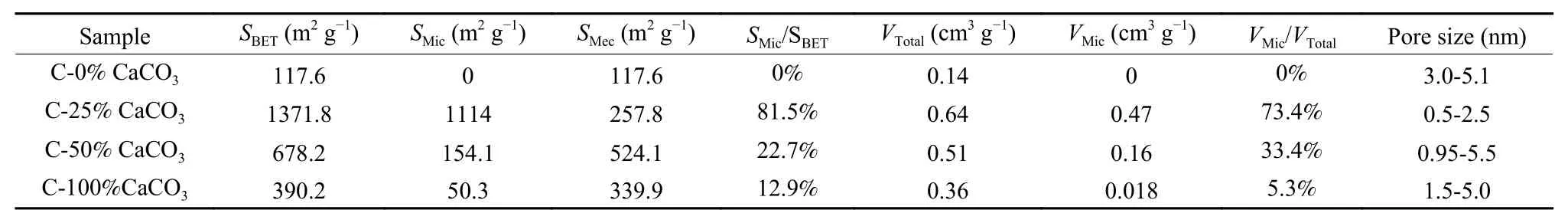
Table 1 Porous properties of the resultant carbons derived from shrimp shell.
To obtain the chemical states of the surface elements, C-X% CaCO3(X=0, 25, 50, 100) samples are further analyzed by XPS spectra (Fig. 5) and corresponding quantitative analysis results are summarized in Table 2. The corresponding C1s spectra of the samples are shown in Fig. 5a. Three obvious peaks located at 284.6, 285.5 and 287.4 eV represent C―C/C=C, C―O, O―C=O functional groups, respectively. The peaks at 531.0 and 532.2 eV correspond to the C=O and C―O in the O 1s spectra, respectively (Fig. 5b). The N1s spectra of the samples are shown in Fig. 5c. The four peaks represent pyrrole nitrogen (N-5, 399.7 eV), pyridinium nitrogen (N-6,398.0 eV), graphitized nitrogen (N-Q, 400.8 eV) and nitrogen oxide (N-X, 402.0 eV). It has been reported in the literature[32]that the functional groups N-5 and N-6 can provide the pseudocapacitance to facilitate the electrochemical performance of carbon. All samples exhibit the N-5 and N-6 contents in the range of 35.1%-41.7%. Particularly, the total amount of N-5 and N-6 of C-25% CaCO3sample can reach 18.3%and 17.8%, respectively. The contents of C, N and O in the samples are shown in Table 2. C-0% CaCO3sample contains about 83.8% C and 12.0% O.However, the C content increases while O content decreases for the obtained carbon when CaCO3remains in the carbon precursors. The maximum C content(88.0%) and least O content (8.4%) are achieved for C-25% CaCO3sample, whereas the O content increases with the increase of the content of CaCO3,which can be attributed to an increased content of oxygen provided by CaO through activation. The N content barely changes for all the obtained samples except C-25% CaCO3which possesses the least N content of 3.6%.
The electrochemical performance of C-X%CaCO3(X=0, 25, 50 and 100) samples is tested in a three-electrode system with 6 mol L−1KOH as the electrolyte. Fig. 6a exhibits a cyclic voltammetric curves of the samples. It is known that the C-25%CaCO3sample has the best electrochemical performance in all samples because of its largest area of the rectangular shape. Furthermore, the electrochemical performance of all the sample follows the order of C-25% CaCO3>C-50% CaCO3>C-100% CaCO3>C-0%CaCO3. The charge and discharge curves are shown in Fig. 6b. The samples all exhibit a good charge and discharge reversibility and the linear shape indicate the presence of EDLCs, where the porous structure dominates the final capacitance. The specific capacitances of the 4 samples follow the order as C-25%CaCO3>C-50% CaCO3>C-100% CaCO3>C-0% CaCO3(Fig. 6c). Such order agrees with that of the surface areas, where the larger is surface area (or micro-surface area), the higher is the specific capacitance. Typically, the capacitances are respectively 273, 231, 186 and 90 F g−1at a current density of 1 A g−1for C-25%CaCO3, C-50% CaCO3, C-100% CaCO3and C-0%CaCO3. Among them, C-25% CaCO3sample with the largest surface area has the highest specific capacitances of 328, 287, 281, 277, 273 and 266 F g−1at 0.05, 0.1, 0.2, 0.5, 1.0 and 2.0 A g−1, respectively. The performance is better than that of the reported N-containing ultra-microporous carbon (269 F g−1at 1.0 A g−1)[33], oxygen-rich hierarchical porous carbon(222.6 F g−1at 0.5 A g−1)[34], hierarchical porous carbons (188 F g−1at 0.04 A g−1)[35], N-doped carbon(205 F g−1at a current density of 0.5 A g−1)[36], N, P, Scodoped hierarchically porous carbon spheres(274 F g−1at a current density of 0.5 A g−1)[37].
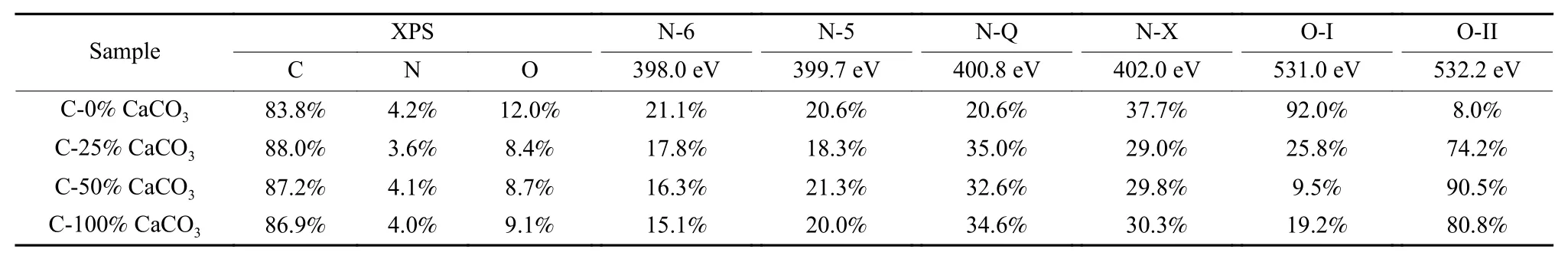
Table 2 The contents of N, C and O in the resultant carbons from XPS analysis.
The EIS curves of the samples (Fig. 6d) are used to understand the capacitive nature of the samples by testing the ion transport behavior of the electrode materials. The half ring diameter in the high frequency region corresponds to the magnitude of the charge transfer resistance, while the oblique line in the lowmid frequency region is related to ion diffusion and capacitance characteristics[38]. The C-25% CaCO3sample exhibits a curve characteristic close to 90° in the low frequency region, indicating the sample has good capacitance performance[38]. The EIS equivalent circuit of the sample is shown in the inset of Fig. 6d.The circuit of the entire capacitor consists ofRsandRct.Rsis the sum of contact resistance and material resistance (including electrode material, electrolyte and current collector) andRctis a charge transfer resistance (corresponding to the adsorption-desorption of ions on the electrode material) and the fitting values are shown in Table 3. TheRctof C-25% CaCO3, C-50% CaCO3, C-100% CaCO3and C-0% CaCO3samples are calculated to be 6.27, 1.55, 0.95 and 3.01 Ω, respectively. The above results indicate the carbon with a higher C content and less N/O content exhibits a better ion transport behavior and a lower charge transfer resistance, thereby improving the electrochemical performance. Fig. 6e shows the electrochemical curve of the sample cycled for 5 000 times at a current density of 1.0 A g−1. The inset is the last 10 charge and discharge curves. The C-25% CaCO3sample has an excellent cycle stability with a retention of over 99% of the initial capacitance in this case.
The electrochemical performance tests are performed on C-X% CaCO3(X=0, 25, 50 and 100)samples under the three-electrode system with 1 mol L−1H2SO4as the electrolyte (Fig. 7). Fig. 7a shows the CV curves of the obtained materials at a scan rate of 10 mV s−1. The electrochemical properties of all the samples are consistent with the conclusions at alkaline conditions as shown in Fig. 6, but the apparent hump at low voltages indicates the redox capacitance derived from the presence of N-/O-functional groups. The irregular linear shape of the curves(Fig. 7b) further indicates the presence of redox capacitance. The results demonstrate that doped N and O heteroatoms contribute lots for the redox capacitance in the H2SO4electrolyte. The specific capacitance curves at different current densities are shown in Fig. 7c. At the current density of 0.05 A g−1, the specific capacitances of C-25% CaCO3, C-50% CaCO3,C-100% CaCO3and C-0% CaCO3are 619, 446, 382 and 166 F g−1, respectively. It is obvious that the specific capacitances under acidic conditions are significantly higher than that in the alkaline electrolyte, which is attributed to the redox capacitance provided by appropriate N/O contents. However, the specific capacitance of the samples has a significant decrease after the current density is higher than 0.5 A g−1due to the large hindrance of ion transport by micropores in the acidic system. As a result, the capacitance of C-25%CaCO3sample decreases from 619.2 at 0.05 A g−1to 252 F g−1at 1 A g−1. The EIS curves (Fig. 7d) show that all samples in the acidic system exhibit a curve characteristic close to 90° in the low frequency region,indicating the good ion transport behavior in this case.Fig. 7e shows the electrochemical curve of the sample cycled 5 000 times at a current density of 1 A g−1,where the capacitance of C-25% CaCO3sample is still up to 266 F g−1, maintaining a capacitance of 96.0% of its initial. The C-25% CaCO3sample exhibits the excellent rate performance and the high cycle stability.
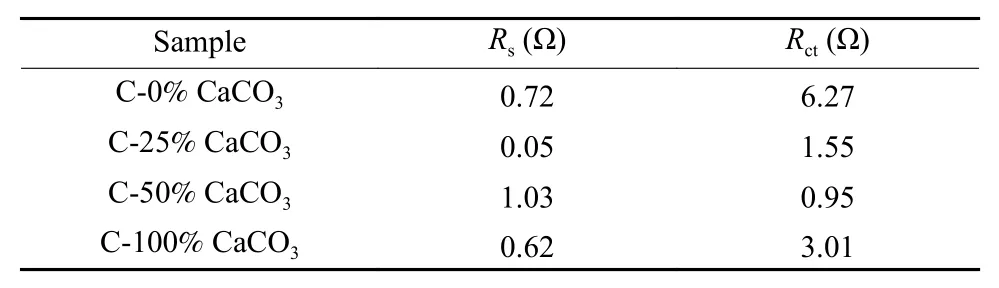
Table 3 Kinetic parameters of the C-X%CaCO3 electrodes.
The C-100% CaCO3and C-25% CaCO3samples are electrochemically tested in a 6 mol L−1KOH electrolyte in a symmetric two-electrode system, as shown in Fig. 8. The good capacitance behavior of the two samples in the wide voltage range of 0-1.0 V can be concluded by the CV curves (Fig. 8a). The CV curve of the C-25% CaCO3shows better rectangular shape and larger area than that of the C-100% CaCO3, which means the better electrochemical performance of C-25% CaCO3. The galvanostatic charge/discharge curves of symmetrical supercapacitors at 50 mA g−1look mostly symmetrical with a slight curvature(Fig. 8b), indicating some pseudocapacitive contribution along with the EDL contribution[7,39]. The EIS curves of the C-100% CaCO3and C-25% CaCO3samples are shown in Fig. 8c. The C-25% CaCO3sample has a smaller semicircle diameter than C-100% CaCO3. It is attributed to the importance of the porous structure to ion diffusion when the sample has a similar nitrogen content. Fig. 8d shows the specific capacitance in a symmetric two-electrode system. According to the total mass of the two electrodes, the specific capacitances of C-100% CaCO3and C-25%CaCO3samples at 0.05 A g−1are 65.5 and 184.0 F g−1,respectively, where the latter is about twice than that of the former. The energy density-power density curves of the samples are shown in Fig. 8e. The energy densities of the C-100% CaCO3and C-25%CaCO3samples are determined to be 9.0 and 26.0 Wh kg−1with power densities of 1 000 and 1 470.9 W kg−1respectively. Our results are significantly higher than that of the reported activated carbon(5.1 Wh kg−1)[40], hierarchical porous carbon (20.1 Wh kg−1)[41], and white clover carbons (25.0-13.1 Wh kg−1)[42](Table 4).
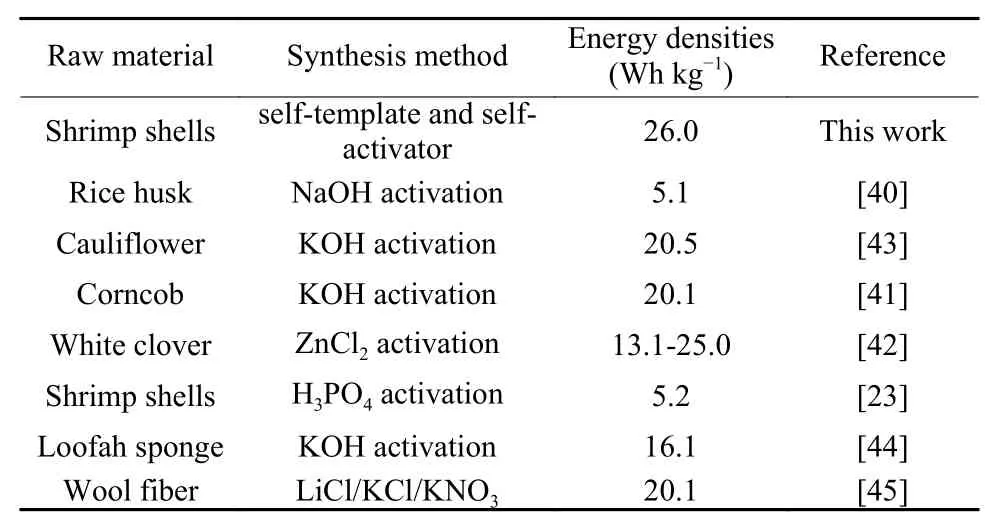
Table 4 Supercapacitor performance comparison ofcarbon materials.
4 Conclusions
In summary, nitrogen-doped porous carbons have been synthesized with adjustable pores using shrimp shell as the carbon/nitrogen source, the CaCO3component as both the template and activator. The process is very efficient and sustainable, where intrinsic CaCO3in shrimp shell plays the role of the activator and the template. The resulting samples have ultrahigh specific surface area and abundant pores, which show the high capacitance (328 F g−1at 0.05 A g−1in KOH), long cycle life, high energy and power densities. The shrimp shell as the waste is cheap and easy to obtain, and it enables the high-value-added utilization of waste.
Acknowledgements
This work is supported by the NSFC (No.51972059, 51901043), Scientific Research Foundation for Leading Scholars in Dongguan University of Technology (DGUT) (GB200902-31), and Research Start-up Funds of DGUT (GC300501-072).
杂志排行
新型炭材料的其它文章
- Guide for Authors
- A correlation of the hydrogen evolution reaction activity to the number of defects formed by the decomposition of doped phosphorus species in carbon nanotubes
- Electrochemical sensing of phenacetin on electrochemically reduced graphene oxide modified glassy carbon electrode
- The synthesis of porous carbons from a lignin-rich residue for high-performance supercapacitors
- 《新型炭材料》征稿简则
- CoN4 active sites in a graphene matrix for the highly efficient electrocatalysis of CO2 reduction
