Active corrosion protection of phosphate loaded PEO/LDHs composite coatings: SIET study
2022-07-13GenZhngJingLingWuAitoTngAnrejAtrensFushengPn
Gen Zhng, E Jing, Ling Wu, Aito Tng, Anrej Atrens, Fusheng Pn
aNuclear Power Institute of China, Chengdu, Sichuan 610213, China
bCollege of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
cNational Engineering Research Center for Magnesium Alloys, Chongqing University, Chongqing 400044, China
d School of Mechanical and Mining Engineering, The University of Queensland, Brisbane Qld 4072, Australia
Abstract
Keywords: Layered double hydroxides; Active corrosion protection; SIET; Phosphate.
1.Introduction
Functional coatings with active corrosion protection have been widely investigated in surface treatment fields, particularly important for Mg alloys with inadequate corrosion resistance [1–5].These are also known as self-healing or selfrepairing coatings.One straight forward production method is to embed healing agents (e.g.healing polymers, catalysts or corrosion inhibitors) into the coating.Two examples are (i)the absorption of corrosion inhibitors into a porous coating(e.g.a PEO coating) or (ii) the mixing directly of healing polymers with the binder, which makes up the coating [6,7].However, the following problems are likely: (i) poor dispersion of the healing agents throughout the coating, (ii) the interaction between the healing agents and the coating itself,and (iii) rapid loss of self-healing functionality, as a result of fast release at initial stage [8].
Another production method is to use encapsulation technology.The healing agents are stored in encapsulating containers and are released using one of the following two basic approaches.(i) The encapsulating containers are broken and release the healing agents because of a mechanical impact on the coating.Polymerization occurs between the healing agents and the coating, resulting in the repair of the barrier property of the coating.A typical example is in White et al.[9].(ii)The encapsulating containers release the healing agents when the coating is subjected to stimuli from local corrosion, such as a pH change, presence of aggressive species, dissolution,humidity or moisture.The corrosion inhibitors leach into the coating defect and inhibit the electrochemical reactions.In this case, ion-exchange material such as layered double hydroxides (LDHs) or zeolites was used as a kind of typical encapsulating container [10,11].

Fig.1.Schematic representation of the LDH structure.
Over the past three decades, LDHs were studied as superhydrophobic coatings [12–14]and as the corrosion inhibiting additive in organic coatings [10,15, especially as the encapsulating container loaded with inhibitors to prepare functional coatings [8,16–20].LDHs can be represented by the general formula [M1-x2+Mx3+(OH)2][An-]x/n•mH2O, where M2+, M3+and An-are divalent cations, trivalent cations and interlayer anions respectively.Fig.1 shows a structure schematic and a typical octahedral unit.Partially M2+cations coordinated octahedrally by hydroxyl groups are replaced by M3+cations, resulting in a positive charge on the host layer.The anions An-(e.g.inhibitor anions) can be incorporated into the interlayer between the brucite-like sheets to compensate for the positive charge of the host layer [21–23].
A large amount of studies used co-precipitation method to prepare LDHs [24].In contrast, the in situ method directly grows LDHs on the metal substrate,which considerably improve the adherence to the substrate and the mechanical.Moreover, combination LDHs with anodic film or PEO coatings has been developed as a new method to prepared highly active protective coatings[25].In such a context,our previous work (i) proposed a novel method to prepare MgAl-LDHs on Mg alloy AZ31 through hydrothermal chemical conversion of a pre-prepared anodic film [18,26,27], and (ii) explained the internal sources of Mg2+and Al3+cations, which are necessary for nucleation and growth of MgAl-LDHs [28].The Stranski-Krastanov (SK) 2d to 3D growth mode explained the transformation from the anodic film to the MgAl-LDHs [28].This novel method of preparing MgAl-LDHs was also developed on electrolytic oxidation (PEO) coatings, and studied the effect of PEO properties on the growth of LDHs [8,29].
Recent unpublished work prepared three different kinds of PEO coatings on AZ31 in aluminate, silicate and phosphatebased electrolytes, followed by hydrothermal treatments to synthesis LDHs in three different growth solutions (deionized water, sodium nitrate and aluminum nitrate containing solution).This work showed that silicate-based PEO specimens had the best corrosion resistance and better stability than aluminate- and phosphate- based PEO specimens.The hydrothermal treatment in Al3+containing growth solution of phosphate-based PEO coatings provided the best corrosion resistance and self-healing ability.An ion-exchange with suitable corrosion inhibitors can be considered as a next step.Ramezanzadeh and Mahdavian [30–34]did a lot of work on modification of MgAl- or ZnAl-LDHs using corrosion inhibitor phosphate solution, and further proved the potential of phosphate loaded LDHs to design efficient protective coating.They suggested that this efficient inhibition behavior toward corrosion was due to i) releasing PO43-anions from LDHs and ii) absorbing the Cl-anions into LDHs.
Previous electrochemical studies provide the average global electrochemical response.Localized techniques can be complementary role in unveiling the corrosion progress at the micro-scale.The scanning ion-selective electrode technique(SIET) is a micro-potentiometric tool that can be used to measure specific free-ion activities at a quasi-constant microdistance over an active surface in solution [35].
This work used the SIET technique to continue to study the localized self-healing performance of phosphate-based PEO/LDHs composite coating loaded with phosphate inhibitors, and in particular (i) the distribution and change of local pH, and (ii) the concentration of Mg2+ions dissolved due to Mg corrosion from artificial defects.
2.Experimental methods
2.1.Materials and reagents
The Mg alloy AZ31 had a nominal composition of Al 2.5–3.5%, Zn 0.6–1.3%, Mn 0.2–1%, Ca 0.04%, Si 0.1%, Cu 0.05% (all in wt.%), and balance Mg.Deionized water was the solvent in this work.
2.2.Specimens preparation
The AZ31 specimens with dimension of 20mm×20mm×5mm were ground up to 1200 grit using SiC papers on all surfaces, and dried with warm air.The phosphate-based PEO specimens were prepared in 8.03g/L Na3PO4and 7.14g/L NaOH electrolytes using a pulsed DC power supply, at a constant applied voltage of 350V for 600s withton:toff=2 ms: 8 ms.The electrolyte was continuously stirred during the PEO treatment and kept at 20±5°C using a water cooling system.The specimens were rinsed in deionized water and dried in warm air.The phosphate-based PEO specimens were hydrothermally treated in a Teflon-lined stainless steel autoclave at 398K for 16h in 0.1M Al(NO3)3aqueous solution with pH=10.5 adjusted using NaOH.Finally, an ion-exchange process was performed by immersion of the phosphate-based PEO/LDHs specimens into the 0.1M sodium phosphate aqueous solution of pH 9 at 90°C for 24h.For clarity, the specimens at this stage are designated as PEO/LDHs-P.For a better comparativeanalysis, silicate-based PEO specimen was the reference sample, rather than phosphate-based PEO specimens, because the silicate-based PEO specimen has almost no self-healing ability, in contrast to the phosphate-based PEO specimens.Furthermore, silicate-based PEO specimens have a corrosion resistance better than other between PEO specimens(including aluminate-, phosphate- and silicate-based PEO specimens).The silicate-based PEO specimen was produced in 5.94g/L Na2SiO3and 7.14g/L NaOH electrolytes using a PEO treatment process was similar to that of the phosphate-based PEO specimens.For clarity of discussion,the silicate-based PEO specimen was designated as Si/PEO.
2.3.Characterization
X-ray diffraction(XRD;Rigaku D/Max 2500X,Japan)was used to clarify if the inhibitor phosphate ions were successfully loaded, using a Cu Kαradiation (40kV, 40mA) at a glancing angle of 1.5°, in the range of 2θfrom 5 to 80°and at a scanning rate of 0.02° s-1.Scanning electron microscopy (SEM; Tescan Vega3, Czech) investigated the surface morphologies of the specimens.Polarization curves were measured using CIMPS-2 Zahner system with a conventional three-electrode system, i.e., a three-electrode cell with a saturated Ag/AgCl reference electrode, a platinum counter electrode and the as-prepared samples as the working electrode with an exposed area of 1 cm2.
The scanning ion-selective electrode technique(SIET)used a commercial device from Applicable Electronics, controlled by the ASET-LV4 software.The preparation of the pH- and Mg2+selective microelectrodes(pH-SME and Mg2+-SME)are presented elsewhere [36–38].A homemade Ag/AgCl/0.05M NaCl mini electrode was the external reference electrode.The QuickGrid (version 5.3) software was used for the SIET map analysis.Before the SIET measurements, a round artificial scratch, whose diameter was approximately 200–300μm, was introduced into the coated specimens by a sharp knife.
3.Results
Fig.2 shows XRD patterns before and after loading the corrosion inhibitor phosphate ions into phosphate based PEO/LDHs specimens.The characteristic diffraction peaks of MgAl-LDHs were located at about 11.3° and 22.2° (correlated with 003 and 006 planes of LDHs), indicating that their interlayers were loaded with hydroxides/carbonate [39].After loading with phosphate ions, there were additional diffraction peaks,corresponding to phosphate loaded MgAl-LDHs,at the peak positions correlated with the 003 and 006 planes shifted to lower angles (8.5° and 17.5°).The basal spacing (c) is the total thickness of the brucite-like sheet and can be calculated from the position of (003) or (006) reflection using Bragg’s equation [33]:


Fig.2.XRD patterns of(a)before and(b)after loading of corrosion inhibitor of phosphate based PEO/LDHs specimens.

Fig.3.SEM surface micrographs of (a) Si/PEO and (b) PEO/LDHs-P.
As we known, the thickness of brucite layer is about 4.77.Hence,the gallery height(d)can be calculated by subtracting the thickness of brucite layer from the basal spacing(c).As for the precursor hydroxide/carbon based LDHs, the calculated gallery heightdwas 3.06.After anion exchange,the calculated gallery heightdincreased to 5.62.That meant that the inhibitor phosphates were intercalated into the LDH interlayer galleries successfully.In addition to the peaks of LDHs, there was a peak corresponding to Mg3(PO4)2•22H2O that may be attributed to the formation of magnesium phosphate during the ion-exchange reaction.
Fig.3 shows the differences in the surfaces of the Si/PEO and PEO/LDHs-P specimens.Fig.3a shows a characteristic morphology of the PEO layer with a random distribution of a large number of pores.The surface morphology was significantly changed after fabrication of the LDHs and the loading with inhibitors.The original pores had disappeared,which significantly increased the coating density.EDS result(not shown) further shows that 11.9 at.% P element existed in PEO/LDHs-P specimens.Fig.4 shows representative polarization curves of Si/PEO and PEO/LDHs-P specimens in 3.5wt.% NaCl aqueous solution.The corrosion current density (icorr) of PEO/LDHs-P was 1.6×10-8A cm-2, and decreased by around 2 orders of magnitude when compared to the Si/PEO specimen, which implied the best corrosion protection for PEO/LDHs-P.
SIET measurements were carried out at a round artificial defect in the surface of coated specimens in order to bet-ter understand the self-healing process for the PEO/LDHs-P.Fig.5 presents the optical images around the defect during the SIET test.Before the SIET test,Fig.5a and b indicate that the diameter of the round artificial defect was between 200μm and 300μm.On initial immersion, a large number of H2bubbles formed around the defect for Si/PEO,while corrosion activity around the defect was relatively low for PEO/LDHs-P(there was only one big bubble).Increasing immersion time,for Si/PEO, indicated a continuing significant hydrogen evolution reaction and extension of the corroding area in Fig.5e,up to ~978min immersion.In contrast, for PEO/LDHs-P, hydrogen evolution almost stopped at the original position of the defect on immersion up to ~202min (Fig.5h), but there was corrosion extension existed (Fig.5f).

Fig.4.polarization potentiodynamic curves measured in 3.5wt.% NaCl solution for Si/PEO and PEO/LDHs-P.
Fig.6 shows the SIET mapping of the pH distribution around the defect after 2h, 8h and 16h immersion in NaCl solution.After 2h, the pH maps for Si/PEO and PEO/LDHs-P revealed significant alkalinization over the entire scanned area.However, for Si/PEO, the pH of the central zone attained values 12.3, which was much higher than that of 10.4 for PEO/LDHs-P.In addition, the minimum pH in Si/PEO was also higher than the maximum pH in PEO/LDHs-P during the whole immersion.Moreover, the strong alkalinization of PEO/LDHs-P was in the lower part of the scanned area, rather than over the entire defect (and was significantly different from that of Si/PEO).These results indicated that the inhibitor containing PEO/LDHs-P lowered the intensity of the corrosion process.With increasing immersion time, the pH in the scanned area continued to decline in both Si/PEO and PEO/LDHs-P, attributed to the following two reasons:i) the consumption of Mg2+, the chemical interaction between Mg2+and OH-or PO43–gave rise to the formation of Mg(OH)2and Mg3(PO4)2; and ii) the spreading of free hydroxide ions to the surrounding solution [38].
For PEO/LDHs-P immersed for 8h, the central of alkalinization area moved to the upper left corner.At 16h immersion, the position corresponding to the original defect did not show a round alkalinized central.Compared to the reference specimen (Si/PEO), The self-healing was almost complete at 8h and the defect was completely healed at 16h.In contrast,the Si/PEO still contained at 16h an obvious alkalinized central region in the original position of the defect.That implied that the corrosion activity of the defect cannot be effectively suppressed only by corrosion product Mg(OH)2.
Figs.7 and 8 show SEM morphologies and corresponding EDS elemental mapping around the defect after immersion for Si/PEO and PEO/LDHs-P, respectively.The corrosion in both Si/PEO and PEO/LDHs-P spread laterally.A lot of corrosion products formed on Si/PEO, but not on PEO/LDHs-P.There was an active corrosion pore, which was in good agreement with the pH mapping.In contrast, less corrosion products formed over the defect of PEO/LDHs-P, and there resulted a more flat surface.The EDS mapping (Fig.7) indicated that the defect of Si/PEO was mainly covered by corrosion product Mg(OH)2and Si did contribute.For PEO/LDHs-P (Fig.8), accumulation of Mg, Al, O, Cl and P over the defect suggested formation of insoluble corrosion products,such as Mg3(PO4)2which can be formed in an alkaline condition.
Fig.9 presents Mg2+distribution around the defect during the immersion period.At initial immersion stage forSi/PEO, the pMg map showed depletion of Mg2+in the central position, where there was strong alkalinization area(shown as pH map) attributed to the formation of sparingly soluble Mg(OH)2products.As the immersion time increased,the original defect was gradually covered by corrosion products Mg(OH)2, leading to suppression of corrosion.However, the Mg2+concentration in the central position remained low, which was similar to the pH map.In addition, the Mg2+concentration gradually increased with immersion time,up to 33mM/L.After 16h immersion, the free Mg2+concentration was between 3 and 33mM/L.These results also implied that corrosion was not completely suppressed.Different to Si/PEO, the free Mg2+reached the highest concentration(24mM/L) at 5h.On increasing the immersion time to 17h,the Mg2+concentration was between 0.3 and 3mM/L, and was similar over the entire surface.

Fig.5.Optical morphology around the defect before immersion and during immersion in 0.05M NaCl.

Fig.6.pH distributions around the defect after different times of immersion in 0.05M NaCl.

Fig.7.SEM morphologies and corresponding EDS elemental mapping of Si/PEO around the defect after 17h immersion in 0.05M NaCl.
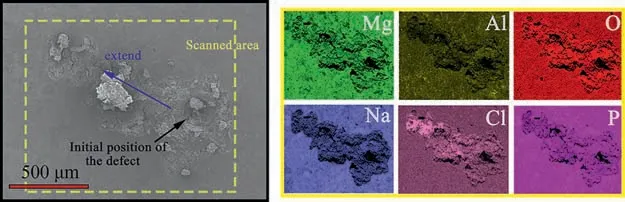
Fig.8.SEM morphologies and corresponding EDS elemental mapping of PEO/LDHs-P around the defect after 17h immersion in 0.05M NaCl.

Fig.9.pMg distributions around the defect after different times of immersion in 0.05M NaCl.

Fig.10.Schematic illustration of the self-healing mechanism for PEO/LDHs-P.
The SIET-data,including pH and pMg maps,indicated that for passive PEO coated specimens,a strong alkalinization area formed in the original position of the defect and led to the depletion of Mg2+in this area.Unusually, SIET is interpreted to indicate that the alkalinization area corresponds to the local cathode and the acidification area corresponds to the local anode.For example, Gnedenkov et al.[35]made a scratch as artificial defect in inhibitor-containing PEO coatings and found that the left side of the scratch was a local anode resulting in acidification, while the right side was a local cathode and showed alkalinization.However, there was no acidification herein, which may be attributed to a too small defect leading in overlap between anode and cathode.Both the pH or pMg data confirmed that the passive PEO coating cannot provide a better corrosion protection, especially at defects in the coating.
This case of PEO/LDHs-P produced a better self-healing ability.The good inhibitive corrosion action of PEO/LDHs-P can be ascribed to i) dissolution of amorphous phosphorus phases, likely Mg3(PO4)2, ii) the release of inhibitive species(PO43–) from the MgAl-LDHs and the precipitation on the anodic regions of Mg3(PO4)2[8]and iii) the precipitation of corrosion products Mg(OH)2.The schematic illustration of this self-healing process are shown as Fig.10.
4.Conclusion
(1) The position of the artificial defect on the passive PEO specimens remained active throughout the immersion period, while hydrogen evolution on the artificial defect of the active PEO/LDHs-P specimens almost stopped at ~202min immersion.Furthermore, both Si/PEO and PEO/LDHs-P suffered corrosion extension.
(2) Due to corrosion of Mg around defects, there was significant alkalinization in both Si/PEO and PEO/LDHs-P in the pH maps.However, the pH of the central zone of PEO/LDHs-P was much lower than that of Si/PEO.With increasing immersion time, the pH in the scanned area continued to decline in both of them.
(3) Due to formation of insoluble Mg(OH)2products in the high pH area, the pMg map of Si/PEO showed depletion of Mg2+in the central position.In contrast,the free Mg2+reached the highest value (24mM/L) at 5h immersion.Further prolonging immersion time, the Mg2+concentration became relatively uniform over the entire surface.
(4) The lower pH or lower pMg results confirmed that PEO/LDHs-P specimens had a better self-healing ability toward defects.
Declaration of Competing Interest
None.
Acknowledgements
This work was supported by the International Cooperation in Science and Technology Innovation between Governments,National Key Research and Development Program of China(No.2018YFE0116200), the National Natural Science Foundation of China (51971040) and the Fundamental Research Funds for the Central Universities (2020CDJQY-A007).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Effect of B4C on strength coefficient, cold deformation and work hardening exponent characteristics of Mg composites
- Understanding pitting corrosion behavior of AZ91 alloy and its MAO coating in 3.5% NaCl solution by cyclic potentiodynamic polarization
- Corrosion protection investigations of carbon dots and polydopamine composite coating on magnesium alloy
- The detailed corrosion performance of bioresorbable Mg-0.8Ca alloy in physiological solutions
- Improving the electrochemical stability of AZ31 Mg alloy in a 3.5wt.%NaCl solution via the surface functionalization of plasma electrolytic oxidation coating
- Analysis of the corrosion performance of binder jet additive manufactured magnesium alloys for biomedical applications
