Rationally Designed Mn0.2Cd0.8S@CoAl LDH S-Scheme Heterojunction for Efficient Photocatalytic Hydrogen Production
2022-08-11ShanchiLiuKaiWangMengxueYangZhiliangJin
Shanchi Liu , Kai Wang , Mengxue Yang , Zhiliang Jin
1 School of Chemistry and Chemical Engineering, North Minzu University, Yinchuan 750021, China.
2 Ningxia Key Laboratory of Solar Chemical Conversion Technology, North Minzu University, Yinchuan 750021, China.
3 Key Laboratory for Chemical Engineering and Technology, State Ethnic Affairs Commission, North Minzu University,Yinchuan 750021, China.
Abstract: Constructing an efficient and stable heterojunction photocatalyst system is a promising approach to achieve solar-driven water splitting to produce hydrogen. In this work, a novel Mn0.2Cd0.8S@CoAl LDH (MCCA) Sscheme heterojunction was successfully prepared through the efficient coupling of Mn0.2Cd0.8S nanorods and CoAl LDH nanosheets, employing a physical mixing method. The photoluminescence and photocurrent-time response results demonstrated that the internal electric field of the constructed MCCA S-scheme heterojunction could successfully accelerate charge separation and electron transfer between the Mn0.2Cd0.8S interface and the CoAl LDH. Critically, the introduction of the CoAl LDH effectively inhibited the recombination of photogenerated electrons and holes, thereby improving the photocatalytic hydrogen production activity of Mn0.2Cd0.8S. A maximum H2 production of 1177.9 μmol in 5 h was obtained with MCCA-3. This represents a significant improvement compared to what can be achieved with the pure Mn0.2Cd0.8S nanorods and CoAl LDH nanosheets individually. This work provides a simple and effective approach for the rational design of S-scheme heterojunction photocatalysts for photocatalytic hydrogen production.
Key Words: Mn0.2Cd0.8S; CoAl LDH; S-scheme heterojunction; Photocatalytic H2 production
1 Introduction
It is urgent to develop clean energy because the massive burning of conventional fossil fuels not only brings a variety of environmental problems but also triggers energy crises1. With the advantage of overwhelmingly high utilization value and nonpolluting nature, hydrogen energy has attracted a lot of attention2. However, traditional hydrogen production methods are faced with the defects of huge energy consumption and harmful by-products production. Since TiO2were discovered could produce H2under ultraviolet light in 19723-5, numerous efforts have been made to design high-performance, low-costing and highly stable photocatalysts to use solar energy to produce H2. Numbers of semiconductors for H2generation by splitting H2O have been known in the past, such as CdS6,7,Zn0.5Cd0.5S8, MoS29, g-C3N410,11, and so on. However, single photocatalyst occurs with poor light response, quick combination of electron and hole, less reactivity sites and severe photocorrosion that severely restrict its hydrogen production efficiency. To prepare photocatalytic materials with superior performance, the researchers at home and abroad keeps great interest on heterojunction photocatalysts12-15.
Metal sulfide semiconductors has been widely attention due to its higher catalytic activity and adjustable band structure16,17.And among them, MnCdS solid solution has attracted wide attention since it was first reported in 201018. Nevertheless, high recombination rate of photogenerated carriers limits the photocatalytic hydrogen production performance of MnCdS. For this reason, it plays a crucial role to build heterojunctions to better separate photogenerated electrons and holes, which closely relate to photocatalytic hydrogen production reaction19. For example, based on Mn0.25Cd0.75S and Co3O4, ap-ntype heterojunction was successful built by Huanget al., the heterojunction photocatalyst could forceful promote the transfer and separation of the interface charges, so as to improve the H2generation ability of Mn0.25Cd0.75S20. Anchoring CoWO4nanoparticles on Mn0.2Cd0.8S could also synthesize a high efficiencyp-ntype semiconductor photocatalysts, the H2production rate of the prepared photocatalysts is very efficient21.
With the characteristic of strong redox capacity and large light absorption capacity, layered double hydroxides (LDHs), a sandwich structure materials formed through non-covalent interactions, have been considered as ideal electrode material22.Transition metal-based LDHs, take CoAl LDH for instance, also showcase prominent advantage in catalytic materials since tunable composition and high dispersion of cations in host layers23. However, recombination of electrons and slow carriers seriously inhibits the application of LDHs in the field of photocatalysis24. Combining LDHs with other semiconductor materials to get photocatalyst material with excellent performance is one of the efficient ways to use layered double hydroxides. By a three-step reaction of kapok carbonization,precursor impregnation, and hydrothermal assembly, MoS2and CoAl LDH were uniformly grown on the carbon-based substrate prepared a heterojunctions equipped with exceptional synergistic adsorption-photocatalytic activity in visible-light degradation of Congo Red25. By coupling Co(OH)2with ZnCr LDHA highly, an efficient bifunctional heterojunction material were architectured, in which photoinduced charge carriers could be effectively separated and transported. So this heterojunction material shows out enhanced photoreactivity compared with ZnCr LDH26.
It is reported by Xuet al.that the S-scheme heterojunction can eliminate useless electrons and holes, and provide more electrons to participate in photocatalytic H2production27. Based on the understanding of S-scheme heterojunction, we anchored CoAl LDH nanoflakes on Mn0.2Cd0.8S nanorods by a classic selfassembly method to get a S-scheme Mn0.2Cd0.8S@CoAl LDH heterojunction with high efficiency in producing hydrogen. The heterostructure formed between Mn0.2Cd0.8S and CoAl LDH possess superior redox performance. Through a series of tests,the structure and the reason for excellent H2production properties of materials were studied.
2 Experimental
2.1 Materials preparation
All reagents were of analytical grade and were used without further purification: thioacetamide (CH3CSNH2, 99.0%),manganous acetate (C4H6O4Mn·4H2O, 99.0%), cadmium acetate (C4H6CdO4·2H2O, 99.0%), ethylenediamine(NH2CH2CH2NH2, 98.0%), cobalt nitrate hexahydrate(Co(NO3)2·6H2O, 99%), aluminum nitrate nonahydrate(Al(NO3)3·9H2O, ≥ 99.5%), sodium hydroxide (NaOH, ≥96.0%), sodium carbonate anhydrous (Na2CO3, ≥ 99.8%).
2.1.1 Fabrication of Mn0.2Cd0.8S nanorods
Adding 12.5 mmol of CH3CSNH2, 7.2 mmol of C4H6CdO4·2H2O and 1.8 mmol of C4H6O4Mn·4H2O into a beaker containing 60 mL of C2H8N2to form a mixed solution.After 30 min of ultrasound treatment, the solution was transferred to an autoclave and kept it 160 °C for 24 h. Using deionized water and anhydrous ethanol cleaning the got mixed solution for several times after it cooled to room temperature.After drying, the yellow powder was named as Mn0.2Cd0.8S21.
2.1.2 Fabrication of CoAl LDH nanosheets
CoAl LDH is synthesized by previous report28. In a typical process, dissolving 1.5 mmol Na2CO3and 4 mmol NaOH into ultrapure water, the obtained homogeneous solution was named as A. At the same time, 0.5 mmol Al(NO3)3·9H2O and 1.5 mmol Co(NO3)2·6H2O was similarly added into a beaker with pure water as the solvent. The resulting solution was uniformly dispersed by ultrasound method and was recorded as B. Solution A was slowly added to solution B under severe agitation. The CoAl LDH was got by washing, centrifuging and drying after keeping the mixed solution at 120 °C for 16 h.
2.1.3 Fabrication of Mn0.2Cd0.8S@CoAl LDH
0.1 g Mn0.2Cd0.8S and 30 mL of ethanol was mixed by ultrasound to form a homogeneous solution. Addingxg (x=0.005, 0.010, 0.015, 0.020, 0.025) CoAl LDH to the beaker contains the above solution. The mixed solution was heated to evaporate ethanol, and dried overnight to get the final sample.Finally, based on the addition of CoAl LDH, the resulting samples were named as MCCA-x(x= 1, 2, 3, 4, 5).
2.1.4 Characterization
By an advanced diffractometer operating (Rigaku RINT-2000, Japan), X-ray diffraction (XRD) measurements of materials was carried out at 40 kV and 30 mA. The microstructures of the samples were tested by scanning electron microscope (SEM, Carl Zeiss AG, Germany), transmission electron microscopy (TEM, FEI, USA) and highresolution transmission electron microscope (HRTEM, FEI, USA). The Xray photoelectron spectroscopy (XPS, ESCALAB 250Xi,Thermo-fisher, USA) measurements were performed to detect the element composition. Using BaSO4as the reference for baseline correction, UV-Vis diffuse reflectance spectra (DRS)were tested by a UV-2550 (Shimadzu, Japan) spectrometer. The Brunauer-Emmett-Teller (BET) specifc surface area was taken using a Micromeritics ASAP2020M (USA) apparatus.Photoluminescence spectroscopy was got by a FLUOROMAX-4 spectrophotometer (France) at room temperature. In addition,using a Princeton electrochemical workstation (USA) to perform photoelectrochemical measurement with 0.2 mol·L-1Na2SO4as electrolyte (pH = 7).
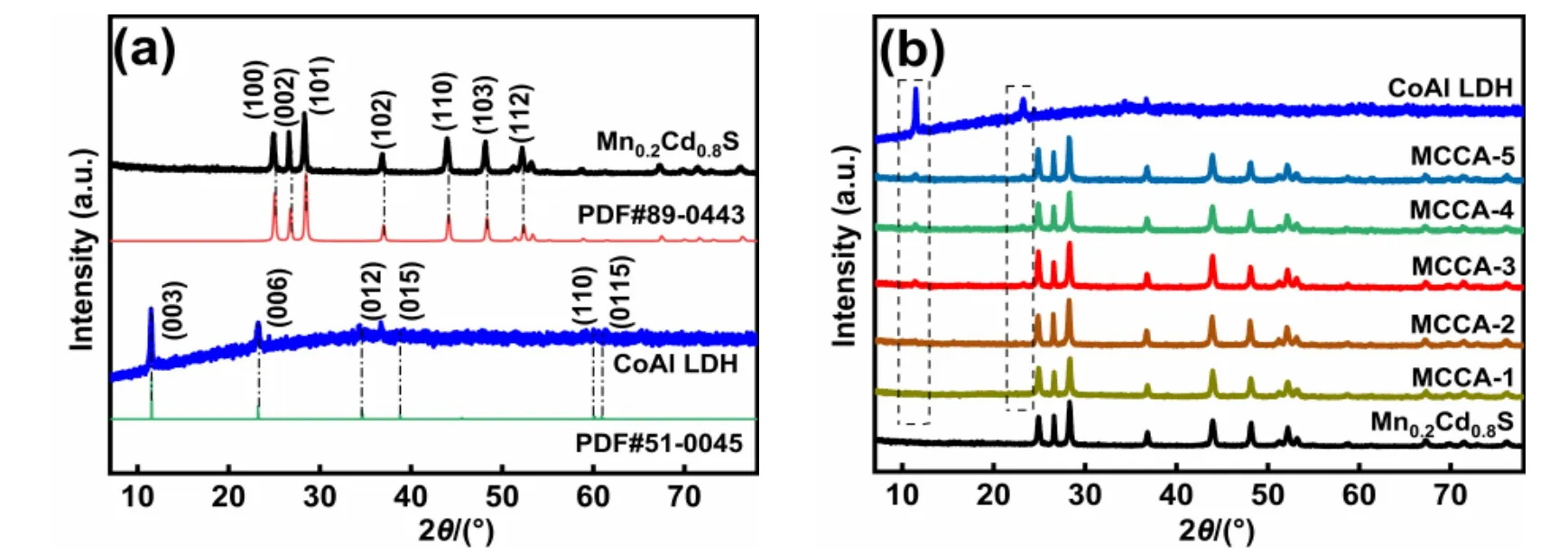
Fig. 1 XRD pattern of the sample (a) Mn0.2Cd0.8S and CoAl LDH; (b) CoAl LDH, Mn0.2Cd0.8S and MCCA-x (x = 1, 2, 3, 4, 5) composite material.
2.1.5 Photocatalytic hydrogen evolution
Taking a 62 mL quartz reaction flask as reaction container, the photocatalytic H2production test was performed. Specific steps were as follows: adding 10 mg sample and 30 mL of Na2S/Na2SO3(composed of 0.35 mol·L-1Na2S and 0.25 mol·L-1Na2SO3) solution into the reaction container and a homogeneous solution is formed by ultrasonic mixing. Using nitrogen to remove air in the system to form anaerobic environment. Taking N2as a carrier, the H2generation was tested by gas chromatography (Tianmei GC7900, TCD, 13X column, USA).
2.1.6 Hydroxyl radical experiment
Taking terephthalic acid (PTA) as probe to detect the surface hydroxyl radicals of Mn0.2Cd0.8S, CoAl LDH and MCCA-3.Dispersing 0.1 g of catalyst in 100 mL of 0.02 mol·L-1PTA and 0.1 mol·L-1NaOH mixed solution. Under the irradiation of 300 W xenon lamp, a certain amount of solution was sucked out and filtered every 15 min for fluorescence test at excitation wavelength of 315 nm.
3 Results and discussion
3.1 XRD analysis
The XRD pattern of Mn0.2Cd0.8S shown in Fig. 1a, in which the diffraction peaks at 25.0°, 26.8°, 28.5°, 37.0°, 44.1°, 42.4°and 48.4° are corresponding to (100), (002), (101), (102), (110),(103) and (112) faces of Mn0.2Cd0.8S (PDF#89-0443).Obviously, Mn0.2Cd0.8S possess clear and sharp diffraction peak,which could indicate it good crystallinity and no other impurities. At the same time, it can be observed that the XRD peaks of CoAl LDH (PDF#51-0045) appearing at 11.3°, 23.0°,34.3°, 38.8°, 59.7° and 61.2° belongs to (003), (006), (012),(015), (110) and (0115) planes. The diffraction peaks of Mn0.2Cd0.8S and CoAl LDH can be found from the XRD patterns of MCCA-3, as shown in Fig. 1b. The diffraction peaks intensity of CoAl LDH gradually increase in MCCA-x(x= 1, 2, 3, 4, 5)with the increase of CoAl LDH addition. According to the above phenomenon, it can be analyzed that both Mn0.2Cd0.8S and CoAl LDH were successfully prepared, and the MCCA-3 were constructed by coupling Mn0.2Cd0.8S and CoAl LDH.
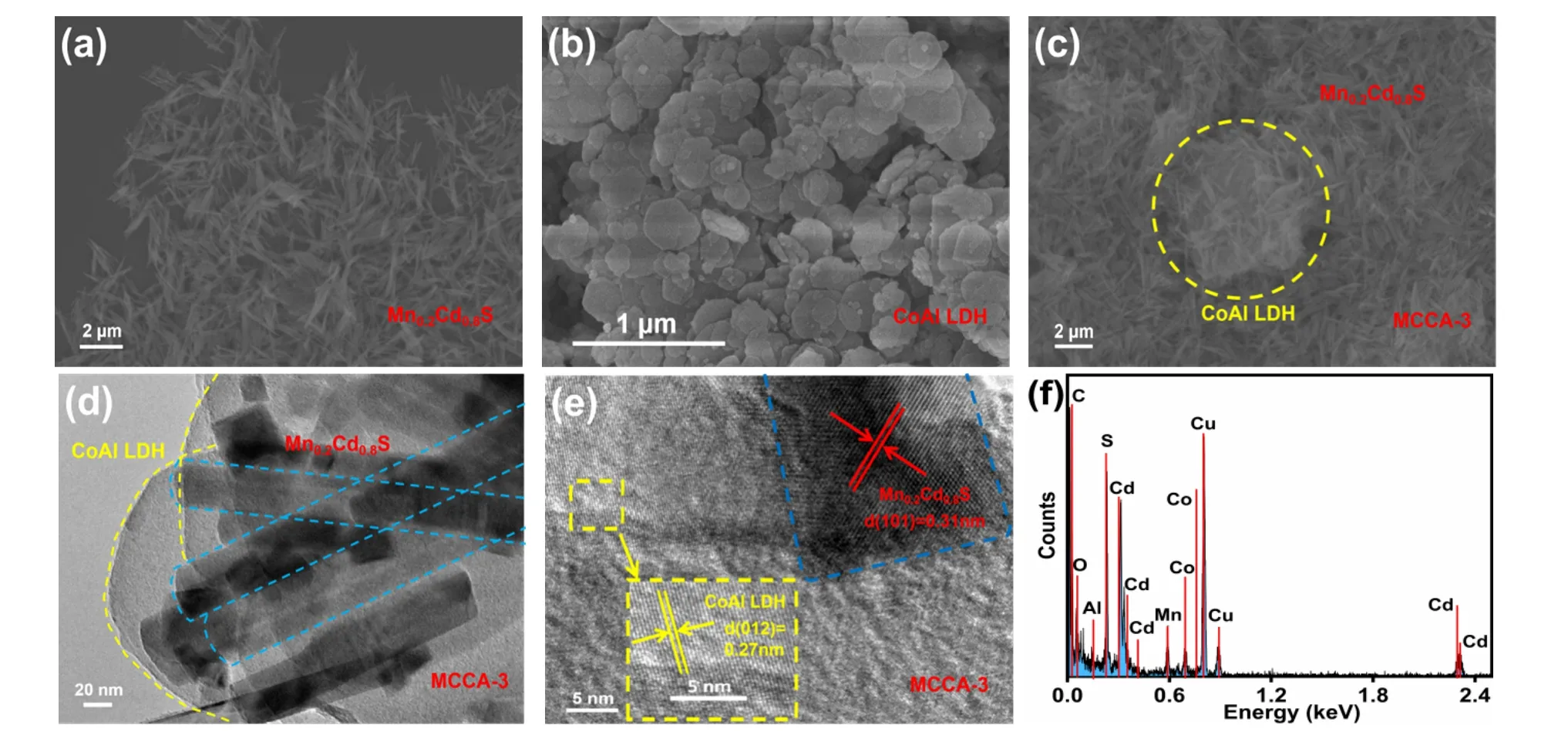
Fig. 2 SEM images of (a) pristine Mn0.2Cd0.8S, (b) CoAl LDH and (c) MCCA-3; (d, e) HRTEM images and (f) EDX of MCCA-3.
3.2 SEM and TEM analysis
The surface morphology and the microstructure of pure Mn0.2Cd0.8S, pristine CoAl LDH and the MCCA-3 composite material were explored by SEM and TEM. In Fig. 2a, pure Mn0.2Cd0.8S exhibits out the typical structure of nanorods,consistent with the previous report29. Different from Mn0.2Cd0.8S, CoAl LDH shows the characteristics of the nanosheets (Fig. 2b). The successful construction of the composite MCCA-3 photocatalyst can be verified by the tight coupling of Mn0.2Cd0.8S and CoAl LDH, as observed in Fig. 2c.The close contact of the two materials can speed up the transfer of photoelectron, which is in favour of the H2generation.Moreover, the successful preparation of MCCA-3 also reflected in the TEM image (Fig. 2d). The HRTEM image of MCCA-3 with clear lattice fringes were displayed in Fig. 2e. Concrete analysis, the crystal distance of 0.31 nm belongs to the (101)crystal planes of Mn0.2Cd0.8S and 0.27 nm marked the (012)plane of CoAl LDH, similar to the XRD results. The above results confirming that Mn0.2Cd0.8S and CoAl LDH is not simple coexist in the composite photocatalyst but a tight connection.The EDX (short for energy dispersive X-ray) spectrum of the prepared composite photocatalyst was exhibited in Fig. 2f, from which, elements such as Mn, Cd, S Co, Al, O, C and O can be clearly observed. To sum up, the MCCA-3 photocatalyst was successful prepared by close coupling Mn0.2Cd0.8S and CoAl LDH.
3.3 X-ray photoelectron spectroscopy
The detailed chemical composition and elemental state of materials were studied by XPS30. The full survey spectrum of Mn0.2Cd0.8S, CoAl LDH and MCCA-3 are displayed in Fig. 3a,
Fig.3 The XPS spectra of Mn0.2Cd0.8S, CoAl LDH and MCCA-3 composite catalyst.from which, both the element in Mn0.2Cd0.8S (Mn, Cd, S, C, O)and CoAl LDH (Co, Al, C, O) could be found in MCCA-3,implying the successful combination of Mn0.2Cd0.8S and CoAl LDH. From Fig. 3b, 780.0 eV and 796.1 eV represent the Co 2p3/2and Co 2p1/2orbital, which may be caused by the small number of cobalt ions in CoAl LDH. And the peak at the positions of 802.9 eV and 786.1 eV corresponding to the satellite peaks of the Co 2p1/2and Co 2p3/2, indicating the oxidation state cobalt31,32. The band energy at 782.2 eV was indicative of cobalt(III), and that at 797.4 eV corresponded to cobalt(II). The Al 2porbital in Fig. 3c is revealed at the position of 74.2 eV,speculating to the appearance of Al(OH)3in CoAl-layered double hydroxide since the band energy of pure Al(OH)3is 74.8 eV33,34. At the same time, the XPS spectra of Mn, Cd, S were revealed in Fig. 3d-f. From Fig. 3d, the Mn 2p1/2and Mn 2p3/2orbit appear at 651.8 eV and 640.8 eV. The peaks centered at 411.2 eV and 404.5 eV belong to Cd 3d3/2and Cd 3d5/2in Fig.3e, respectively, implying the Cd2+in the material35. The XPS spectrum of S 2pin Mn0.2Cd0.8S can be divided into S 2p1/2(162.0 eV) and S 2p3/2(160.8 eV) in Fig. 3f. Compared with Mn0.2Cd0.8S and CoAl LDH, the shift of peak position of MCCA-3 could be attribute to the strong electron transfer between Mn0.2Cd0.8S and CoAl LDH.
3.4 The Brunauer-Emmett-Teller study
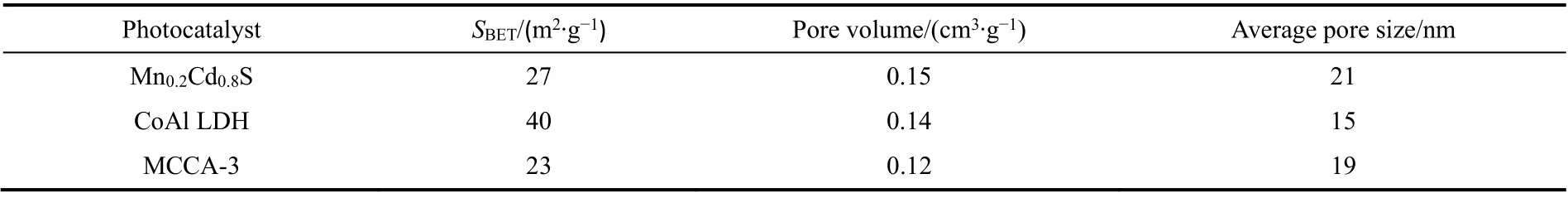
The Brunauer-Emmett-Teller (BET) characteristic of photocatalysts can be observed from Fig. 4a-c, from which,Mn0.2Cd0.8S, CoAl LDH and MCCA-3 shows typical type IV isotherm, implying its multilayer adsorption process. In the embedded diagram, all materials pores are concentrated in 2-50 nm, stating its characteristic of mesoporous structure36. Making use of Eq. (1), the adsorption isotherm of samples is calculated and displayed in Table 1.
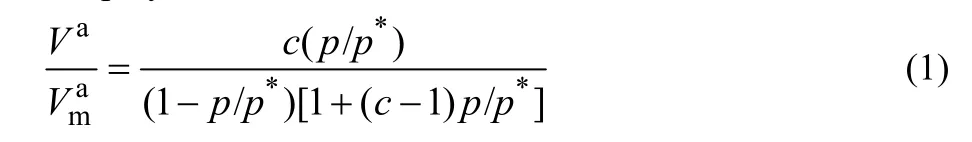
WhereVais the adsorption amount under pressurep;p* is adsorbed liquid saturated vapor pressure;Vamis the monolayer saturated adsorption amount;Cis the constant of adsorption. It is worth noting that the BET characteristic of MCCA-3 is different from Mn0.2Cd0.8S and CoAl LDH, illustrating that a new complex MCCA-3 was successfully prepared.
3.5 Photocatalytic H2 production activity of catalyst
The photocatalytic properties of materials were investigated with the Na2S/Na2SO3solution as the sacrificial agent. Fig. 5a show the H2generation rate of Mn0.2Cd0.8S, CoAl LDH and MCCA-3, from which, Mn0.2Cd0.8S exhibits non-ideal H2generation activity since its rapid photon-generated carriers recombination. At the same time, the hydrogen producing performance of CoAl LDH is also pretty low. However, the hydrogen production of MCCA-3 could reach to 1177.9 μmol in 5 h, which is equal to 23 times of pristine Mn0.2Cd0.8S (51.0 μmol) and 502 times of CoAl LDH (2.3 μmol). The excellent photocatalytic performance of MCCA-3 put down to the architecture of S-scheme heterojunction, the heterojunction structure can efficient use generate electrons to produce H2.
The catalytic activity of MCCA-x(x= 1, 2, 3, 4, 5) also was conducted to explore the optimal coupling mass ratio of Mn0.2Cd0.8S and CoAl LDH. As can be found in Fig. 5b, the hydrogen production performance of MCCA-x(x= 1, 2, 3, 4, 5)presents the trend like a mountain, which rises first and then decreases, and MCCA-3 showcases the optimum performance.When a small amount of CoAl LDH coupling with Mn0.2Cd0.8S,with the increase of CoAl LDH, the constructed S-scheme heterojunction between Mn0.2Cd0.8S and CoAl LDH is gradually increased, the composite photocatalyst shows gradually enhanced catalytic activity. Nevertheless, when the addition of CoAl LDH exceeds the optimum coupling amount, the light absorption capacity of the composite photocatalyst is weakened,and the performance of hydrogen evolution is reduced.
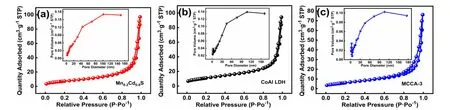
Fig. 4 Nitrogen adsorption-desorption isotherms and corresponding pore size distribution curves (inset) of (a) Mn0.2Cd0.8S,(b) CoAl LDH and (c) MCCA-3.
Photocatalyst SBET/(m2·g-1) Pore volume/(cm3·g-1) Average pore size/nm Mn0.2Cd0.8S 27 0.15 21 CoAl LDH 40 0.14 15 MCCA-3 23 0.12 19

Fig. 5 Photocatalytic H2 evolution amount of (a) Mn0.2Cd0.8S, CoAl LDH and MCCA-3, (b) MCCA-x (x = 1, 2, 3, 4 and 5);(c) Cycle test of the MCCA-3; (d) the zeta potential of Mn0.2Cd0.8S, CoAl LDH and MCCA-3.
In addition, the H2production stability of MCCA-3 are shown in Fig. 5c. The first three cycles were performed without repeated addition of catalyst and hole sacrifice reagent, and N2was used to expel air from the reaction flask. Obviously, in the first three photocatalytic operations, the rate of hydrogen production gradually decreased from the first cycle to the last cycle. When 25 mL sacrificial reagent was replaced in the fourth cycle, the hydrogen production of the catalyst increased. Thus,the decrease of catalytic activity can be attributed to the consumption of sacrificial reagents, that result in a large number holes accumulate in CoAl LDH. The low separation efficiency of holes and electrons has a negative effect on photocatalytic reaction.
In addition, the sample was tested for zeta potential in aqueous solution, as shown in Fig. 5d. Mn0.2Cd0.8S showed positive zeta potential value (3.4 mV), and CoAl LDH showed negative zeta potential value (-12.0 mV), which is very beneficial for the tight coupling of Mn0.2Cd0.8S and CoAl LDH.The zeta potential of MCCA-3 (-5.2 mV) lies between CoAl LDH and Mn0.2Cd0.8S, revealing electron migration from the surface of CoAl LDH to Mn0.2Cd0.8S, which is consistent with the results of UV-Vis and Mott-Schottky analysis.
3.6 Optical performance
In Fig. 6a, the absorption band edge of Mn0.2Cd0.8S located at approximately 519 nm, consistent with literature reports37. The charge transfer transition of O2→ Co2+caused the 250 nm characteristic band edge of CoAl LDH, and the absorption peaks at 320 and 380 nm put down to the octahedral coordination of Co2+38. The MCCA-3 composite photocatalyst represents an enhanced light absorption capacity compared with CoAl LDH.In Fig. 6b, all composite photocatalysts show similar absorption curves with pure Mn0.2Cd0.8S, verifying that CoAl LDH is not embedded into the crystal structure of Mn0.2Cd0.8S but loaded on its surface39. Furthermore, the band gap of materials can get by the Eq. (2):

Fig. 6 UV-Vis DRS spectra of (a) Mn0.2Cd0.8S, CoAl LDH and MCCA-3, (b) MCCA-x (x = 1, 2, 3, 4, 5);(c) the band gap energy of Mn0.2Cd0.8S and CoAl LDH materials.

Whereα,ʋ,EgandArespectively are absorption coefficient,light frequency, bandgap energy, and a constant. As displayed in Fig. 6c, the band gap energy of pristine Mn0.2Cd0.8S and pure CoAl LDH are 2.30 and 2.09 eV28. It can be clearly noted that both Mn0.2Cd0.8S pure CoAl LDH are employed with favorable band gap for photocatalytic H2evolution.
3.7 PL and TRPL spectra
To study the photogenerated carrier dynamics, the photoluminescence (PL) spectra of Mn0.2Cd0.8S, CoAl LDH and MCCA-3 was carried out. As is exhibited in Fig. 7a, Mn0.2Cd0.8S showcases the strongest PL intensity under excitation wavelength at 390 nm, which could attribute to its intrinsic bondto-bond recombination40. It is believed that the strong fluorescence intensity symbolizes abundant ground state electrons which result in the recombination of e-and h+, further result in a decline of photogenerated electron participating in photocatalytic H2production41,42. Therefore, the decrease of PL intensity stands for the recombination of e-and h+was effectively suppressed. The MCCA-3 composite photocatalyst exhibits a decreased PL intensity compared with original Mn0.2Cd0.8S and CoAl LDH, indicating that the S-scheme heterojunction is beneficial to the interface charges transfer between Mn0.2Cd0.8S and CoAl LDH. The effective charges transfer plays an active role in photocatalytic H2generation.
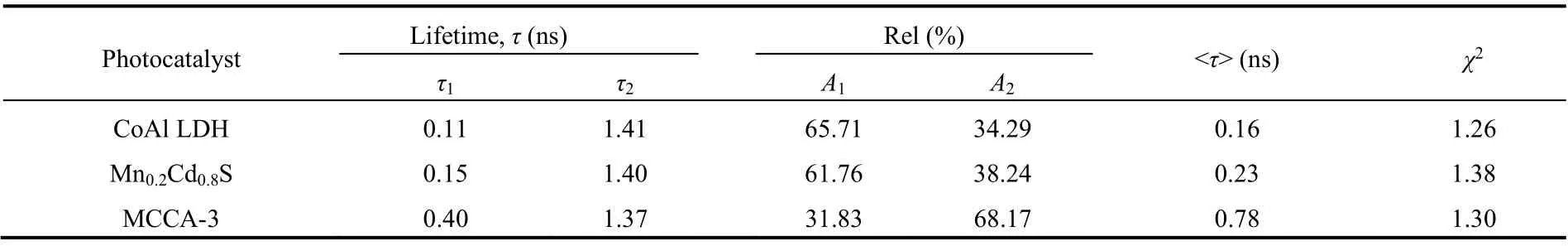
Employing the time-resolved photoluminescence (TRPL)decay spectra to explore the energy transfer kinetics of photocatalysts, as displayed in Fig. 7b. Using Eq. (3) to fit traces,the fitting parameters were displayed in Table 2. The average lifetime of Mn0.2Cd0.8S, CoAl LDH and MCCA-3 was calculated by the Eq. (4).
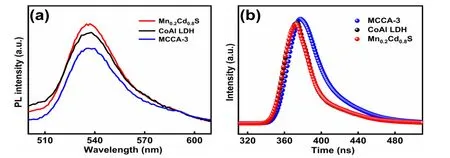
Fig. 7 (a) PL spectra of Mn0.2Cd0.8S, CoAl LDH and MCCA-3; (b) TRPL spectra of Mn0.2Cd0.8S, CoAl LDH and MCCA-3.

Whereτis emission lifetimes;Ais the corresponding amplitude. Visibly, the MCCA-3 possesses the longer PL lifetimes (0.78 ns) than CoAl LDH (0.16 ns) and Mn0.2Cd0.8S(0.23 ns). The significantly extended average lifetime of carriers suggest the better separation of electrons and holes, prompting more electrons participated in photocatalytic reactions43.
3.8 Electrochemical characterization
To further comprehend the transfer behavior of photogenerated charges between Mn0.2Cd0.8S and CoAl LDH,the photocurrent response curves of Mn0.2Cd0.8S, CoAl LDH and MCCA-3 were measured44-46. The transient photocurrent responses could reflect the ability of semiconductor materials to produce charge carriers since the cathodic photo-current peak is created from the separation of electrons and holes47. According to Fig. 8a, MCCA-3 possesses highest photocurrent density at the bias of 0.2 Vvs. SCE, which is put down to the coupling of Mn0.2Cd0.8S and CoAl LDH, reflecting that the architecture of the S-scheme MCCA-3 is be conducive to the production of photo-generated carriers48,49. The electrons utilization of samples consistent with the trend of H2production of photocatalysts.
The electrochemical impedance spectroscopy (EIS) plots were adopted to study the charges transfer capabilities of the photocatalysts50,51. As can be observed from Fig. 8b, the MCCA-3 composite photocatalyst shows out the least radius curve compared with Mn0.2Cd0.8S and CoAl LDH. It is believedthat the curve with small radius is the behalf of efficient charge transfer in the sample52. Apparently, MCCA-3 possess the weakest charge transfer impedance, attributing to its fast transfer of charges53,54. That is to say, the constructed S-scheme heterojunction between Mn0.2Cd0.8S and CoAl LDH plays a noticeable rule in improving photocatalytic activity.
Photocatalyst Lifetime, τ (ns) Rel (%) <τ> (ns) χ2 τ1 τ2 A1 A2 CoAl LDH 0.11 1.41 65.71 34.29 0.16 1.26 Mn0.2Cd0.8S 0.15 1.40 61.76 38.24 0.23 1.38 MCCA-3 0.40 1.37 31.83 68.17 0.78 1.30

Fig. 8 (a) Transient photocurrent response, (b) Nyquist plots, (c) Linear sweep voltammograms curves of Mn0.2Cd0.8S, CoAl LDH and MCCA-3;Mott-Schottky curves of (d) Mn0.2Cd0.8S and (e) CoAl LDH; (f) The obtained band gap patterns of samples.
The linear sweep voltammetry (LSV) plots of photocatalysts tested from -0.7 to -0.3 V were displayed in Fig. 8c to explore the mechanism of enhanced photocatalytic activity. As can be observed that the three samples appear increased cathode current, the MCCA-3 composite photocatalyst exhibits the smallest cathodic current response, which may be attribute to the fast electron transfer at the interface of Mn0.2Cd0.8S and CoAl LDH55,56. The result indicating that the MCCA-3 S-scheme heterojunction possesses superior photocatalytic hydrogen production performance than original Mn0.2Cd0.8S and CoAl LDH. Therefore, the effective separation and transfer of charges is of extraordinary significance in enhancing photocatalytic activity57.
In Fig. 8d,e, the Mott-Schottky plots of Mn0.2Cd0.8S and CoAl LDH are shown, in which the flat-band potential (Efb) of semiconductors could be observed. TheE-C2plots with positive slopes stating that both Mn0.2Cd0.8S and CoAl LDH are typical n-type photocatalyst58,59, and theirEfbare -1.07 and -0.59 eV.The conduction band (CB) position in n-type semiconductors is 0.1 or 0.2 eV less than theirEfb, therefore, the CB site of Mn0.2Cd0.8S is -1.27 eV and CoAl LDH is -0.79 eV. Based onENHE=ESCE+ 0.241 eV, after converted into normal hydrogen electrode, theENHEof Mn0.2Cd0.8S and CoAl LDH are -1.03 and-0.55 eV. From Fig. 6c, the band gap energy of Mn0.2Cd0.8S is 2.30 eV while CoAl LDH is 2.09 eV. According toEVB=ECB+Eg, theEVBof Mn0.2Cd0.8S is 1.27 eV and CoAl LDH is 1.54 eV.Finally, Fig. 8f shows out clear band structure of photocatalysts.
3.9 Hydroxyl radical test
In the solution, hydroxyl radical (·OH) on the surface of the sample easily reacts with TA to form high fluorescence 2-hydroxyl terephthalic acid (PTA-OH). Therefore, the photoluminescence intensity of the sample is proportional to the number of hydroxyl radicals generated in the solution. As can be seen from Fig. 9a-c, CoAl LDH exhibits stronger fluorescence intensity than Mn0.2Cd0.8S, and MCCA-3 shows the strongest PL peak. This phenomenon indicates that MCCA-3 has the ability to produce more ·OH than pure Mn0.2Cd0.8S, and further indicates that S-scheme heterojunction is established between Mn0.2Cd0.8S and CoAl LDH.

Fig. 9 PL intensity of different species during reaction process with PTA under illumination (a) Mn0.2Cd0.8S; (b) CoAl LDH; (c) MCCA-3.
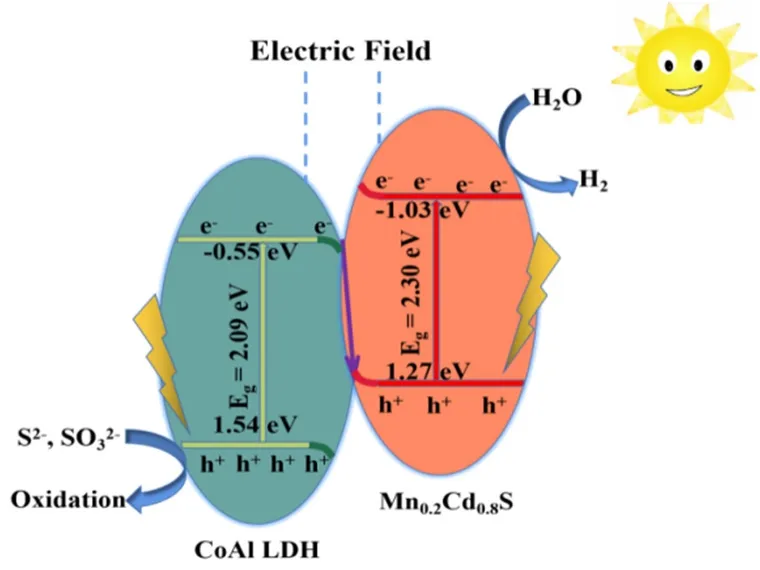
Fig. 10 Photocatalytic mechanism of MCCA-3 S-scheme heterojunction photocatalyst for hydrogen evolution.
3.10 Possible photocatalytic mechanism
Through the above analysis, the reaction mechanism of MCCA-3 in water splitting with Na2S/Na2SO3aqueous solution as an electron donor was proposed. It is analyzed that the photocatalytic H2evolution process of MCCA-3 is a typical Sscheme process (Fig. 10)60. From Fig. 8f, it is easy to find that compared with CoAl LDH, Mn0.2Cd0.8S possess higher CB and VB positions and less work function. After Mn0.2Cd0.8S and CoAl LDH closely contacted, the electrons transfer spontaneously from Mn0.2Cd0.8S to CoAl LDH in their contact surface. Thus, Mn0.2Cd0.8S has a positive charge after losing electrons while CoAl LDH emerges negative charged after getting electrons, an internal electric field is created at the interface61. When photocatalysts is irradiated by visible light,the electrons transfer from the VB of photocatalysts to its CB that leaving holes in the VB. Under the combined action of the band edge bending, internal electric field and coulomb interaction, the electrons from CoAl LDH (CB) are recombined with the holes in Mn0.2Cd0.8S (VB). Therefore, a great amount of e-from the CB of Mn0.2Cd0.8S could participate in hydrogen generation. Meanwhile, the h+in the VB of CoAl LDH are consumed by Na2S/Na2SO3. The MCCA-3 S-scheme heterojunction photocatalyst possess strong redox capacity that it can showcase excellent H2-production property.
4 Conclusions
A Mn0.2Cd0.8S@CoAl LDH composite photocatalyst was successfully prepared by ultrasonic mixing Mn0.2Cd0.8S and CoAl LDH. The composite photocatalyst possesses enhanced light absorption performance comparing with pure CoAl LDH. In addition, at the interface between the two materials constructed a S-scheme heterojunction. Combing electrochemical, transientstate and steady-state fluorescence test of photocatalyst, the Sscheme heterojunction not only provides a fast electron transfer channel, but also eliminates useless electrons and holes so that useful electrons are retained for photocatalytic H2generation reactions. The hydrogen production rate of the optimal Mn0.2Cd0.8S@CoAl LDH could reach to 1177.9 μmol in 5 h,higher than Mn0.2Cd0.8S and CoAl LDH.
杂志排行
物理化学学报的其它文章
- Insights into Mechanism of CsPbBr3 Nanocrystal Interfacial Modifier in Perovskite Solar Cells
- Enhanced Photocatalytic CO2 Reduction over 2D/1D BiOBr0.5Cl0.5/WO3 SScheme Heterostructure
- Hollow NiCo2S4 Nanospheres as a Cocatalyst to Support ZnIn2S4 Nanosheets for Visible-Light-Driven Hydrogen Production
- A 0D/2D Bi4V2O11/g-C3N4 S-Scheme Heterojunction with Rapid Interfacial Charges Migration for Photocatalytic Antibiotic Degradation
- P-Doped g-C3N4 Nanosheets with Highly Dispersed Co0.2Ni1.6Fe0.2P Cocatalyst for Efficient Photocatalytic Hydrogen Evolution
- Enhancement of Photocatalytic H2-Evolution Kinetics through the Dual Cocatalyst Activity of Ni2P-NiS-Decorated g-C3N4 Heterojunctions
