非晶含氢碳薄膜本征结构对退火行为的影响
2022-07-27贾倩张斌赖振国张俊彦
贾倩,张斌,赖振国,张俊彦
摩擦磨损与润滑
非晶含氢碳薄膜本征结构对退火行为的影响
贾倩1,2,张斌1,2,赖振国1,2,张俊彦1,2
(1.中国科学院兰州化学物理研究所 中国科学院材料磨损与防护重点实验室,兰州 730000;2.中国科学院大学 材料与光电技术学院,北京 100049)
为在高温工况下服役的含氢碳(a–C:H)薄膜的制备提供新思路。首先利用DP–PECVD和BiP–PECVD两种方法分别在Si基底上制备了两种本征结构不同的a–C:H薄膜,分别在350、450、550、650 ℃下进行退火处理。通过纳米硬度、X射线光电子能谱、傅里叶转变红外光谱、激光共聚焦拉曼光谱、场发射扫描电镜及CSM摩擦试验机等,分别评价了未退火和不同退火温度下两种不同结构a–C:H薄膜的结构、表面形貌、力学及摩擦学等性能。研究了不同本征结构a–C:H薄膜对退火行为的影响。DP–PECVD方法在制备a–C:H薄膜(A薄膜)的过程中具有更高的沉积速率,是BiP–PECVD法(B薄膜)的1.52倍。随着退火温度的增加,两种方法制备的a–C:H薄膜均发生H脱附,但是A薄膜的脱H转变点为450 ℃,B薄膜的脱H转变点为350 ℃。DP–PECVD法制备的a–C:H薄膜在H脱附过程中更容易形成sp3–C,而BiP–PECVD法制备的a–C:H薄膜在此过程中形成sp3–C和sp2–C杂化键的概率基本相同。BiP–PECVD法制备的a–C:H薄膜在退火过程中更容易失去H,且在450 ℃以上出现大面积剥离,摩擦失效。而DP–PECVD法制备的碳薄膜则表现出更好的热和摩擦学稳定性,在350~650 ℃均可保持薄膜的完整性,并且在350~ 550 ℃退火后保持低至约0.06的摩擦因数。DP–PECVD方法制备的a–C:H薄膜具有更好的热稳定性、力学稳定性及摩擦学稳定性。
非晶含氢碳薄膜;退火;双极脉冲;直流脉冲;等离子体增强化学气相沉积
DLC薄膜是主要以sp3–C和sp2–C杂化键结合的非晶碳材料。根据2005年德国工程师学会发布的“碳薄膜”标准,DLC薄膜可以被分为7类,即:非晶碳(a–C)、四面体非晶碳(ta–C)、金属掺杂非晶碳(a–C:Me)、含氢非晶碳(a–C:H)、四面体含氢非晶碳(ta–C:H)、金属掺杂含氢非晶碳(a–C:H:Me)、改性含氢非晶碳(a–C:H:X)[1-3]。a–C:H薄膜具有独特的高硬度、高热导率、低介电常数、良好的光学透过性、优异的化学惰性和生物相容性以及优异的减摩耐磨等性能,被广泛应用于机械、电子、光学、生物医学、航空航天等领域[4-10]。
将化学气相沉积和辉光等离子体放电过程综合用于制备薄膜的方式称为等离子体化学气相沉积(PECVD)技术,是常用的制备a–C:H薄膜的方法之一[11-15]。该方法能够方便地控制薄膜厚度和结构,制备的薄膜质量均匀,结构致密,稳定性好。值得注意的是,许多研究表明a–C:H薄膜在惰性或真空环境下的摩擦因数可以达到10‒3量级,磨损率低于10‒9mm3/(N·m),展现出很好的应用前景。但是,在一些使用温度较高(≥350 ℃)的工况条件下,如活塞环、气门、摩擦副等,则要求a–C:H薄膜具有更好的热稳定性。
通常采用退火研究a–C:H薄膜的热稳定性及高温下的摩擦学行为,较高的退火温度通常会促使a–C:H薄膜的结构发生变化[16-17]。研究表明在温度高于200 ℃时,H会开始逸出[18],300 ℃以上薄膜会发生相转变,出现石墨化[19-21]等。Li等[22]研究了a–C:H薄膜的退火行为,认为200 ℃以上薄膜开始释放H,硬度下降,磨损增加,但是摩擦因数(~0.1)在大气中基本保持不变。Wu等[23]研究了真空下a–C:H薄膜的退火行为,结果与Li等人的研究一致。但是Wang等[24]结合弹性反冲散射(ERD)、拉曼光谱(Raman)和高分辨电镜对比研究了10纳米级和亚微米级类富勒烯含氢碳(FL–C:H)薄膜在500 ℃以内的退火行为,认为薄膜的厚度对结构转化有一定的影响,且含氢量在退火过程中变化不大。Wang等[25]进一步研究表明,退火温度在300 ℃以内时,硬度随温度的升高而增加,随后降低;在高载荷下,摩擦因数随着温度的升高而降低,在250 ℃以上趋于稳定,摩擦因数低至0.004。上述研究结果表明,不同的a–C:H薄膜表现出不同的退火行为。因此,研究不同本征结构a–C:H薄膜的热稳定性对适用于高温服役的a–C:H薄膜的制备及应用具有指导性意义。
虽然目前国内外已经开展了一些高温退火对a–C:H薄膜热稳定行为影响的研究,但是尚缺乏对不同制备方法下获得的不同本征结构的a–C:H薄膜退火行为进行系统对比研究,尤其是温度高于500 ℃以上的a–C:H薄膜热稳定性研究几乎未见报道。因此,本文分别使用直流脉冲等离子体增强化学气相沉积(DP–PECVD)和双极脉冲等离子体增加化学气相沉积(BiP–PECVD)法制备了两种不同结构的a–C:H薄膜,在同样的条件下进行退火处理,研究了薄膜本征结构对退火行为的影响。
1 试验
1.1 样品制备
试验使用等离子体增强化学气相沉积镀膜设备,如图1所示。薄膜沉积前,首先将真空腔预抽到10‒4Pa,然后在100 ml/min的Ar等离子体环境中清洗衬底30 min,调节脉冲偏压为‒900 V,Ar 20 Pa。使用的基体材料为单晶Si(100)片,尺寸为直径20 mm,厚度725 μm。制备过程中两电极间距离为50 mm,衬底不加热。
1.1.1 a–C:H薄膜的制备
1)DP–PECVD。反应气体为Ar、CH4和H2混合气体,流量比为1∶1∶2,偏压为‒800 V,脉冲频率为40 kHz,占空比为0.6,此时通过样品盘的电流约0.1 A,沉积2 h,称之为A样品。
2)BiP–PECVD。关闭Ar,通入反应气体CH4,调节压强至10 Pa。将恒流双极对称脉冲电源加载到负极板上,调控电压至‒500 V,脉冲频率为200 kHz,占空比为0.6,此时通过样品盘的电流约0.8 A,沉积3 h,称之为B样品。
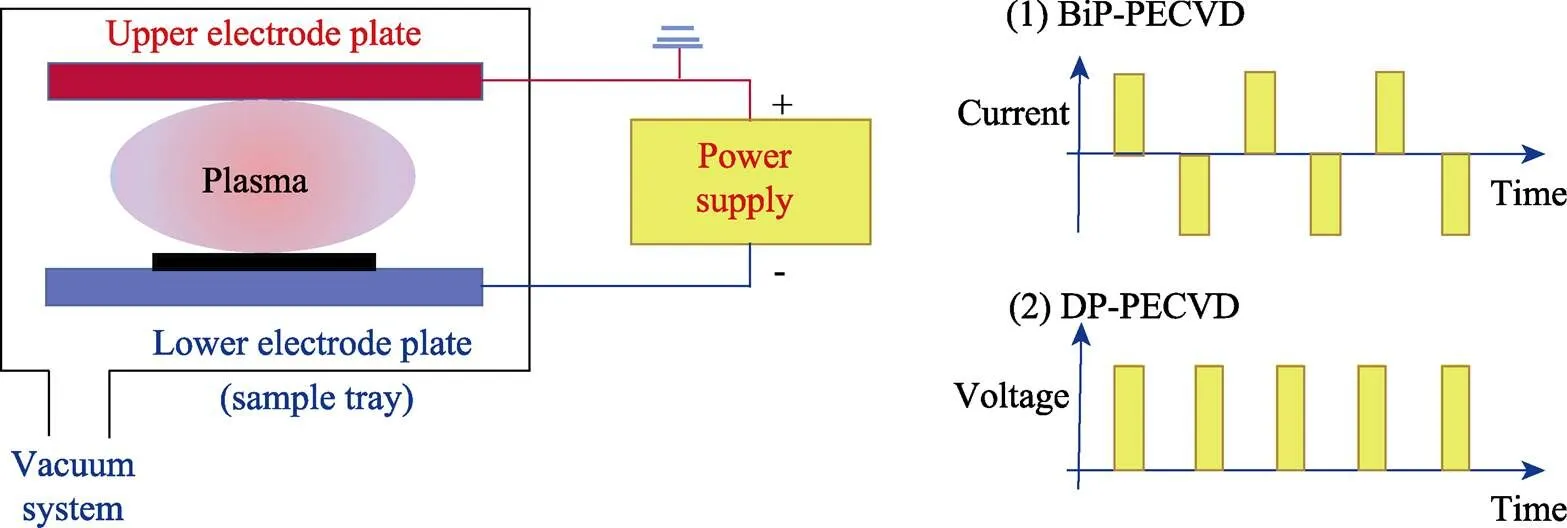
图1 沉积系统及供电模式示意图
1.1.2 退火处理
将制备好的两种a–C:H薄膜在管式炉仪器中进行退火处理。退火处理在Ar保护下进行,升温速率设定为5 ℃/min,退火时间定为1 h。退火温度分别为350、450、550、650 ℃,将未退火(室温25 ℃)与不同温度(350、450、550、650 ℃)退火后的样品分别标记为A25、A350、A450、A550、A650、B25、B350、B450、B550和B650。
1.2 结构及性能表征
利用X射线光电子能谱(XPS,ESCALAB 250Xi,美国)来获得薄膜表面元素组成及C的键合组成,腔体压力10‒6Pa,Al–Ka 辐射。借助傅里叶转变红外(FTIR,V70,Bruker,德国)光谱仪来确定a–C:H薄膜中的C—H基团,扫描范围700~4 000 cm−1,分辨率为1 cm−1。通过场发射扫描电子显微镜(FE– SEM,JSM–6701F,日本)对a–C:H薄膜厚度及表面形貌进行观察,加速电压300 kV。利用纳米压入仪(Ti–950,Hysitron,美国)对a–C:H薄膜的力学性能进行测量,压入深度为薄膜厚度的10%~12%。利用显微共聚焦拉曼(Raman)光谱(LabRAM HR Evolution,法国)获得薄膜的键合信息,激发波长为532 nm。
使用摩擦试验机(Tribometer 3,瑞士)对A、B两种a–C:H薄膜退火前后的摩擦因数进行了测量。测试参数为:载荷3 N,相对湿度25% ~ 30%,Ar氛围,频率5 Hz,摩擦时间30 min。
2 结果及分析
图2给出了分别用DP–PECVD和BiP–PECVD制备的A、B两种a–C:H薄膜的FE–SEM断面照片,证明这两种方法制备的薄膜均具有致密的内部结构。A、B两种a–C:H薄膜厚度分别为728、718 nm,沉积速率分别为6.06、3.99 nm/min,这说明DP–PECVD方法制备的薄膜具有更快的沉积速率,约是BiP– PECVD法的1.52倍。纳米压痕测量结果表明A薄膜硬度为15.88 GPa,弹性模量为100.65 GPa;B薄膜硬度为22.49 GPa,弹性模量为174.11 GPa。这说明B薄膜在退火前具有更好的力学性能。
图3给出了A、B两种a–C:H薄膜在退火前后的FTIR吸收光谱。对于a–C:H薄膜,由于1 800 cm‒1以下的C—H弯曲振动和C—C伸缩振动模式强度弱,叠加严重而不考虑。在2 700~2 950 cm‒1处出现的吸收带主要归因于sp3杂化C—H伸缩震动模式。在2 855 cm‒1和2 920 cm‒1附近出现两个较强的吸收峰,分别是sp3–CH2(sym)对称振动吸收峰和sp3–CH(asym)非对称振动模式,在2 955 cm‒1附近出现一个弱的肩峰,可以归结为sp3–CH3(asym)的非对称振动模式[26-28]。并且可以从图3a和图3b得知,H在非晶碳网络中主要以sp3–CH2和sp3–CH的形式存在,较少的H以sp3–CH3的形式存在。随着退火温度的增加,在2 700~2 950 cm‒1区间的吸收峰逐渐变弱。对于A薄膜,在550 ℃下峰强度突然变弱,而对于B薄膜,这种变化发生在450 ℃左右。为了进一步说明H键合结构的变化,如图3c和图3d所示,对所有样品的特征FTIR吸收峰进行了高斯拟合,分别对应sp3– CH2(sym)、sp3–CH(asym)、sp3–CH3(asym)振动模式。进一步地,通过分析sp3–CH2(sym)/sp3–CH3(asym)和sp3–CH(asym)/sp3–CH3(asym)的比值随退火温度变化的趋势,可以分析H原子更容易从哪个键合位置脱附较快,结果如图3e和图3f所示。综合考虑图3c和图3d的结果,可以认为,随着退火温度的增加,不同键合结构的H均发生脱附行为。对A薄膜,在550 ℃之前,sp3–CH2和sp3–CH3的脱附速率基本一致,在550 ℃之后sp3–CH2脱附变快,且在450 ℃开始sp3–CH脱附变快。这说明在450 ℃之前,sp3–CH2和sp3–CH3脱附H后可能变为sp3–CH,在450 ℃之后sp3–CH脱附H转化为纯碳键合结构。对B薄膜,在350 ℃之前,sp3–CH2和sp3–CH3脱附速率基本一致,在350 ℃之后sp3–CH2和sp3–CH脱附H变快。这说明在350 ℃之前,sp3–CH2和sp3–CH3脱附H后可能变为sp3–CH,在350 ℃之后sp3–CH脱附H转化为纯碳键合结构。上述结论说明A薄膜具有更好的温度稳定性,而B薄膜更易脱氢。
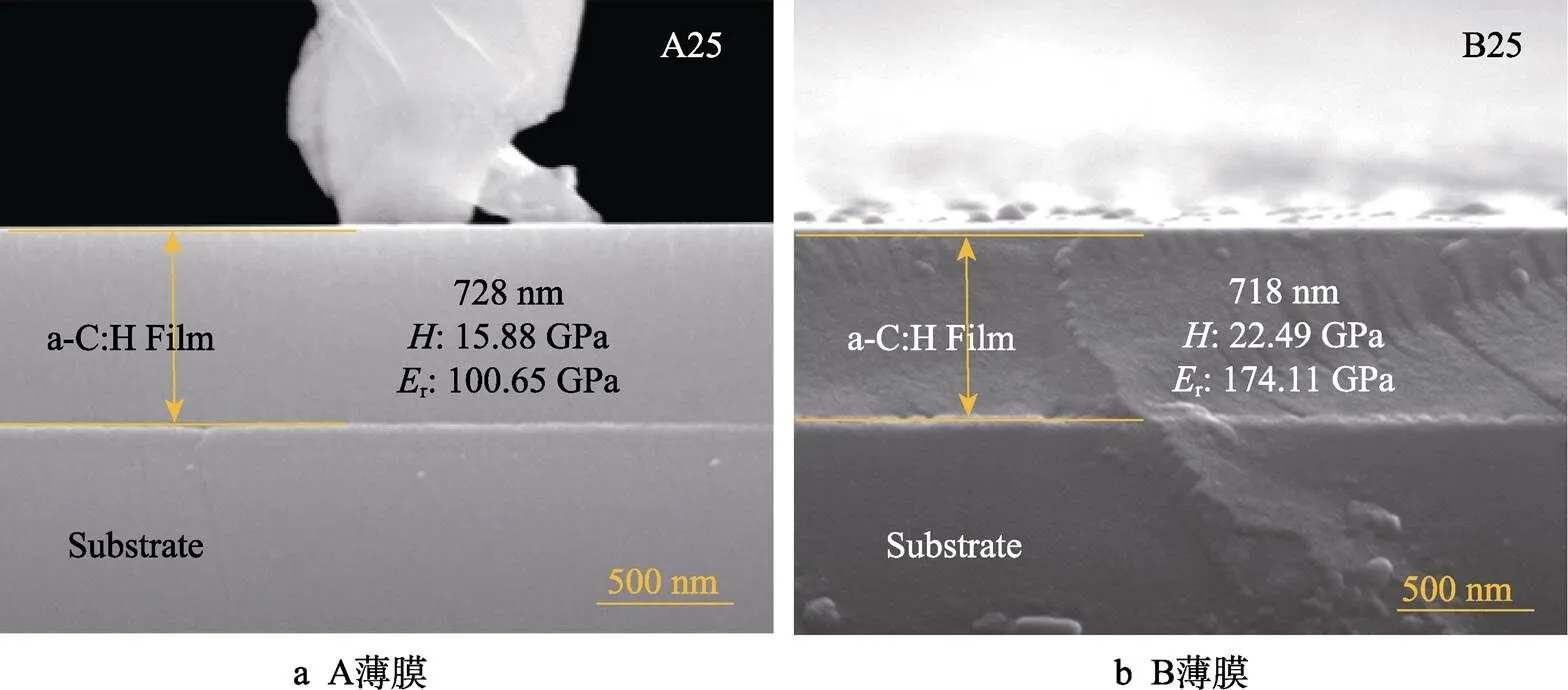
图2 A、B两种a–C:H薄膜断面厚度
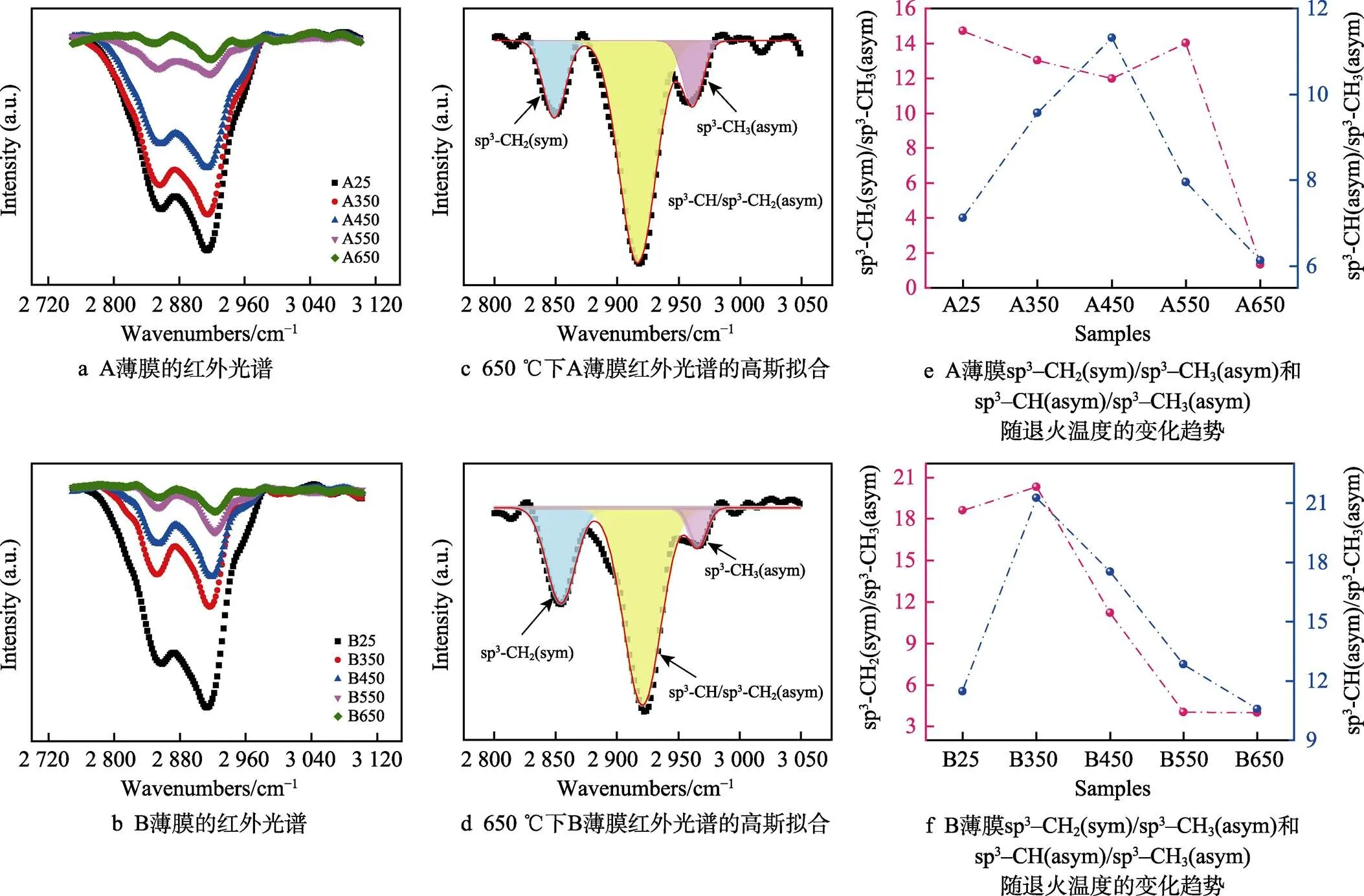
图3 A、B薄膜退火前后红外吸收光谱
如图4所示,将A、B两种a–C:H薄膜退火前后XPS的C 1s峰利用洛伦兹–高斯拟合为3个峰,分别出现在284.3~284.8、285.5~285.9、287.3~287.9 eV,分别对应sp2C==C键、sp3C—C键以及C==O键[28-30]。为了更系统地研究薄膜中sp2–C和sp3–C杂化键的相对含量,表1统计了A、B两种a–C:H薄膜退火前后的C 1s分峰结果。从表1中可以看出,退火前,A、B两种a–C:H薄膜中的sp3–C杂化键含量基本一致。但是在不同温度下进行退火处理后,A薄膜sp3–C杂化键含量随着退火温度的升高而增大,B薄膜中sp3–C杂化键含量变化不大。对比图3e和图3f,可以看到A薄膜sp3/sp2比值的变化趋势与其红外分峰的变化恰好相反,说明A薄膜在H脱附过程中更容易形成sp3–C,而B薄膜中形成sp3–C杂化键和sp2–C杂化键的概率基本一样。这一结果说明,不同方法制备的碳薄膜,其退火过程中的结构变化依赖于含氢碳薄膜的本征结构。
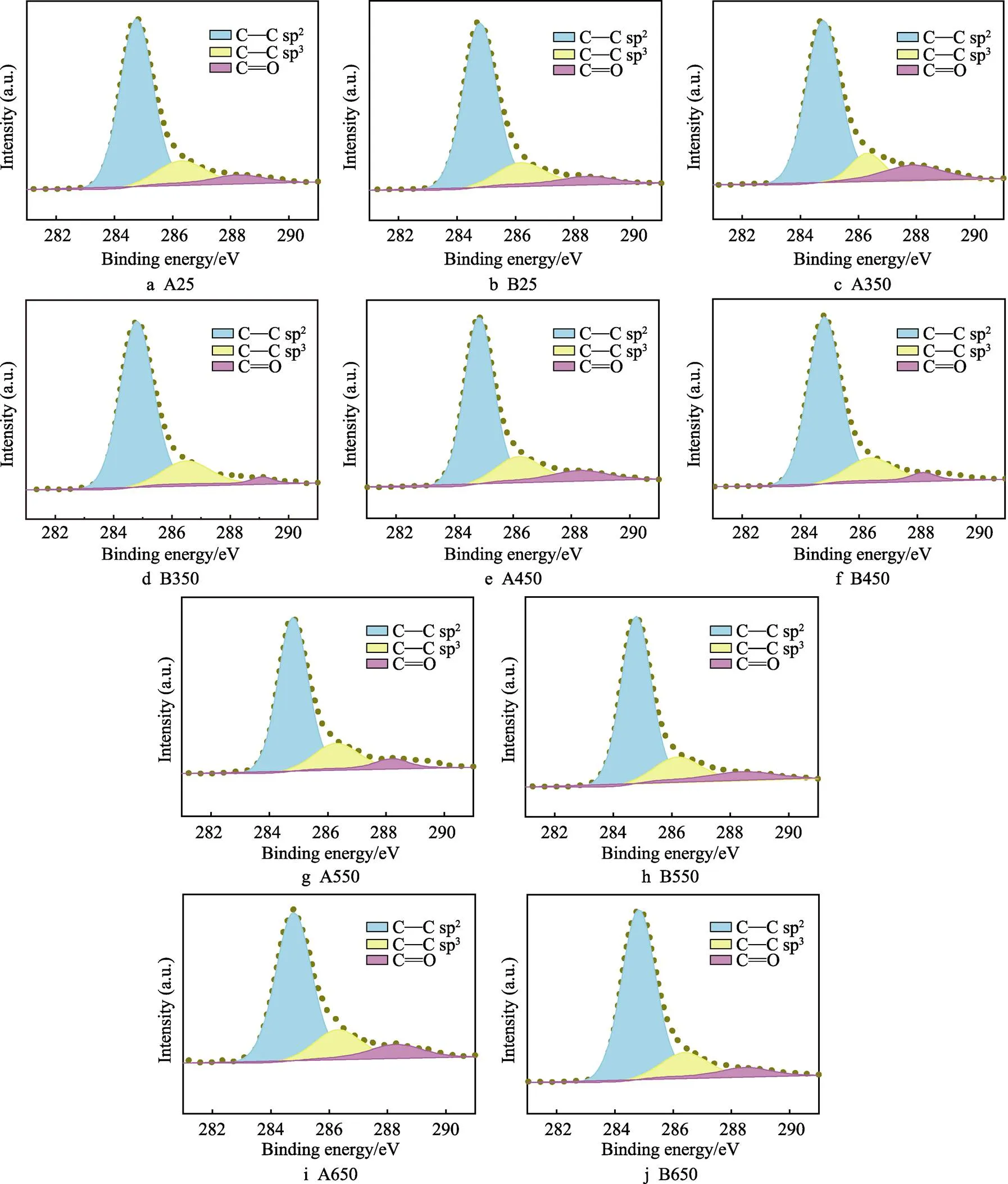
图4 A、B两种薄膜退火前后C 1s的分峰结果
表1 A、B两种含氢碳薄膜退火前后的XPS结果
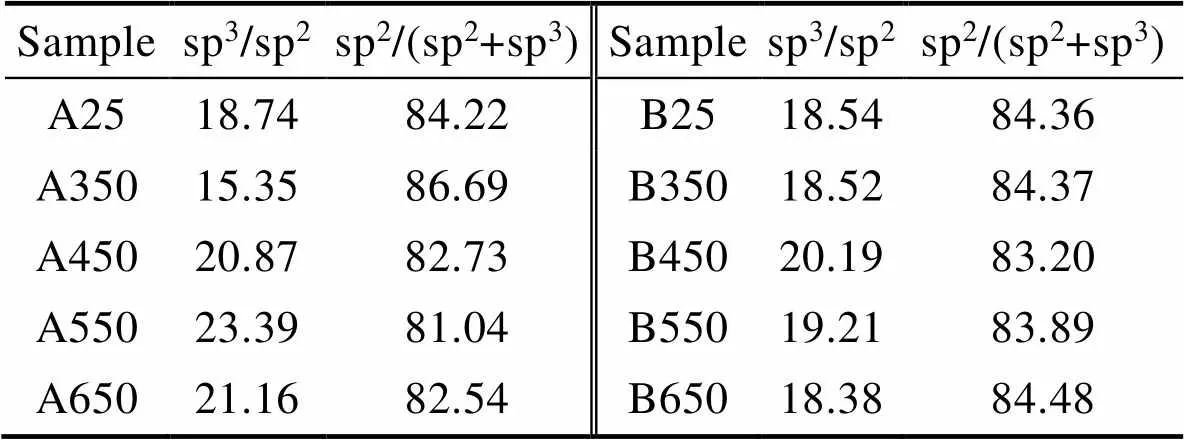
Tab.1 The statistical results from XPS of A and B film before and after annealing %
Raman光谱可以有效地区分碳薄膜中sp2–C(G峰,1 560 cm−1)和sp3–C(D峰,1 350 cm−1)杂化结构[31-33]。如图5a和图5b所示,利用高斯拟合将退火前后A、B两种a–C:H薄膜的Raman光谱从1 000 cm−1到2 000 cm−1分为2个峰,分别对应G峰和D峰。可以看出随着退火温度的增加,G峰随着退火温度的增加逐渐向高波数移动,说明薄膜内部结构的有序化程度增加。但是,A薄膜Raman峰向高波数移动的速度较慢,且在550 ℃左右才出现明显的两个波峰;不同于A薄膜,B薄膜Raman峰向高波数移动的速度较快,在450 ℃左右就出现明显的两个波峰,这一结果同红外及XPS光谱获得的结果一致。D/G值是表示sp2–C团簇有序度和尺寸大小的证据之一。图5c和图5b给出了G峰位置和D/G值变化的曲线,进一步证实了薄膜中的sp2–C有序度增加,sp2–C相对含量增加,sp2–C团簇尺寸增加。
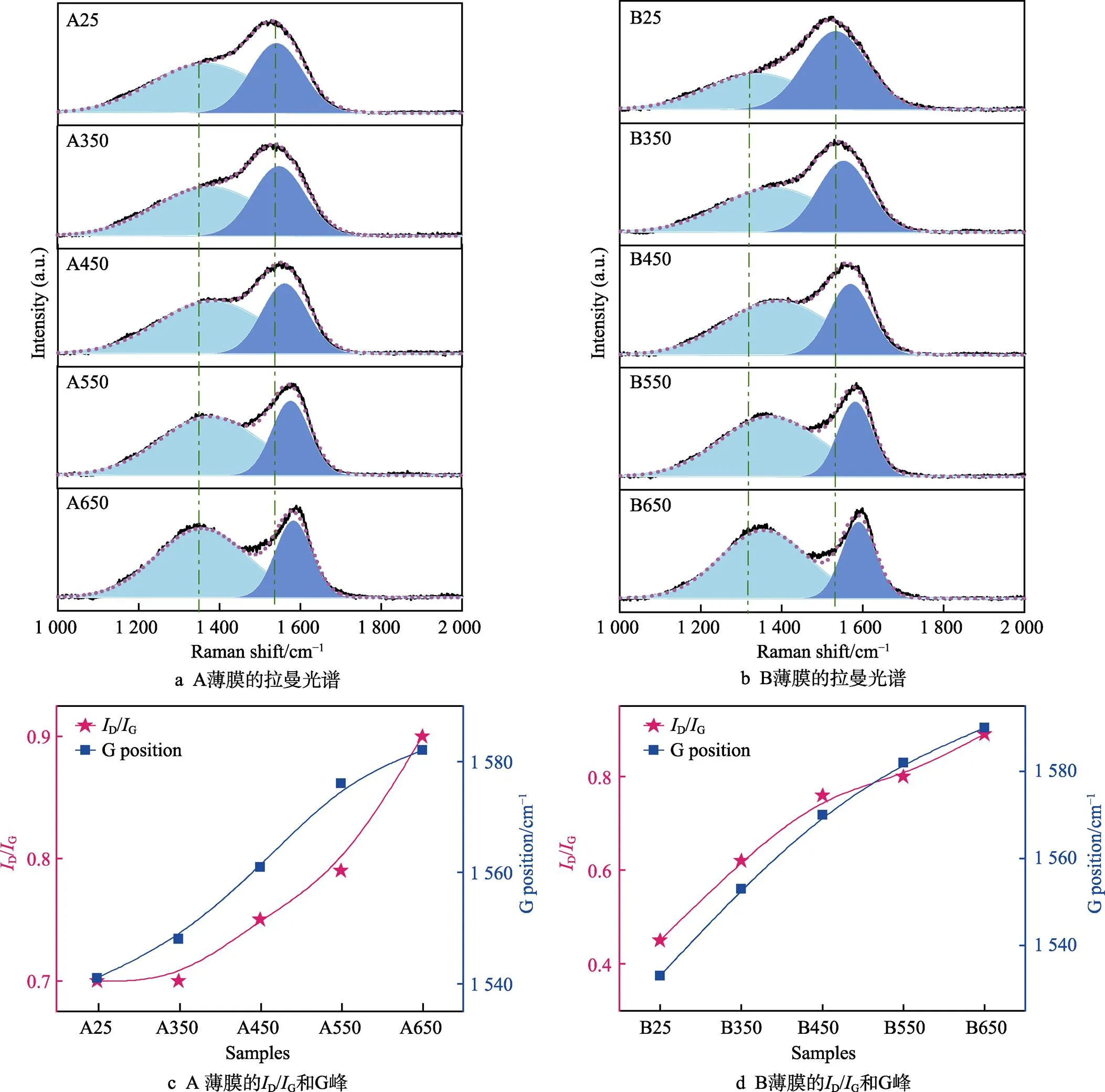
图5 A、B两种薄膜退火前后拉曼光谱分析
表面形貌照片能够直观展示退火前后薄膜表面的变化,图6为A、B两种a–C:H薄膜退火前后表面FE–SEM形貌照片。其中A薄膜在退火前后表面始终保持光滑,退火处理并未使其脱落。B薄膜随着退火温度的升高,出现愈发严重的脱落现象,在450 ℃时薄膜开始从基底剥离,550 ℃时剥离严重,且部分开始脱落,退火温度进一步升高至650 ℃时表面大面积脱落。这说明在高温下,B薄膜的结合力变差。此外,对薄膜退火前后的厚度进行了研究,结果见表2,随着退火温度的升高,薄膜的厚度发生变化,对于A薄膜,退火处理使得薄膜厚度增大,当退火温度在550 ℃时厚度达到最大,为1 020 nm。B薄膜厚度同样随着退火温度的升高而呈现出增大趋势,在450 ℃时厚度最大为850 nm。
此外,如表2所示,纳米测试结果表明,A薄膜的纳米硬度在450 ℃之前基本不变,但是当温度高于550 ℃时,薄膜硬度下降,这与sp3–C增加(XPS)的结果相反。对比红外结果,可以发现其变化同红外特征峰强度一致(2 700~2 950 cm‒1),这可能是因为虽然sp3增加,但是FTIR结果表明大部分为sp3–CH饱和的端基,导致sp2团簇不能形成sp3–C键以形成桥接的互锁结构,团簇之间容易因外力而发生相对位移。对于B薄膜,在退火温度450 ℃以下纳米硬度的变化基本同A薄膜一致,但是更高退火温度导致其脱落而不能测量纳米硬度。因此,A薄膜具有更好的热稳定性。
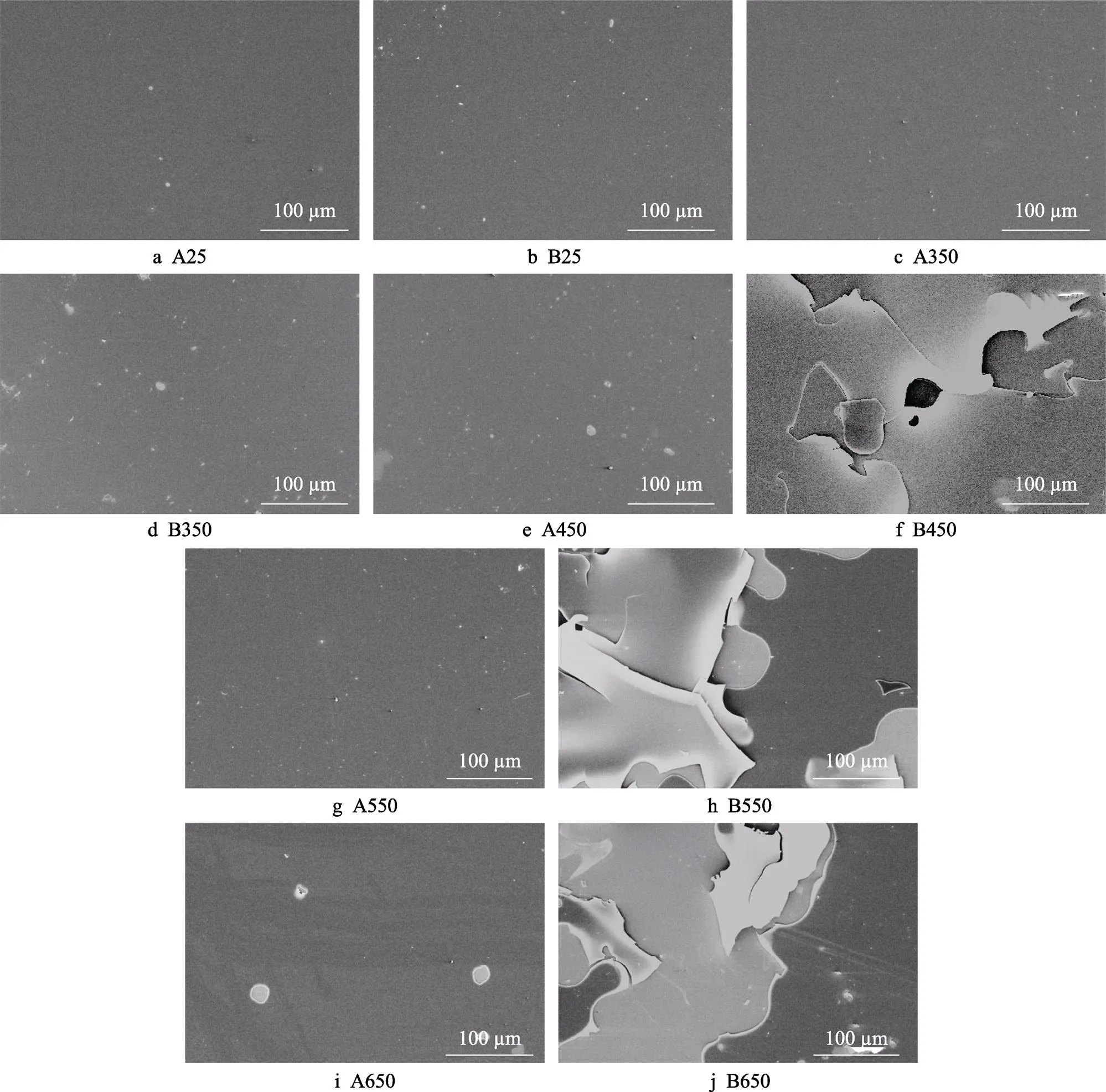
图6 退火前后A、B薄膜的表面形貌
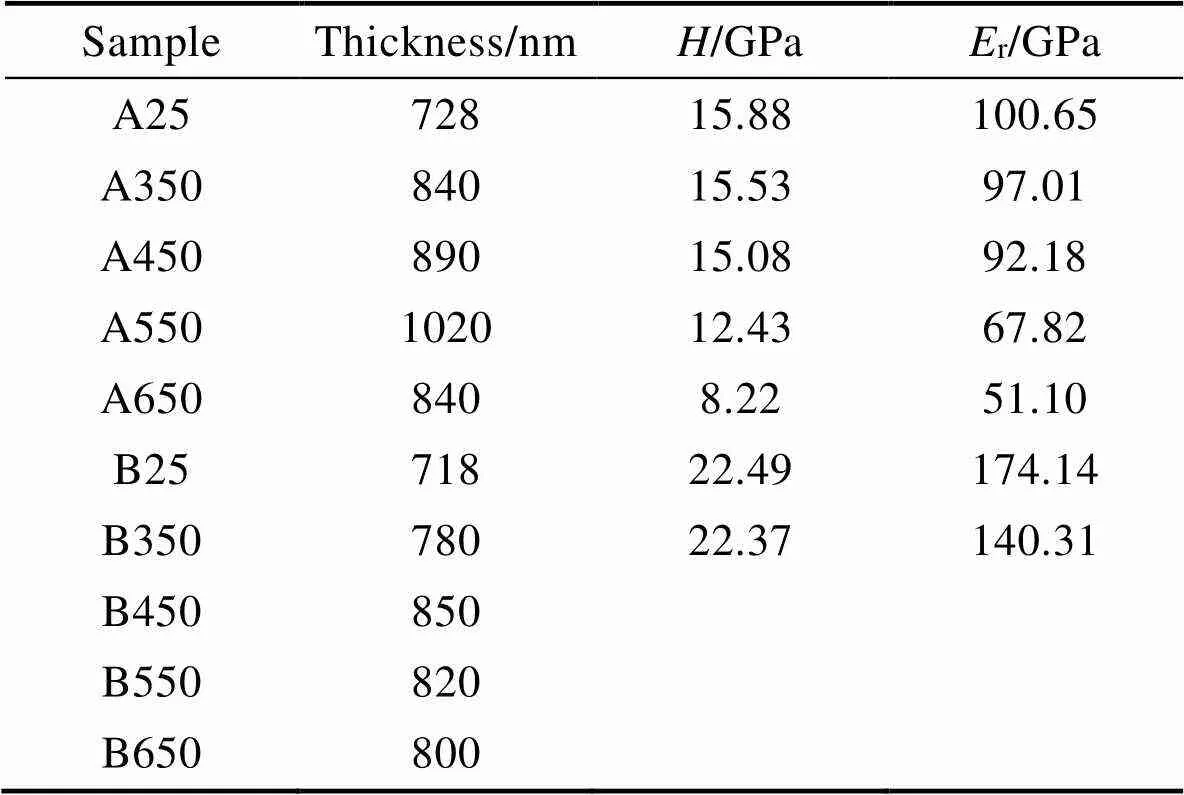
表2 退火前后A、B薄膜机械性能测试结果
进一步地,对A、B两种薄膜退火前后的摩擦因数进行了测量,结果如表3所示。A、B两种薄膜在退火前摩擦因数均为0.10,其中A薄膜的摩擦因数在退火温度为350~550 ℃时基本保持在0.06左右,但是当退火温度高达650 ℃时,摩擦测试失效。对于B薄膜,在室温和退火温度为350 ℃时均为0.10,更高温下的摩擦测试均失效。说明A薄膜在550 ℃范围内均有很好的摩擦学性能,适用于在550 ℃以下变温条件下的润滑服役。
表3 退火前后A、B薄膜摩擦因数
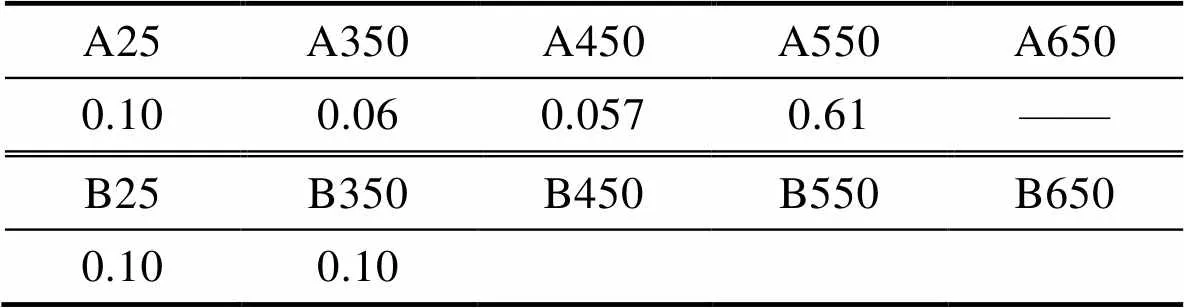
Tab.3 The friction coefficient of A and B film before and after annealing with 3 N
3 结论
1)本文分别使用DP–PECVD和BiP–PECVD两种方法成功制备了A、B两种本征结构不同的a–C:H薄膜,其中DP–PECVD方法制备a–C:H薄膜的速率更快(6.06 nm/min),是BiP–PECVD法制备a–C:H薄膜(3.99 nm/min)的1.52倍。
2)对不同方法制备的的薄膜,随着退火行为的发生均出现脱H现象。A和B薄膜的脱H转变温度不同,对于A薄膜,在450 ℃之前,sp3–CH2和sp3–CH3脱附速率基本一致,主要以sp3–CH2和sp3–CH3脱H为主,脱氢后可能变为sp3–CH,在450 ℃之后sp3–CH2和sp3–CH脱附H变快。对于B薄膜,在350 ℃之前,sp3–CH2和sp3–CH3脱附速率基本一致,在350 ℃之后sp3–CH2和sp3–CH脱附H变快。说明A薄膜具有更高的热稳定性。
3)在本文考察的退火温度范围内,A薄膜几乎没发生剥离,硬度在退火温度升高至550 ℃时急剧下降,且摩擦因数在550 ℃之前稳定在0.06左右,在650 ℃时失效。B薄膜在退火温度达到450 ℃即开始大面积剥离脱落,摩擦因数在350 ℃前稳定在0.10,自450 ℃起失效,说明A薄膜具有更好的热、力学和摩擦学稳定性。
[1] DONNET C, ERDEMIR A. Tribology of diamond-like carbon films: fundamentals and applications[M].
[2] 赵飞. 多环境适应性超润滑复合类金刚石薄膜的制备及其性能研究[D]. 北京: 中国科学院研究生院, 2010.
ZHAO Fei. Preparation and Properties of Composite Diamond-Like Carbon Films with Multi-Environmental Adaptability and Super-Lubrication[D]. Beijing: Graduate University of Chinese Academy of Sciences, 2010.
[3] OHTAKE N, HIRATSUKA M, KANDA K, et al. Properties and Classification of Diamond-Like Carbon Films [J]. Materials (Basel, Switzerland), 2021, 14(2): 315.
[4] BAI Li-chun, ZHANG Guan-gan, LU Zhi-bin, et al. Tribological Mechanism of Hydrogenated Amorphous Carbon Film Against Pairs: A Physical Description[J]. Journal of Applied Physics, 2011, 110(3): 033521.
[5] DONNET C, ERDEMIR A. Historical Developments and New Trends in Tribological and Solid Lubricant Coatings [J]. Surface and Coatings Technology, 2004, 180-181: 76-84.
[6] ROBERTSON J. Diamond-Like Amorphous Carbon[J]. Materials Science and Engineering: R: Reports, 2002, 37(4-6): 129-281.
[7] NOVIKOV N V, GONTAR A G, KHANDOZHKO S I, et al. Protective Diamond-Like Coatings for Optical Materials and Electronic Devices[J]. Diamond and Related Materials, 2000, 9(3-6): 792-795.
[8] GRILL A. From Tribological Coatings to Low-Dielectrics for ULSI Interconnects[J]. Thin Solid Films, 2001, 398-399: 527-532.
[9] 左潇, 孙丽丽, 汪爱英, 等. 高功率脉冲磁控溅射制备非晶碳薄膜研究进展[J]. 表面技术, 2019, 48(9): 53-63.
ZUO Xiao, SUN Li-li, WANG Ai-ying, et al. Research Progress on Preparation of Amorphous Carbon Thin Films by High Power Impulse Magnetron Sputtering[J]. Surface Technology, 2019, 48(9): 53-63.
[10] LU L, JONES M W, WU R L. Diamond-Like Carbon as Biological Compatible Material for Cell Culture and Medical Application[J]. Bio-Medical Materials and Engineering, 1993, 3(4): 223-228.
[11] CHOU L H. Hydrogenated Amorphous Carbon Films Prepared by Plasma-Enhanced Chemical-Vapor Deposition[J]. Journal of Applied Physics, 1992, 72(5): 2027- 2035.
[12] KIM Y T, CHO S M, CHOI W S, et al. Dependence of the Bonding Structure of DLC Thin Films on the Deposition Conditions of PECVD Method[J]. Surface and Coatings Technology, 2003, 169-170: 291-294.
[13] CAPOTE G, PRIOLI R, JARDIM P M, et al. Amorphous Hydrogenated Carbon Films Deposited by PECVD: Influence of the Substrate Temperature on Film Growth and Microstructure[J]. Journal of Non-Crystalline Solids, 2004, 338-340: 503-508.
[14] BRUNO P, CICALA G, LOSACCO A M, et al. Mechanical Properties of PECVD Hydrogenated Amorphous Carbon Coatings via Nanoindentation and Nanoscratching Techniques[J]. Surface and Coatings Technology, 2004, 180-181: 259-264.
[15] SUGIURA H, OHASHI Y, ISHIKAWA K, et al. Gas- Phase and Film Analysis of Hydrogenated Amorphous Carbon Films: Effect of Ion Bombardment Energy Flux on Sp2Carbon Structures[J]. Diamond and Related Materials, 2020, 104: 107651.
[16] 王林青, 周永涛, 王军军. 退火处理对DLC薄膜结构及摩擦学性能的影响[J]. 表面技术, 2019, 48(2): 133-138.
WANG Lin-qing, ZHOU Yong-tao, WANG Jun-jun. Effects of Annealing Treatment on the Structure and Tribological Properties of Diamond-Like Carbon Thin Films[J]. Surface Technology, 2019, 48(2): 133-138.
[17] 崔龙辰, 余伟杰. 类金刚石碳薄膜的高温摩擦学研究进展[J]. 表面技术, 2019, 48(12): 150-159.
CUI Long-chen, YU Wei-jie. Progress on High-Temperature Tribology of Diamond-Like Carbon Films[J]. Surface Technology, 2019, 48(12): 150-159.
[18] HUANG B R, YEH C S, LEE S F, et al. Effects of Pretreatment and Post-Annealing on the Field Emission Property of Diamond-Like Carbon Grown on a Titanium/ Silicon Substrate[J]. New Carbon Materials, 2008, 23(3): 209-215.
[19] FRIEDMANN T A, MCCARTY K F, BARBOUR J C, et al. Thermal Stability of Amorphous Carbon Films Grown by Pulsed Laser Deposition[J]. Applied Physics Letters, 1996, 68(12): 1643-1645.
[20] TALLANT D R, PARMETER J E, SIEGAL M P, et al. The Thermal Stability of Diamond-Like Carbon[J]. Diamond and Related Materials, 1995, 4(3): 191-199.
[21] CLIN M, BENLAHSEN M, ZEINERT A, et al. Effect of Annealing on the Structural and Electrical Properties of D.C. Multipolar Plasma Deposited A-C: H Films[J]. Thin Solid Films, 2000, 372(1-2): 60-69.
[22] LI Hong-xuan, XU Tao, WANG Cheng-bing, et al. Annealing Effect on the Structure, Mechanical and Tribological Properties of Hydrogenated Diamond-Like Carbon Films[J]. Thin Solid Films, 2006, 515(4): 2153-2160.
[23] WU Yan-xia, LI Hong-xuan, JI Li, et al. Effect of Vacuum Annealing on the Microstructure and Tribological Behavior of Hydrogenated Amorphous Carbon Films Prepared by Magnetron Sputtering[J]. Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology, 2013, 227(7): 729-737.
[24] WANG Zhao-long, GAO Kai-xiong, ZHANG Bin, et al. Verification Study of Nanostructure Evolution with Heating Treatment between Thin and Thick Fullerene- Like Hydrogen Carbon Films[J]. Coatings, 2019, 9(2): 82.
[25] WANG Zhao-long, GONG Zhen-bin, ZHANG Bin, et al. Heating Induced Nanostructure and Superlubricity Evolution of Fullerene-Like Hydrogenated Carbon Films[J]. Solid State Sciences, 2019, 90: 29-33.
[26] XIAO Ye-quan, TAN Xin-yu, JIANG Li-hua, et al. The Effect of Radio Frequency Power on the Structural and Optical Properties of A-C: H Films Prepared by PECVD [J]. Journal of Materials Research, 2017, 32(7): 1231-1238.
[27] VERES M, KOÓS M, PÓCSIK I. IR Study of the Formation Process of Polymeric Hydrogenated Amorphous Carbon Film[J]. Diamond and Related Materials, 2002, 11(3-6): 1110-1114.
[28] TERZYK A P. The Influence of Activated Carbon Surface Chemical Composition on the Adsorption of Acetaminophen (Paracetamol) in Vitro[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 177(1): 23-45.
[29] PANDIYAN R, DELEGAN N, DIRANY A, et al. Correlation of Sp2Carbon Bonds Content in Magnetron- Sputtered Amorphous Carbon Films to Their Electrochemical H2O2Production for Water Decontamination Applications[J]. Carbon, 2015, 94: 988-995.
[30] MÉREL P, TABBAL M, CHAKER M, et al. Direct Evaluation of the Sp3Content in Diamond-Like-Carbon Films by XPS[J]. Applied Surface Science, 1998, 136(1-2): 105-110.
[31] ZHANG Bin, YU Yuan-lie, ZHANG Jun-yan. Magnetron Sputtering Deposition of Carbon Nitride Nanocolumns at Low Temperature[J]. Journal of Physics D: Applied Physics, 2009, 42(18): 185304.
[32] BAI Chang-ning, LIANG Ai-min, CAO Zhong-yue, et al. Achieving a High Adhesion and Excellent Wear Resistance Diamond-Like Carbon Film Coated on NBR Rubber by Ar Plasma Pretreatment[J]. Diamond and Related Materials, 2018, 89: 84-93.
[33] FILIK J, MAY P W, PEARCE S R J, et al. XPS and Laser Raman Analysis of Hydrogenated Amorphous Carbon Films[J]. Diamond and Related Materials, 2003, 12(3-7): 974-978.
Annealing Treatment of Hydrogenated Amorphous Carbon Film is Affected by Its Intrinsic Structure
1,2,1,2,1,2,1,2
(1. Key Laboratory of Science and Technology on Wear and Protection of Materials, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China; 2. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China)
Hydrogenated amorphous carbon (a-C:H) film was used in different field due to its excellent electrical, optical and tribological properties. With the evolution of the mechanic systems, the a-C:H film is required not only to have the performance of low friction coefficient, but also to meet the high temperature (≥350 ℃) service conditions. Thus, the thermal stability of the a-C:H film is the key point. The stability of a-C:H films is closely related to their inner structure, which depends on the preparation methods. So it is important to study the effects of different preparation methods on the nano structure of a-C:H films with variation of temperatures. The previous work mainly focused on the structural changes of specific a-C:H films at different annealing temperatures. However, there still lack of attention to the effect of film’s intrinsic structure on the annealing results. Thus, in this work, DC Pulsed Plasma Enhanced Chemical Vapor Deposition (PD-PECVD) and Bipolar Pulsed Plasma Enhanced Chemical Vapor Deposition (BiP-PECVD) were used to deposit two kinds of a-C:H films with different structures, then of which were annealed at 350 ℃, 450 ℃, 550 ℃ and 650 ℃, respectively. The variation of structural, mechanical properties, surface morphology and tribological properties of two kinds of a-C:H films were evaluated by appropriate testing methods. The results show that the deposition rate of a-C:H films, deposited via PD-PECVD method is 1.52 times higher than that of the BiP-PECVD method. With the increase of the annealing temperature, the a-C:H films prepared by the two methods all desorbed H but the transitional temperature point of desorption of H at 450 ℃ and 350 ℃, respectively. One can be also confirmed that the a-C:H film prepared by the PD-PECVD method is easier to form sp3-C during the H desorption process, while the a-C:H film prepared by the BiP-PECVD method has the same probability of forming sp3-C and sp2-C hybrid bonds. In addition, the a-C:H film, prepared by the BiP-PECVD method, is easier to lose H during the annealing process and peeled off in a large area beyond the annealing temperature of 450 ℃, resulting in friction failure. The a-C:H film prepared by the PD-PECVD method is unpeeled off and keep the friction coefficient as low as 0.06 when annealing at 350-550 ℃. To sum up, the film prepared by PD-PECVD method has better thermal, mechanical and tribological stability, which can work under high-temperature conditions.
hydrogenated amorphous carbon film; annealing; bipolar pulse; DC pulse; plasma-enhanced chemical vapor deposition
TG174.442
A
1001-3660(2022)07-0098-09
10.16490/j.cnki.issn.1001-3660.2022.07.009
2021–05–21;
2021–11–17
2021-05-21;
2021-11-17
国家自然科学基金(U1737213)
Supported by National Natural Science Foundation of China (U1737213)
贾倩(1995—),女,博士研究生,主要研究方向为碳薄膜异质配副界面行为及超滑机制。
JIA Qian (1995-), Female, Doctoral student, Research focus: heterogeneous interfacial behavior and superlubricity mechanism of carbon films.
张俊彦(1968—),男,博士,研究员,主要研究方向为薄膜材料的微细结构构筑与摩擦学,材料表面、界面及功能可控的物理化学。
ZHANG Jun-yan (1968-), Male, Doctor, Professor, Research focus: microstructure construction and tribology properties study of thin film materials, physical chemistry of material surface, interface and function controllable.
贾倩, 张斌, 赖振国, 等. 非晶含氢碳薄膜本征结构对退火行为的影响[J]. 表面技术, 2022, 51(7): 98-106.
JIA Qian, ZHANG Bin, LAI Zhen-guo, et al. Annealing Treatment of Hydrogenated Amorphous Carbon Film is Affected by Its Intrinsic Structure [J]. Surface Technology, 2022, 51(7): 98-106.
责任编辑:万长清
