P-Doped g-C3N4 Nanosheets with Highly Dispersed Co0.2Ni1.6Fe0.2P Cocatalyst for Efficient Photocatalytic Hydrogen Evolution
2022-08-11RongchenShenLeiHaoQingChenQiaoqingZhengPengZhangXinLi
Rongchen Shen , Lei Hao , Qing Chen , Qiaoqing Zheng , Peng Zhang , Xin Li ,*
1 Institute of Biomass Engineering, Key Laboratory of Energy Plants Resource and Utilization, Ministry of Agriculture and Rural Affairs, South China Agricultural University, Guangzhou 510642, China.
2 College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China.
3 State Center for International Cooperation on Designer Low-Carbon & Environmental Materials (CDLCEM), School of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450001, China.
Abstract: Throughout the twentieth century, temperatures climbed rapidly as the use of fossil fuels proliferated and greenhouse gas levels soared. Thus, the need to develop environmentally friendly energy sources to replace traditional fossil fuels is urgent. Clean and highly efficient, hydrogen is considered the most promising energy source to replace traditional fossil fuels. The production of hydrogen by photocatalytic water splitting is environmentally friendly, and is considered the most promising method for producing hydrogen energy. Enhancing the separation efficiency of photogenerated electron-hole pairs has been identified as a key milestone for constructing high-efficiency photocatalysts. However, the construction of efficient and stable hydrogenevolution photocatalysts with highly dispersed cocatalysts remains a challenge. Here, we succeeded, for the first time, in fabricating P-doped CNS (PCNS) with a highly dispersed non-noble trimetallic transition metal phosphide Co0.2Ni1.6Fe0.2P cocatalyst (PCNS-CoNiFeP), by a one-step in situ high-temperature phosphating method. Remarkably, the CoNiFeP in PCNS-CoNiFeP demonstrated no aggregation and high dispersibility compared with CoNiFeP prepared by the traditional hydroxide-precursor phosphating method (PCNS-CoNiFeP-OH). X-ray diffraction, X-ray photoelectron spectroscopy,element mapping images, and high-resolution transmission electron microscopy results demonstrate that PCNS-CoNiFeP was successfully synthesized. The UV-Vis absorption results indicate a slight increase in absorbance for PCNS-CoNiFeP in the 200-800 nm wavelength region compared with that of PCNS. Photoluminescence spectroscopy, electrochemical impedance spectroscopy, and photocurrent results demonstrated that CoNiFeP cocatalysts could effectively promote the separation of photogenerated electron-hole pairs and accelerate the migration of carriers. The linear sweep voltammetry results also demonstrate that the CoNiFeP cocatalyst loading could significantly decrease the overpotential of CNS.Therefore, the maximum hydrogen evolution rate of PCNS-CoNiFeP was 1200 μmol·h-1·g-1, which was approximately four times higher than that of pure CNS-Pt (320 μmol·h-1·g-1) when using TEOA solution as a sacrificial agent. The apparent quantum efficiency of PCNS-CoNiFeP was 1.4% at 420 nm. The PCNS-CoNiFeP also exhibited good stability during the photocatalytic reaction. In addition, the TEM results indicate that CoNiFeP with a size of 6-8 nm are highly dispersed on the PCNS surface. The highly dispersed CoNiFeP demonstrated better charge-separation capacity and higher intrinsic electrocatalytic hydrogen-evolution activity than the aggregated CoNiFeP. Thus, the hydrogen evolution rate of aggregated CoNiFeP-PCNs (300 μmol·h-1·g-1) was much lower than that of PCNS-CoNiFeP. Furthermore, P doping of CNS could improve electric conductivity and charge transport. It is expected that loading highly dispersed CoNiFeP and P doping could be extended to promote photocatalytic hydrogen production using various photocatalysts.
Key Words: Trimetallic phosphide; Photocatalytic hydrogen evolution; Non-noble metal cocatalyst; P doping;Graphitic carbon nitride
1 Introduction
Recently, various studies have been carried out for developing efficient semiconductor photocatalysts for hydrogen evolution in solving various energy and environment problems1-4.Among them, depositing cocatalysts on semiconductor photocatalysts had been regarded as a desired scheme for boosting their photocatalytic hydrogen production, due to the sluggish electrocatalytic hydrogen-evolution kinetics on the surface of bare semiconductors5-8. Although noble metal cocatalysts show high activity and stability, unaffordable price significantly restricts their practical application. Therefore, nonnoble metal-based cocatalysts such as NiS9-13, MoS25,14,15, and Ni3C16,17have been selectively employed as the highly efficient hydrogen-evolution cocatalyts over various semiconductor photocatalysts. Recently, TMPs such as CoxP18,19, NixP19-21,FexP22,23, and MoP24-26, have been attractively emerged as the promising cocatalysts, although such monometallic TMPs cannot provide the ideal photocatalytic activity in some cases.To further improve the catalytic activity of monometallic TMPs,Li and his coworkers found that NiCoP, bimetallic TMP, with hollow polyhedron structure, shows higher electrocatalytic hydrogen evolution than monometallic TMPs such as CoP and Ni2P27,28. Moreover, the intrinsic activity of bimetallic TMP as cocatalyst can be further improved by metal doping. For example, Fe doping has been proven to evidently increase the electrocatalytic activity and change the electronic structure of bimetallic TMPs29,30. In theory31, suitable Fe doping increases the intrinsic activity of bimetallic NiCoP due to the expected multicomponent synergistic effects, thus leading to higher hydrogen-evolution efficiency than the bimetallic TMPs as the cocatalyst. However, so far, there have been few reports regarding the trimetallic TMPs as cocatalysts for highly active and stable photocatalytic hydrogen evolution.
Previous studies on CNS doped by TMPs show low photocatalytic activity owing to some un-intrinsic factors, such as dispersibility of cocatalysts depending on their diameter.Typically, Liet al. reported that CNS doped by CoP with size of 10-12 nm, prepared by the method of two-step hydroxideprecursor evaporate and then phosphating, shows hydrogenproduction rate of 750 μmol·h-1·g-132. Similarly, Chenget al.reported that CNS doped by CoP with size of 5-10 nm prepared by hydrothermal and phosphating method shows the hydrogenproduction rate of 1074 μmol·h-1·g-133. On the contrary, Yiet al.reported that CNS doped by CoP with a large size of 25 nm prepared by a high-temperature phosphating method shows the low hydrogen-production rate of 474 μmol·h-1·g-134. Especially,Sunet al.reported that ZIF-67 modified CNS doped by CoP with a larger size of 60-80 nm displays lower hydrogen-production activity of 67 μmol·h-1·g-135. From those results, it is obviously concluded that the size of CoP as cocatalysts has a strong influence on the photocatalytic activity, implying that smaller size could lead to the higher photocatalytic performance, which could be explained by higher dispersibility and activity of smaller size CoP cocatalysts over CNS. However, beyond monometallic TMPs, it is still challenging to achieve the highly dispersed bimetallic or trimetallic TMP cocatalysts with small sizes and efficient activity through the simple and facile fabrication approaches.
Additionally, it is known that the P atoms could preferentially replace the C atoms or be doped into the interstitial (cave) sites of single-layer g-C3N4nanosheets, both of which could achieve the intrinsic P―N bond polarization and delocalization of lonepaired electrons in electron-rich P atom, which create strong Lewis acid sites (P+) in the CNS36. Accordingly, herein, we, for the first time, designed and constructed P-doped CNS (PCNS)composited with trimetallic transition metal phosphide (TMP),Co0.2Ni1.6Fe0.2P (CoNiFeP) as a Schottky-based cocatalyst(PCNS-CoNiFeP) byin situhigh-temperature phosphating method, where CoNiFeP cocatalysts with the size of 6-8 nm are highly dispersed on the surface of PCNS. The resulting PCNSCoNiFeP exhibits higher photocatalytic hydrogen-production rate of 1200 μmol·h-1·g-1with AQE of 1.4% at 420 nm. The desirable photocatalytic activity could be attributed to the following two main reasons: (1) P doping could induce more delocalization of electrons in PCNS than in CNS, resulting in increasing the electric conductivity and electron transport, (2)CoNiFeP, trimetallic cocatalyst, show higher intrinsic electrocatalytic hydrogen-evolution activity than those of bimetallic and monometallic TMPs, due to higher dispersibility and more exposed active sites. It is anticipated that both loading CoNiFeP and P doping can be extended to enhance the photocatalytic hydrogen production over various nanostructured semiconductor photocatalysts.
2 Experimental section
2.1 Materials
2.1.1 Raw materials
All chemical reagents are of analytic grade and used as received without further purification. Urea (≥ 99%), Na2HPO4(≥ 99%), Co(NO3)2·6H2O (≥ 99%), Ni(NO3)2·6H2O (≥ 99%),Fe(NO3)3·9H2O (≥ 98.5%), Triethanolamine (TEOA) (≥ 98%),H2SO4(95.0%-98%), NaOH (≥ 95%).
2.1.2 Synthesis of CNS
Firstly, urea was heated at 550 °C for 4 h. Then the CNS were obtained after heating the bulk CNS at 550 °C in air for another 2 h.
2.1.3 Synthesis of PCNS
500 mg of as-prepared CNS and 100 mg of Na2HPO4were placed in an alumina crucible and heated at 350 °C under N2flow for 2 h. Then the mixture was washed with distill water several and ethanol.
2.1.4 Synthesis of PCNS-Co0.2Ni1.6Fe0.2P
300 mg of as-prepared CNS and Co(NO3)2·6H2O,Ni(NO3)2·6H2O, or Fe(NO3)3·9H2O with different weight ratios were dispersed in 50 mL of distill water. In detail, the mass of Co(NO3)2·6H2O, Ni(NO3)2·6H2O, and Fe(NO3)3·9H2O were 4,32, 4 mg, respectively. The suspension was ultrasonicated for 30 min, and evaporated in an 85 °C water bath to yield CNSCo0.2Ni1.6Fe0.2. Finally, PCNS-Co0.2Ni1.6Fe0.2P (CoNiFeP) was prepared by heating as-prepared CNS-Co0.2Ni1.6Fe0.2and Na2HPO4at 350 °C under N2flow for 2 h.
2.1.5 Synthesis of PCNS-Co0.2Ni1.6Fe0.2P-OH
300 mg of as-prepared CNS and Co(NO3)2, Ni(NO3)2, or Fe(NO3)2were dispersed in 50 mL of distill water. The mass of Co(NO3)2·6H2O, Ni(NO3)2·6H2O, and Fe(NO3)3·9H2O were 4,32, 4 mg, respectively. The suspension was ultrasonicated for 30 min. Next, 2 mol·L-1NaOH (7.5 mL) was added into the suspension to yield CNS-CoNiFe(OH)x. PCNS-Co0.2Ni1.6Fe0.2POH (CoNiFeP-OH) was prepared by heating as-prepared CNSCoNiFe(OH)xand Na2HPO4at 350 °C under N2flow for 2 h.
2.2 Characterization
XRD, XPS, and TEM and HRTEM, were measured with Ultima IV-ray diffractometer (Rigaku, Japan), VG ESCALAB250 (Thermo-fisher, USA), and JEM-2100HR at 200 kV (Thermo-fisher, USA), respectively. The diffuse reflection spectra were measured with UV-Vis spectrometer (Shimadzu UV-2550, Japan).
2.3 Photocatalytic hydrogen production
Photocatalytic hydrogen production was carried out in a 100 mL three neck flat-bottom Pyrex flask. In a typical process,PCNS-CoNiFeP (10 mg) was dispersed in 10 mL water together with TEOA. The system irradiated with a 350 W Xe lamp (PLSSXE300, Beijing Perfect Light Technology,λ> 420 nm). During the photocatalytic reaction. The production hydrogen was detected by GC-9500 online chromatograph. The following equation was used to calculate the AQE.

2.4 Photoelectrochemical measurement
Photoelectrochemical experiments were carried out on a CHI660E electrochemical station (Chenhua, Shanghai). The detailed of photoelectrochemical measurement are displayed in our previous work37.

Fig. 1 Schematic illustration of preparation of PCNS-CoNiFeP.
3 Results and discussion
TPCNS-CoNiFeP was prepared byin situhigh-temperature phosphating method (Fig. 1). First, CNS was prepared by directly heating urea. Then, CNS-CoNiFe was obtained by evaporating CNS, Co(NO3)2, Ni(NO3)2, and Fe(NO3)3suspension in a water bath. CNS-CoNiFe was successfully converted into PCNS-CoNiFeP by one-stepin situhightemperature phosphating method. Details are described in the experimental section.
The crystalline structure of PCNS-CoNiFeP was characterized by XRD (Fig. 2). Two obvious diffraction peaks could be observed at around 27° and 13° for both CNS and PCNS-CoNiFeP to be assigned to (002) and (100) planes of CNS, respectively38. Due to high dispersibility of CoNiFeP, the same XRD pattern was observed for CNS and PCNS-CoNiFeP without any peak shifts, indicating that CoNiFeP don’t affect the crystal structure of CNS39. Pure XRD spectrum of Co0.2Ni1.6Fe0.2P are displayed in Fig. 2B, these peaks are attributed of the (111), (201), (210), (002), (300) planes of Fe2P(PDF51-0943) and (111), (201), (210), (002), (211), (212) planes of the CoNiFeP (PDF71-2336).
The dispersibility of CoNiFeP and the morphology of PCNSCoNiFeP, were further studied by TEM (Fig. 3A). No CoNiFeP was found on the surface of PCNS, suggesting high dispersibility of CoNiFeP. Since no lattice fringe of CNS was observed in the HRTEM image due to the low crystallinity, while that of CoNiFeP was observed. The red squares show CoNiFeP deposited on the surface of PCNS have size of 6-8 nm (Fig. 3B).In the case of PCNS-CoNiFeP-OH prepared by the traditional hydroxide-precursor phosphating method, CoNiFeP-OH are inclined to aggregate on the surface of CNS to have size of 30-60 nm (Fig. 3C,D). One-stepin situhigh-temperature phosphating method does not result in the unexpected aggregation of CoNiFeP, compared to the traditional hydroxideprecursor phosphating method. The lattice spacing values of 0.203 and 0.215 nm are attributed to the (2 0 1) and (1 1 1) planes of CoNiP, respectively (PDF#71-2336). The results are the same as the previous report about iron-doped nickel cobalt phosphide electrocatalyst40. The elemental mappings on STEM image show that Co, Ni, Fe, and P elements are uniformly dispersed on the surface of PCNS-CoNiFeP (Fig. 4A-G). All these results confirm that CoNiFeP have been highly dispersed on the surface of P-doped CNS.
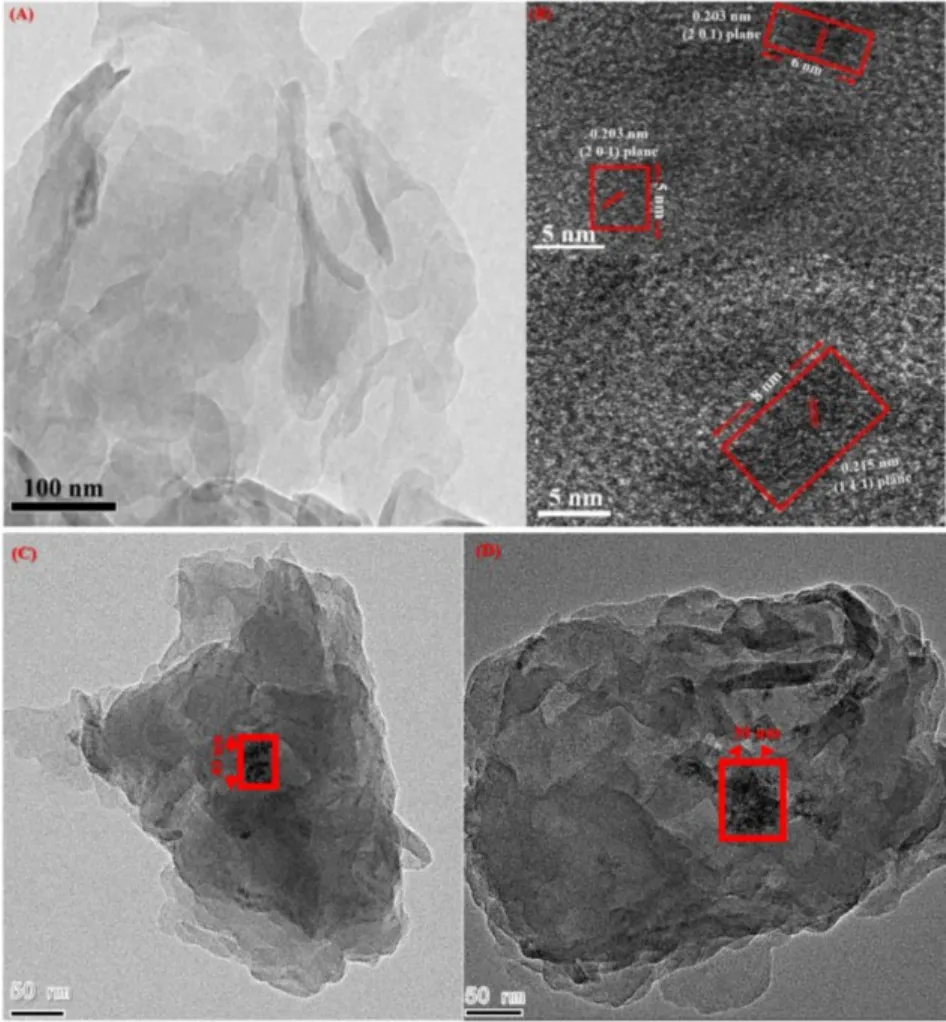
Fig. 3 (A) TEM and (B) HRTEM of PCNS-CoNiFeP where red squares represent CoNiFeP with the lattice fringes of the crystal planes. (C) and (D) TEM images of CNS-CoNiFeP-OH where the squares represent CoNiFeP-OH aggregates. Color online.
The surface chemical properties of PCNS-CoNiFeP were studied by XPS (Fig. 5A). Two peaks in C 1sspectrum were assigned to N―C=N and graphitic carbon, respectively41-43. In N 2pregion (Fig. 5B), four peaks at 398.6, 399.9, 401.2, and 404.1 eV were observed to be assigned to N atoms of C―N=C, N―C3, and C―N―H, respectively44-46. In P 2pregion (Fig.5C), two peaks at were assigned to oxidized phosphorus species and P―N bond, respectively47. The formation of P―N bond is resulted from substitution of C by P in triazine rings of CNS48,49,indicating that P doping for CNS means the substitution of C by P in a part of CNS. No peak was observed for P―metal bond.The traditional hydroxide-precursor phosphating method also shows a peak at 133.0 eV, suggesting P-doping in PCNSCoNiFeP-OH (Fig. 5D). All results confirm that P atoms have been successfully doped into the CNS.

Fig. 2 XRD patterns of (A) CNS and PCNS-CoNiFeP as well as (B) Co0.2Ni1.6Fe0.2P.

Fig. 4 (A) STEM images and (B-G) elemental mappings of PCNS-CoNiFeP.
Photocatalytic H2evolution was examined in the presence of TEOA as a sacrificial agent under visible light irradiation. Pure CNS showed poor photocatalytic hydrogen-production rate of 0.5 μmol·h-1·g-1. The hydrogen-production rate of PCNS was 6 μmol·h-1·g-1, which was 12 times higher than that of CNS. More surprisingly, PCNS-CoNiFeP displayed the hydrogenproduction rate of 1200 μmol·h-1·g-1, which was 2400 times higher than that of CNS. Furthermore, the photocatalytic activity of PCNS-CoNiFeP is higher than those of bimetallic and monometallic TMPs modified PCNS (Fig. 6A). The photocatalytic hydrogen-production rate of CNS-Pt was 320 μmol·h-1·g-1, which was lower than that of PCNS-CoNiFeP (Fig.6B), indicating that CoNiFeP work as a more efficient cocatalyst for PCNS compared with Pt for CNS. PCNS-CoNiFeP showed higher photocatalytic H2evolution activity than PCNSCoNiFeP-OH (Fig. 6C), due to higher dispersibility of CoNiFeP in the former than the latter. The H2evolution rate of PCNSCoNiFeP was constant after 8-cycle experiments, indicating the good stability of CoNiFeP on the surface of PCNS (Fig. 6B).PCNS-CoNiFeP showed AQE of 1.4% at 420 nm. The photocatalytic hydrogen-production rate of PCNS-CoNiFeP was higher than those in most previously reported TMP-doped CNS photocatalytic reactions (Table 1)14,34,35,37,50-61.
UV-Vis absorption spectrum of as-prepared PCNS-CoNiFeP shows a slight increase in the wavelength region of 200-800 nm compared with that of PCNS (Fig. 7A). The increase is consistent with the color of PCNS-CoNiFeP (Fig. 7B). The absorption edge of CNS does not shift after CoNiFeP loading and P doping, which could be attributed to the low content of P in the PCNS-CoNiFeP and PCNS. The bandgap energies of CNS and PCNS-CoNiFeP are calculated to be 2.61 and 2.63 eV,respectively (Fig. 7C). Mott-Schottky (MS) was used to determine the energy levels of valence band (VB) and conduction band (CB). The flat bands (FB) level of CNS and PCNS-CoNiFeP are -1.39 and -1.20 eV (vs. Ag/AgCl),respectively (Fig. 7D). Therefore, the CB level of CNS and PCNS-CoNiFeP are -1.59 and -1.4 eV, respectively. The VB level of CNS and PCNS-CoNiFeP are 1.02 and 1.23 eV,respectively62. Since Ag/AgCl electrode has a potential of 0.61 Vvs. RHE at pH 7, CB and VB energies of CNS are estimated to be -0.98 and 1.63 eV, while those of PCNS-CoNiFeP are calculated to be -0.79 and 1.84 eVvs. RHE, respectively.

Fig. 5 XPS of (A) C 1s, (B) N 1s, (C) P 2p of PCNS-CoNiFeP, and (D) P 2p of PCNS-CoNiFeP-OH.
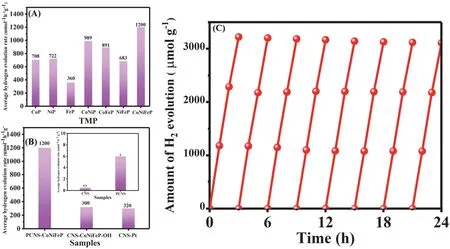
Fig. 6 (A) Average hydrogen evolution rate for PCNS-TMP, (B) Compared average hydrogen evolution rate for CNS, PCNS, PCNS-CoNiFeP,CNS-Pt, and PCNS-CoNiFeP-OH. (C) Repeated photocatalytic hydrogen evolution for PCNS-CoNiFeP.

Photocatalyst Light source Activity (μmol·h-1·g-1) AQE (%) Reference PCNS-CoNiFeP 300 W Xe lamp, λ > 420 nm 1200 1.4% (420 nm) this work CNS-Ni2P-MoS2 300 W Xe lamp, λ > 420 nm 532.41 1.4% (420 nm) 14 CNS-CoP 300 W Xe lamp, λ > 420 nm 956 50 CNS-CoP 300 W Xe lamp, λ > 420 nm 948 4.3% (420 nm) 51 CNS-CoP 300 W Xe lamp, λ > 420 nm 201.5 0.12% (420 nm) 35 CNS-CoP 300 W Xe lamp, λ > 420 nm 840 52 CNS-CoP 300 W Xe lamp, λ > 420 nm 474.4 34 CNS-Co2P-K2HPO4 300 W Xe lamp, λ > 420 nm 556 53 CNS-CoNiP 300 W Xe lamp, λ > 420 nm 230 54 Ni2P/Ni@C-CNS 300 W Xe lamp, λ > 420 nm 210 55 EY-CNS- Ni2P 300 W Xe lamp, λ > 400 nm 1540 56 CNS-Ni2P 300 W Xe lamp, λ > 420 nm 270 2.85% (420 nm) 57 CNS-Ni2P 300 W Xe lamp 3344 9.1% (420 nm) 58 Cu3P-CNS 300 W Xe lamp, λ > 420 nm 159.41 37 CNS-Ni2P 300 W Xe lamp, λ > 420 nm 474.7 3.2% (420 nm) 59 CNS-Ni12P5 300 W Xe lamp, λ > 420 nm 126.61 60 CNS-Ni2P Xe lamp, λ > 420 nm 162 61
To understand the improved photocatalytic performance of PCNS-CoNiFeP, photoluminescence (PL) was also carried out(Fig. 8A). In general, lower PL intensity means efficient charge separation, indicating higher photocatalytic performance63-65.Since PL intensity of PCNS-CoNiFeP and PCNS is lower than that of CNS, indicating that P doping of CNS and loading of highly dispersed CoNiFeP decrease the recombination of photogenerated electron-hole pairs, due to the improved charge trapping by the introduced P and CoNiFeP cocatalysts as the electron traps. The photocurrents of PCNS-CoNiFeP and PCNS are higher than that of CNS (Fig. 8B), suggesting the higher conductivity induced by CoNiFeP loading and P doping on CNS.Lower resistance during current in the Nyquist plots is observed for PCNS-CoNiFeP and PCNS than CNS (Fig. 8C), suggesting the promoted interfacial charge separation66-68. The surface electrocatalytic hydrogen-evolution overpotential of PCNSCoNiFeP is found to be more negative than those of PCNS and CNS, indicating that CoNiFeP enhance the surface electrocatalytic HER reaction (Fig. 8D). It has been previously reported that P doping into CNS increases the conductivity and charge mobility49,69. Thus, the high photocatalytic activity of PCNS-CoNiFeP is ascribed to two main reasons, 1) the P doping causes more delocalization of electrons in PCNS than in CNS,to increase the electric conductivity and electron transport, 2)CoNiFeP shows higher intrinsic activity than those of bimetallic and monometallic TMPs due to higher dispersibility and more exposed active sites.

Fig. 7 (A) Diffuse reflectance spectra, (B) pictures of CNS, PCNS, and PCNS-CoNiFeP, (C) bandgap analyses, and(D) Mott-Schottky plots for CNS and PCNS-CoNiFeP.

Fig. 8 (A) photoluminescence (PL) spectra, (B) photocurrents, (C) EIS spectra, and (D) polarization curves for CNS, PCNS, and PCNS-CoNiFeP.

Fig. 9 Diffuse reflectance spectra of CNS-CoNiFeP-OH and PCNS-CoNiFeP.

Fig. 11 Schematic illustration of photocatalytic H2 evolution for PCNS-CoNiFeP.

Fig. 10 (A) PL spectra and (B) photocurrents for CNS-CoNiFeP-OH and PCNS-CoNiFeP.
To further confirm high dispersibility of CoNiFeP, we compared the UV-Vis absorption (Fig. 9), PL and photocurrent of PCNS-CoNiFeP and PCNS-CoNiFeP-OH. Both show the similar light absorption spectra, while PCNS-CoNiFeP exhibits weaker PL intensity and higher photocurrent than PCNSCoNiFeP-OH (Fig. 10A,B). These results can be explained by the high aggregation of CoNiFeP in PCNS-CoNiFeP-OH photocatalysts, while CoNiFeP are highly dispersed for PCNSCoNiFeP. Since it is well known that CoNiFeP generally act as the surface electrocatalytic hydrogen-evolution sites, the observed photocatalytic H2evolution rate is higher for PCNSCoNiFeP than for PCNS-CoNiFeP-OH, indicating higher dispersibility and more exposed active sites of CoNiFeP in the former.
As shown in Fig 11, the plausible photocatalytic H2-evolution mechanism for PCNS-CoNiFeP with band levels of CNS and PCNS-CoNiFeP and potential energies for hydrogen and oxygen production from water. Under visible light irradiation, CNS is excited to generate electron-hole pairs in CB and VB, respectively. Due to fast recombination of photogenerated electrons and holes, CNS shows a low photocatalytic hydrogen-production performance. However,after loading CoNiFeP on PCNS, the photogenerated electrons in CB of PCNS are effectively trapped by CoNiFeP due to the formation of favorable Schottky-based heterojunctions. The trapped electrons in CoNiFeP were then dominantly used for reducing water to generate H2on the surface of CoNiFeP, due to its intrinsic electrocatalytic H2-evolution activity. Furthermore,P doping into CNS increases the electric conductivity of CNS due to more delocalization of electrons in PCNS with P―N bonds than in CNS, leading to the efficient transport of electrons from PCNS to CoNiFeP69. Meanwhile, the holes in VB of PCNS are quenched by the sacrificial agent of TEOA. Consequently, it is concluded that P doping and loaded CoNiFeP improve the photocatalytic hydrogen-production performance of CNS.
4 Conclusions
In summary, PCNS-CoNiFeP was successfully synthesized by one-stepin situhigh-temperature phosphating method, which shows highly efficient photocatalytic H2evolution, resulted from the excellent synergistic effects of high dispersed CoNiFeP as a Schottky-based cocatalyst and P doping of CNS. PCNSCoNiFeP show the photocatalytic hydrogen-production rate of 1200 μmol·h-1·g-1with AQE of 1.4% at 420 nm. The high dispersibility of CoNiFeP on CNS leads to enhance charge trapping, separation, and transport to CoNiFeP, thus boosting the efficient photocatalytic H2evolution. More delocalization of electrons induced by P doping in PCNS could improve the electric conductivity, resulting in more efficient photogenerated electron transport form PCNS to CoNiFeP. It is anticipated that developing TMP as cocatalysts and P doping can be extended to boost the hydrogen production activity over various kinds of semiconductor photocatalysts.
杂志排行
物理化学学报的其它文章
- Insights into Mechanism of CsPbBr3 Nanocrystal Interfacial Modifier in Perovskite Solar Cells
- Enhanced Photocatalytic CO2 Reduction over 2D/1D BiOBr0.5Cl0.5/WO3 SScheme Heterostructure
- Hollow NiCo2S4 Nanospheres as a Cocatalyst to Support ZnIn2S4 Nanosheets for Visible-Light-Driven Hydrogen Production
- A 0D/2D Bi4V2O11/g-C3N4 S-Scheme Heterojunction with Rapid Interfacial Charges Migration for Photocatalytic Antibiotic Degradation
- Rationally Designed Mn0.2Cd0.8S@CoAl LDH S-Scheme Heterojunction for Efficient Photocatalytic Hydrogen Production
- Enhancement of Photocatalytic H2-Evolution Kinetics through the Dual Cocatalyst Activity of Ni2P-NiS-Decorated g-C3N4 Heterojunctions
