Preparation and Photophysical Properties of o-Carboranyl AIEgens Containing Regioisomeric N-Phenyl-Carbazole∗
2022-08-02JINXiaopingWUXueyanLYUYanGUOJixi
JIN Xiaoping,WU Xueyan,LYU Yan,GUO Jixi
(State Key Laboratory of Chemistry and Utilization of Carbon Based Energy Resources,Key Laboratory of Advanced Functional Materials,Xinjiang Uygur Autonomous Region of China,Institute of Applied Chemistry,School of Chemistry,Xinjiang University,Urumqi Xinjiang 830017,China)
Abstract:This work described here a new design strategy for obtaining o-carboranyl compounds containing regioisomeric N-phenyl-carbazole with AIE properties.Two o-carborane functionalized N-phenyl-carbazole compounds (C3DC and C9DC)were synthesised and fully characterised.Photoluminescence experiments confirmed both compounds have strong AIE activity.The two o-carborane-functionalised N-phenyl-carbazole isomers have high solid-state fluorescence quantum yields,reaching 99%and 93%,respectively.In addition,based on the results of DFT calculation,the emission bands could be attributable to ICT transitions from N-phenyl-carbazolemoieties to the o-carborane units.
Key words:o-carborane;N-phenyl-carbazole;regioisomeric;aggregation-induced emission;intramolecular charge transfer
0 Introduction
Solid-state luminescent materials,which pose excellent photoluminescence (PL) quantum yields,higher electroluminescence efficiencies and small efficiency roll-offare becoming hot topics of current research worldwide[1].The efficient solid-state luminescent materials are relatively insufficient because the usual design strategy of it is to introduce and extend aryl or heteroaryl groups to broaden π-conjugation and have a red-shift of the spectra.However,the usual design strategy often causes aggregation-caused quenching(ACQ)which left lots of troublesomes for their application.In 2001,Tang and his co-workers proposed the concept of AIE,which emphasize that the materials significantly promoted bright luminescence in the aggregate or solid state[2].Therefore,many researchers have always centered on the development of novel efficient solidstate luminescent materials,among which the o-carborane containing derivatives are a kind of efficient materials exhibiting great potential in solid-state luminescent materials emission.
o-Carborane,an icosahedralboron-cluster via the three-center two-electron bonds,with high thermal and electrochemical stabilities has been extensively applied in boron neutron capture therapy(BNCT)[3],OLEDs[4],chemical sensing[5],and optical functional materials[6]in recent years.o-Carborane considered as a three-dimensional(3D)variant of benzene shows great electron-withdrawing ability which is in favor of design of D-A (D=donor,A=acceptor) conjugated materials which exhibit intriguing luminescent features due to the intramolecular charge transfer (ICT) process[7−9].Indeed,o-carboranes appending to various electron donors such as naphthyl[10],anthracenyl[11],tetraphenyl ethenyl[12],pyrenyl[13]chrysenyl[14],and carbazolyl[15]groups exhibit multiple photoluminescence emissions features,such as aggregation-induced enhanced emission(AIEE),crystallization-induced emission enhancement(CIEE),thermally activated delayed fluorescence(TADF),aggregation-induced delayed fluorescence (AIDF) and so on,which triggered by twisted intramolecular charge transfer(TICT) states.Furthermore,our group has reported a series of aryl or heteroaryl groups have been applied to electron donors in D-A dyads/triads to enhance the ICT transition due to their electron-rich features[13,15−16].Among them,o-carborane functionalized carbazole compounds have attracted significant attention due to their unusual multiple photoluminescence properties that derive from their structural rigidity.However,o-carborane functionalized carbazole compounds vary considerably in the photophysical behavior by altering the electronic environment of the carbon atoms of the carbazole moiety in such D-A triads,which rarely been investigated.
To insight into the detailed relationships between molecular structure and photophysical properties of o-carborane functionalized carbazole compounds,particularly the effect of the substitution position of the o-carborane in carbazole on the emissive character via ICT transitions,we herein design synthesis and photophysical properties of two o-carborane functionalized N-phenyl-carbazole compounds(C3DC and C9DC)in which regioisomeric N-phenyl-carbazole were introduced to the o-carboranes,respectively.This enabled us to elucidate the impact of the o-carborane substitution position on photophysical properties involving ICT transitions.The photoluminescent properties of the o-carborane functionalized carbazole compounds were studied with steady-state UV-Vis absorption fluorescence spectra and DFT calculation.
1 Experimental Section
1.1 General Procedures
All reactions were carried out under a dry N2atmosphere.Solvents of THF and toluene were distilled from sodium/benzo-phenone and stored over molecular sieves.Other chemicals were purchased from Energy and Aladdin chemical reagent company and used without further purification.All reactions were monitored by thin layer chromatography(TLC).Column chromatography was conducted using silica gel(300∼400 mesh).
1.2 Analytical Measurements
NMR spectra were recorded on a Bruker ADVANCE III 400 MHz spectrometer(1H NMR:400 MHz,13C NMR:100 MHz).All chemical shifts were reported in δ units with references to the residual solvent resonances of the deuterated solvents for proton and carbon chemical shifts.Mass spectra were obtained on an Ultimate 3000/Q-Exactive spectrometer.Melting points were measured using a Nikon Polarizing Microscope ECLIPSE 50i POL equipped with an INTEC HCS302 heating stage.UV-Vis absorption spectra were recorded on a Hitachi U-3900H.Fluorescence spectra were performed on Horiba FluoroLog-3 spectrofluorometer(Horiba–Jobin–Yvon,Edison,NJ,USA).Luminescence lifetime measurements were carried out by using a FluoroLog-3 spectrofluorometer,absolute PL quantum yield (ΦPL) was determined using a Horiba FL-3018 Integrating Sphere.The temperature dependent luminescence spectra were measured with Janis VPF-100 liquid nitrogen low temperature thermostat(Janis,USA).
1.3 DFT Calculation
The optimized structures and frontier orbitals(HOMO:highest occupied molecular orbital;LUMO:lowest unoccupied molecular orbital) were calculated by using density functional theory (DFT) and time-dependent DFT (TD-DFT) at the B3LYP/6-31 G(d,p)level by using the Gaussian 09 suite of programs in the ground and excited states.Structure optimization was performed before the calculation of the HOMO/LUMO energies[15,17].
1.4 Synthesis
1.4.1 Synthesis of C3DC
To a THF solution(10 mL)of o-carborane(288 mg,2.0 mmol)was slowly addediPrMgCl(2.0 M in THF,2.4 mL,4.8 mmol) at 0 ℃under N2atmosphere for 3 h,and the mixture was stirred at room temperature for 10 h.After replacement of THF with toluene(10 mL),addition of 3-bromo-9-phenylcarbazole(1.55 g,4.8 mmol,2.4 equiv)and NiCl2(46 mg,0.2 mmol),the reaction mixture was heated at 115 ℃under stirring for 12 h in a closed flask.Then,the reaction was quenched with water (10 mL) and the organic layer was extracted with CH2Cl2(3×30 mL) and dried over MgSO4.The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography using DCM/petroleum ether(1/6,v/v)as the eluent to obtain pale yellow compounds C3DC(526 mg,63%).Mp:182.6∼184.1 oC,1HNMR(400 MHz,CDCl3):δ 8.32(d,J=4.0 Hz,2 H),8.09(t,J=4.0 Hz,1 H),8.07(t,J=4.0 Hz,1 H),7.56∼7.50(m,6 H),7.43∼7.39(m,2 H),7.38∼7.34 (m,6 H),7.29∼7.24 (m,4 H),7.09 (d,J=8.0 Hz,2 H),3.15∼1.60 (10 H,br,B–H).13C NMR (100 MHz,CDCl3):δ 141.93,141.24,136.79,129.83,128.39,127.76,126.84,126.57,123.37,123.02,1 222.78,120.44,120.35,109.99,109.20,88.01.HRMS:m/z calcd for C38H34B10N2[M]−:626.371 97,Found:626.374 51.
1.4.2 Synthesis of C9DC
This compound was prepared in a manner analogous to the synthesis of C3DC using 9-(4-bromophenyl)-carbazole(1.55 g,4.8 mmol,2.4 equiv)to replace 3-bromo-9-phenylcarbazole to give acolorless powder of C9DC(788 mg,63%.).Mp:>250℃1HNMR(400 MHz,CDCl3):δ 8.11(t,J=4.0 Hz,2 H),8.09(t,J=4.0 Hz,2 H),7.75(d,J=8.0 Hz,4 H),7.48(d,J=8.0 Hz,4 H),7.28∼7.19(m,12 H),3.50∼1.60(10H,br,B–H).13C NMR(100 MHz,CDCl3):δ 141.71,141.18,136.89,130.05,128.02,126.97,125.52,124.93,123.29,122.58,120.68,120.41,120.01,110.19,109.75,77.97,61.33.HRMS:m/z calcd for C38H34B10N2[M]−:626.371 97,Found:626.378 36.
2 Results and Discussion
2.1 Design and Synthesis of the Compounds
Carbazole has been one of the most important and thoroughly investigated organic chromophore in previous years due to their high thermal and photochemical stability,pure blue fluorescence,planar geometry and natural high charge carrier mobility[18].In addition,the carbazole is a strong electron donor,with a large Stokes shift,long excited lifetime and considerable chemical stability,making them valuable members of a panel of conventional fluorophores o-carborane was selected to tune the excited state of organic chromophores.Two regioisomeric N-phenyl-carbazole were introduced to the carbon atoms of the o-carborane to synthesize C3DC and C9DC via a modified nickel-catalyzed cross-coupling reaction indifferent reactive sites(Fig 1)[13,15−16,19].All compounds were obtained with moderate to satisfactory yields and the products exhibited fine stability under ambient conditions and stability high enough for spectroscopic measurements.The molecular structures were fully verified with NMR and HRMS spectra.
2.2 Steady-State Electronic Spectroscopy(UV-Vis absorption and emission spectra)
The UV-Vis absorption spectra of C3DC and C9DC were studied.As shown in Fig 2 and Table 1,UV-Vis absorption spectra of C3DC and C9DC were measured in different solvents.o-Carborane as an electron withdrawing unit in C3DC and C9DC does not show any significant absorption in the absorption spectra.The absorption bands in the region of 320 nm∼360 nm in C3DC and C9DC were assigned to the π-π∗transitions on the carbazole groups,and no significant electronic coupling between carbazole and o-carborane moiety in the ground state[20]Solvent dependency studyof UV-Vis absorption spectra for C3DC and C9DC hardly observed,further indicating that the ground state was not affected by the solvent polarity.

Table 1 Photophysical properties of C3DC and C9DC
To elaborate the emission mechanism of C3DC and C9DC,optical spectra in various solvents were measured (Fig 3 and Table 1).The C9DC exhibited dual-emissive property in the solution (Fig 3b).Sharp emission bands with vibrational structures and broad ones were observed with peaks at around 370 nm and 550 nm,respectively,which indicated that the emission bands of C9DC around 370 nm and 550 nm are from the locally excited(LE)and ICT states[15−16,21].However,for C3DC,the emission intensity is too low to measure(Fig 3a).Moreover,we measured the emission spectra at low temperature of 77 K in 2-MeTHF solution where the molecular motions are completely suppressed(Fig 3c and 3d).In contrast to these characters from emission at room temperature in 2-MeTHF,C3DC and C9DC showed strong broad emission in the longerwavelength region,but the short wavelength emission bands with vibration structures is similar to that at room temperature.The above results revealed that the substitution position of the o-carborane in carbazole have effect on the emissive character via ICT transitions.
Next,the aggregation and solid-state emission properties of C3DC and C9DC were estimated (Fig 4 and Table 1).Thanks to the introduction of regioisomeric N-phenyl-carbazole into two carbon atoms of optically functional “elementblock”o-carborane in different reactive sites,C3DC and C9DC showed typical AIE characteristic.The AIE characteristic of C3DC and C9DC was further proved by studying their PL behaviors in THF and THF/water mixtures with different water fractions (fw)(Fig 4 and Table 1).In dilute THF solution,C3DC and C9DC were weakly emissive owing to the vibrations of Ccage–Ccagebond in o-carborane which quenched the emission in solution[22].With the increase of water in THF solutions results in the formation of insoluble aggregates which can enhance the emission of compounds.The highest emission was achieved at fw=99%.The quantum yields of C3DC and C9DC in dilute THF solutions were determined to be less than 0.1%by a calibrated integrating sphere.In the aggregated state,their quantum yields were measured to be 56.8%and 34.7%.Correspondingly,an intense broad emission band around 548 nm and 556 nm was obtained for C3DC and C9DC,respectively,and with bright yellow emission under the 365 nm UV light irradiation(Fig 4 insert).However,accompanying the increase in the water content,blue-shifted emission bands were observed in Fig 4,which attributed to the molecules aggregate into nanoparticles,whose polarity inside is lower than that outside.And the emission bands are blue-shift similarly to typical ICT emission[15].The above results indicate that C3DC and C9DC are all AIEgens.
The emission properties in the solid and film states were examined(Fig 5 and Table 1).From the emission spectra in the solid state and in film state,the C3DC and C9DC were pale yellow and white under daylight illumination.In Fig 5a,the solid-state emission peaks of C3DC and C9DC are 530 nm and 545 nm,respectively,and the emission peaks are shifted by 15 nm.As depicted in Fig 5b,under 365 nm UV light irradiation,strong yellow emissions for C9DC,bright yellow green emission for C3DC were observed from the solid and film state under 365 nm UV light irradiation.The absolute emission quantum yields for C3DC and C9DC in the solid state and film state were also evaluated(Table 1).Interestingly,the emission efficiency with QY up to 99% and 93.7% were achieved for C3DC and C9DC,respectively.This fact strongly suggests that the emission band in the solid-state should be induced by the ICT state and the reactive sites play an important role in manipulating the degree of ICT process.
Thermochromic behavior in luminescence from C3DC and C9DC were investigated with the powder samples(Fig 6).In order to avoid the powder samples decomposing when heated,we evaluated the thermochromic luminescence properties below melting point.Emission spectra were monitored on the liquid nitrogen low temperature thermostat by increasing the temperature from 77 K to 333 K (before melting).The C3DC and C9DC in solid presented a narrow and sharp bands attributable to the ICT emissions at 550 nm and 565 nm,respectively.The intramolecular rotation was likely prohibited because of the steric hindrances of the substitutes in o-carborane units[23].Moreover,the compounds are insensitive to the temperature due to their rigid structure,only enhanced emission intensity observed for all compounds rather than change the emission wavelength during cooling from 333 K to 77 K.From the above results,it can be concluded that o-carborane acts as a versatile“element-block”not only for constructing ICT emission but also for designing AIE luminogens with stronger fluorescence in solid.
To better understand the AIE characteristics and stronger fluorescence of C3DC and C9DC,the fluorescence lifetime was measured in various states and the radiative and nonradiative decay processes were also investigated(Fig 7 and Table 2).The radiative decay rate and nonradiative decay rate in solution compared to that of the rigid state,the radiative decay rate(krad) of C3DC increased by around 3 times (krad,solid=1.52×107s−1and krad,film=1.53×107s−1),while the nonradiative decay rate(knr)decreased almost by 300 times(knr,solid=0.15×105s−1and knr,film=0.13×105s−1).This suggests that the inhibition of nonradiative decay of excited state in the solid state is accounted for its high fluorescence.In sharp contrast,the kradof C3DC increased over 3 times than that of C9DC in the solid state,while knrdecreased 20 times.In a word,the different reactive sites have a noticeable impact on photophysical properties of o-carborane containing carbazole derivative compounds.

Table 2 The photophysical properities of C3DC and C9DC in different states
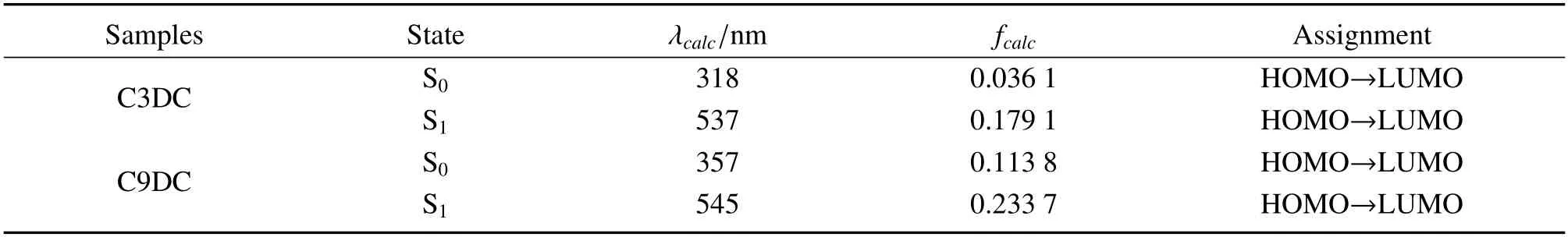
Table 3 The major low-energy electronic transitions for C3DC and C9DC in their ground state (S0) and first excited singlet(S1)states as calculated using TD-B3LYP with the 6-31 G(d,p)basis seta
2.3 DFT Calculation
To ensure electronic alteration in functional carbazole groups driven by o-carborane,geometry optimization as well as the orbital contribution from HOMO and LUMO energies were performed on the B3LYP functional and 6-31 G(d,p)basis sets using Gaussian and the results are shown in Fig 8.
The energy gap between HOMO and LUMO of C3DC and C9DC are 2.73 eV and 3.30 eV,respectively,and its energies changes concerning the reactive sites of carbazole groups with o-carborane.As expected,the electron cloud of HOMOs in C3DC mainly located in the carbazole groups and LUMOs on the Ccage–Ccagebond in o-carborane.LUMOs are mixed with carbazole groups and cage carbon orbitals and depending on the reactive sites of carbazole groups.However,for C9DC,the electron cloud of HOMOs mainly located in the carbazole groups,while the LUMOs lie on the entire molecules.LUMOs are mixed with carbazole groups and cage carbon orbitals and depending on the reactive sites of carbazole groups.This finding is another clear evidence for the difference electronic communication between carbazole and the o-carborane unit.
Time-dependent DFT(TD-DFT)calculations were performed according to the optimized geometries to locate the origin of the low energy absorptions of each type of carbazole groups (Fig 9).The low energy absorptions were attributed to the transitions from HOMO to LUMO,shifting from π(carbazole)to π∗(Ccage–Ccagebond)and transitions observed at λmaxas well as their relative energies and oscillator strength values are consistent with the UV-Vis absorption spectra(Fig 1 and Table 3).In addition,we optimized the excited state structures of C3DC and C9DC through TD-DFT calculations in the gas phase(Fig 9).It was revealed that the S0–S1electronic transition was mainly derived from HOMO to LUMO.The HOMOs in C3DC and C9DC were localized on the π-conjugated carbazole moiety.In contrast,the LUMOs were significantly delocalized to the o-carborane moiety.This result also strongly supports that the emission should be derived from the CT states.
3 Conclusion
We demonstrate that the introduction of skeletal distortion by o-carborane with regioisomeric N-phenyl-carbazole is a facile strategy for obtaining AIE-active molecules.From the comparison with regioisomeric N-phenyl-carbazole based o-carborane complexes,it was found that none of the o-carborane functionalized N-phenyl-carbazole compounds exhibited emission in the solution state at room temperature the emission peak intense location in the region of 540 nm∼560 nm in rigid states(i.e.in THF at 77 K,in solid and films state),and all demonstrated the enhanced ICT-based emission.The two o-carborane-functionalised N-phenyl-carbazole compounds have high solid-state fluorescence quantum yields,reaching 99%and 93%,respectively.DFT calculation results show that the intense emission bands attributable to ICT transitions from Nphenyl-carbazolemoieties to the o-carborane units.Consequently,the results of this study confirm the o-carborane attached to the different sites of the carbazole moiety can regulate the emission process based on ICT transitions.
