Novel soybean peptide iglycin ameliorates insulin resistance of high-fat diet fed C57BL/6J mice and differentiated 3T3L1 adipocytes with improvement of insulin signaling and mitochondrial function
2022-07-11YinghunWuRnZhoMinxiLiHuiyunLiZhengwngChenYnyingZho
Yinghun Wu, Rn Zho, Minxi Li, Huiyun Li, Zhengwng Chen, Ynying Zho,*
a Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization of Ministry of Education, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization of Sichuan Province, College of Life Science and Technology, Southwest Minzu University, Chengdu 610041, China
b Shandong Tianheng Inspection Co., Ltd., Heze 274000, China
c Key Laboratory of Molecular Biophysics of Ministry of Education, School of Life Science and Technology, Huazhong University of Science and Technology, Wuhan 430074, China
ABSTRACT
Soy consumption has been associated with potential health benefits in reducing chronic diseases. These physiological functions have been attributed to soy proteins or more commonly to bioactive peptides. Thus,more studies are required to identify these bioactive peptides, and elucidate their biological mechanisms of action. In the present study, a novel peptide iglycin was purified from soybean seeds with a molecular mass of 3.88 kDa. Thereafter, iglycin reduced fasting blood glucose and restored insulin sensitivity of C57BL/6J mice on a high-fat diet with increased phosphorylation of insulin receptor substrate 1 (IRS1) and AKT in adipose tissue. Furthermore, it improved glucose uptake, induced translocation of intracellular GLUT4 to plasma membrane and activation of insulin signaling in adipocytes under insulin-resistant condition. In addition, it decreased reactive oxygen species production, lipid peroxidation and inhibited adipocyte apoptosis with improved mitochondrial function as evidenced by up-regulation of succinate dehydrogenase activity,mitochondrial membrane potential and intracellular ATP store. These data suggested that iglycin ameliorated insulin resistance via activation of insulin signaling, which was associated with inhibition of oxidative stress,adipocyte apoptosis, and improvement of mitochondrial function.
Keywords:
Soybean peptide
Iglycin
Insulin resistance
Insulin signaling
1. Introduction
Insulin resistance describes that the usual actions of insulin are not mediated in various target tissues such as the fat, muscle and liver [1].It is the primary cause of type 2 diabetes and several factors have been proposed to explain the mechanisms of insulin resistance including obesity [2,3], inflammation [4,5], mitochondrial dysfunction [6,7],hyperinsulinemia, lipotoxicity/hyperlipidemia [8], genetic background, endoplasmic reticulum stress [9], aging, oxidative stress [10,11], fatty liver, hypoxia, lipodystrophy and pregnancy.
Soybean (Glycine max) is one of the most important crops and it is a rich source of high-quality proteins that contain all the essential amino acids without cholesterol and with less saturated fat than animal proteins. Soybean consumption is associated with many potential benefits for human health in reducing many chronic diseases [12-14],such as obesity [15], cardiovascular disease [16], insulin-resistance/type 2 diabetes [17] and cancers [18]. These physiological functions have been attributed to soy proteins or more commonly to functional bioactive peptides derived from soybean processing [19]. Soy bioactive peptides are small fragments of soybean proteins produced by enzymatic hydrolysis, food processing, fermentation, and/or gastrointestinal digestion [20]. Interestingly, several soy peptides possess anti-diabetic activity in different experimental models.For example, soy peptides LPYP, IAVPGEVA, and IAVPTGVA increased glucose uptake into hepatic cells via glucose transporters 1 and 4 [21]. Similarly, soymorphin-5 (YPFVV) lowered the levels of glucose and triglyceride in diabetic KKAy mice [22]. Our previous study also showed that the soybean peptide, vglycin, normalized fasting glucose levels, increased insulin sensitivity, restored insulin signaling and pancreatic function in type 2 diabetic Wistar rats [23].In addition, it prevented liver injury, in flammation and insulin resistance by inhibiting fat accumulation in liver [24]. In the present study, a novel peptide iglycin was purified from soybean seeds and its regulation on glucose homeostasis of high-fat diet fed C57BL/6J mice was explored.
2. Materials and methods
2.1 Peptide purification
Iglycin was isolated from soybean seeds as we previously described [25]. Brie fly, soybean seeds were soaked and subsequently homogenized. After the fractions were pre-treated with alginic acid adsorption, NaCl salting-out, acetone extraction, the peptide was purified by reverse phase-high performance liquid chromatography (RP-HPLC) using an Agilent 1200 system (Agilent Technologies, Wilmington, DE, USA). Then, molecular mass of the peaks recovered from RP-HPLC was determined using MALDI-TOF-MS in an Applied Biosystems Voyager 4307 instrument (Foster City, CA,USA) and amino acid sequence of the target peak was analyzed by Edman degradation.
2.2 Animal experiment
Animal studies were approved by the Southwest Minzu University Institutional Committee for the Care and Use of Animals.7-week aged, 32 male C57BL/6J mice were free access to normal chow diet and water for 1 week under the following conditions:specific pathogen free, 12 h light-dark cycles at 22–24 °C. Then the mice (20 ± 2) g were randomly equally divided into 4 groups: the mice (n = 8) in group A were fed with chow diet (Chow), the mice (n = 8)in group B were fed with a high fat diet (HFD, D12492, Research Diets, New Brunswick, NJ, USA), the mice (n = 8) in group C were fed with a high fat diet supplemented with 40 μg iglycin per gram of body weight (bw) per day (40 μg/(g·day)), and the mice (n = 8)in group D received a high fat diet supplemented with 80 μg/(g·day)iglycin. On the 0, 28th, 56th, 84th, 112th, 140thday, fasting blood glucose was measured by the One Touch®Ultra®Blood Glucose Test System Kit (Lifespan Company, USA). In addition, on the 140thday,4 mice in per group underwent oral glucose tolerance test (OGTT).After an overnight fast, they were orally fed glucose at 2 g/kg bw.Blood sample was collected from caudal vein at the 0th, 30thand 60thand 120thmin for blood glucose test. Meanwhile, insulin tolerance tests (ITT) were taken, the remaining 4 mice in each group were fasted for 6 h and insulin was intraperitoneally injected at 0.5 U/kg bw. The concentration of blood glucose was measured at the 0th, 30thand 60thmin.
2.3 Culture of adipocytes
Murine 3T3L1 pre-adipocytes, obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), were fully differentiated into adipocytes as we described previously [26]. In brief, 2 days after the cells reached confluence (day 0), the cells were induced to differentiate by Dulbecco’s modified Eagle’s medium (DMEM, Gibco, NY, USA) supplemented with 10% fetal bovine serum, 0.5 mmol/L 3-isobutyl-1-methyl-xanthine, 0.25 μmol/L dexamethasone and 10 μg/mL insulin (Sigma, St. Louis, MO,USA) for another 2 days. Then, the cells were cultured in DMEM containing 10% fetal bovine serum and 10 μg/mL insulin for 2 days and replenished with DMEM containing 10% fetal bovine serum until full differentiation into adipocytes.
2.4 Glucose consumption
Insulin resistance model of cultured 3T3-L1 adipocytes was induced according to the references [27]. In detail, 20 nmol/L dexamethasone was incubated with 3T3L1 pre-adipocytes from day 8 to day 14 of differentiation. The media was refreshed every other day. Insulin resistance was verified by the following insulin-stimulated glucose consumption. These 3T3-L1 adipocytes were exposed to 0, 50, 100 or 200 nmol/L iglycin and 100 nmol/L insulin for 48 h in DMEM with 10% fetal bovine serum. The concentration of glucose in the culture medium was analyzed with the glucose oxidase method.
2.5 Western blot
Western blot was employed to measure AKT, insulin receptor substrate 1 (IRS1) phosphorylation and insulin-responsive glucose transporter 4 (GLUT4) translocation in differentiated 3T3L1 adipocytes and/or epididymal fat depots. The proteins were prepared with lysis buffer containing protease and phosphatase inhibitors (Sigma, St. Louis, MO, USA). Protein concentrations were determined using a Pierce™ BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). These proteins were applied to SDS-PAGE, and transferred to nitrocellulose membranes (Whatman,London, UK). Then the membranes were blocked with 20 mmol/L Tris-HCl, pH 7.6, 0.14 mol/L NaCl, 0.1% Tween 20 containing 5% bovine serum albumin for 1 h at room temperature. After that, the proteins on the membranes were incubated overnight at 4 °C with the specific primary antibodies against GLUT4 (1:1 000,abcam, Cambridge, MA, USA), AKT (1:1 000, abcam, Cambridge,MA, USA), p-AKT (phospho S473, 1:1 000, abcam, Cambridge,MA, USA), IRS1 (1:1 000, abcam, Cambridge, MA, USA),p-IRS1 (phospho S307, 1:1 000, abcam, Cambridge, MA, USA),GAPDH (1:1 000, Proteintech, Wuhan, China). Membranes were washed thoroughly before the corresponding horseradish peroxidase conjugated secondary antibodies were added. Thereafter, specific immune complexes were detected by chemiluminescence (West Pico kit, Pierce, Loughborough, UK) and quantified using ChemiDoc™XRS+(Bio-Rad, Hercules, CA, USA).
2.6 Assay of cell apoptosis
After 3T3L1 adipocytes were treated with iglycin for 48 h. Cell apoptosis was analyzed using an annexin V-FITC/PI apoptosis assay kit (Beyotime, Nanjing, China) according to the manufacturer’s protocol. The cell percentage of different apoptotic stages was determined by flow cytometer (Beckman Coulter FC500, Brea, CA).
2.7 Reactive oxygen species and lipid peroxidation
The adipocytes were harvested by centrifuging at 500 g for 5 min and lysed after iglycin treatment. The reactive oxygen species production was measured using fluorometric intracellular reactive oxygen species kit (Sigma-Aldrich, St. Louis, MO, USA) and assayed by a flow cytometer (Beckman Coulter FC500, Brea, CA).The lipid peroxidation was assessed by examining malondialdehyde as an end product of lipid peroxidation with malondialdehyde assay kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions.
2.8 Mitochondrial succinate dehydrogenase activity
Activity of mitochondrial succinate dehydrogenase activity was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Specifically, adipocytes were treated with iglycin as above in 96-well plate, then 0.5 mg/mL of MTT (Sigma-Aldrich,St. Louis, MO) dissolved in 20 μL PBS was added. Plate was incubated at 37 °C for 4 h and centrifuged at 2 500 × g for 10 min,150 μL isopropanol was used to solubilize the resulting formazan crystals. The optical density was read on a microplate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 490 nm.Succinate dehydrogenase activity was normalized to the control group, which was considered to be 100%.
2.9 Assessment of mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential was estimated using fluorescent probe JC-1 (Beyotime, Nanjing, China) as described previously [28]. Briefly, adipocytes were incubated with 5 μg/mL JC-1 for 20 min at 37 °C. The plate was centrifuged at 400 × g for 5 min at room temperature and the supernatant was discarded, then washed twice with PBS and suspended in 0.5 mL PBS for flow cytometry analysis (Beckman Coulter FC500, Brea, CA). JC-1 monomer signal was excited at 488 nm and emitted at 525 nm (green).JC-1 aggregates were formed with excitation and emission at 560 and 590 nm (red). The ratio of red/green fluorescent intensity was calculated for the level of mitochondrial membrane potential.
2.10 Intracellular ATP content
Intracellular ATP concentrations were assayed with an ATP assay kit (Beyotime, Nanjing, China). The cells were lysed in 200 μL ATP assay buffer followed by deproteinization of cell lysates. Then, using ATP as standard, intracellular ATP contents were determined by chemiluminescence (West Pico kit, Pierce, Loughborough, UK) and normalized to protein concentrations.
2.11 Statistical analysis
Data were presented as means ± standard error of the mean (SEM). Statistical analyses were performed using SPSS Statistics V17.0 software and comparisons between iglycin treated group and 0 nmol/L iglycin treated group under the same condition were analyzed by t-test, while differences among iglycin + HFD administrated groups and HFD group of the mice were analyzed by two-way ANOVA and comparisons between different dose of iglycin treated groups were made by one-way ANOVA, P < 0.05 was considered as statistical significance.
3. Results and discussion
3.1 Peptide purification
The peptide was purified by RP-HPLC as presented in Fig. 1.The three peaks recovered from RP-HPLC were collected and applied to MALDI-TOF-MS. The molecular mass of the labeled fragment was 3.88 kDa, and the sequence of amino acid was ISCNG VCSPF DIPPC GTPLC RCIPA GLFVG KCRHP YG, which was similar to vglycin [23].Thus, the peptide was named as iglycin. Given the antidiabetic property of vglycin, C57BL/6J mice were orally administrated with iglycin and insulin sensitivity was tested.
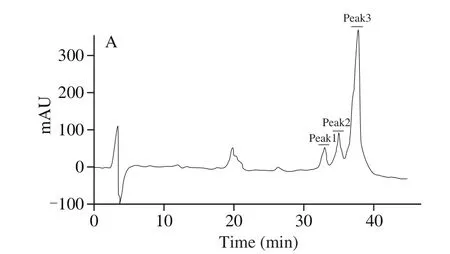
Fig. 1 RP-HPLC for iglycin purification. Peak 1, peak 2 and peak 3 were selected to molecular mass, then peak 1 was applied to amino acid sequence identification.
3.2 Insulin sensitivity and glucose homeostasis of C57BL/6J mice
Animal experimental strategy was presented in Fig. 2A. High fat diet significantly increased fasting blood glucose of C57BL/6J mice on the 28th, 56th, 84th, 112th, and 140thday as shown in Fig. 2B. While long-term oral administration of iglycin inhibited the increase of fasting blood glucose caused by high fat diet at a dose of 40 and 80 μg/(g·day)on the 112thand 140thday. The levels of fasting blood glucose of the mice in iglycin treatment groups were close to that in control group on the 112thand 140thday. Furthermore, on the 140thday,iglycin also decreased the contents of fasting blood glucose of the mice compared with that in high fat diet group at 30 and 60 min with 40 and 80 μg/(g·day) iglycin treatment (Figs. 2C, D).Moreover, it also reduced blood levels of the mice in insulin tolerance test compared with that in high fat diet group at 30 min at the concentration of 40 and 80 μg/(g·day) (Figs. 2E, F). There were no noticeable differences between the levels of blood glucose in iglycin treatment groups and the chow group in glucose tolerance test and insulin tolerance test. Thus, iglycin improved insulin sensitivity and glucose homeostasis in C57BL/6J mice.

Fig. 2 Fasting blood glucose, insulin tolerance and oral glucose tolerance of C57BL/6J mice on a high-fat diet. (A) Animal experimental, (B) fasting blood glucose,(C, D) OGTT, and (E, F) ITT. Values are presented as percentages of the initial blood glucose level. **P < 0.01 iglycin treated group compared with HFD group.
3.3 Insulin signaling in adipose tissue
Concomitant with the increase in obesity prevalence in recent decades, there has been an increase in prevalence of type 2 diabetes mellitus. Specifically, Brandon et al. [29] suggested fat tissue as a source of insulin resistance and type 2 diabetes. In our study, iglycin improved insulin sensitivity of high-fat diet fed C57BL/6J mice.Therefore, the insulin signaling in adipose tissue was investigated by Western blot in Fig. 3A. Then, the relative average optical density of the blots of phosphorylation of IRS1 (Fig. 3B) and AKT (Fig. 3C)from 3 independent Western blot experiments was presented,respectively. As we expected, iglycin enhanced phosphorylation of IRS1 (Fig. 3B) and AKT (Fig. 3C) in a dose dependent manner compared with the high fat diet administration. Insulin stimulates glucose uptake by producing IRS1 phosphorylation and alteration in AKT function [30]. AKT is a major hub in the insulin signaling network, AKT phosphorylates numerous intracellular substrates,which orchestrates the translocation of GLUT4 to the plasma membrane leading to glucose uptake [31]. Therefore, improved insulin sensitivity by iglycin may be the result of insulin signaling reconstruction.

Fig. 3 Phosphorylation of IRS1 and AKT in epididymal fat depots. (A) Western blot. On the 140th day, phosphorylation of IRS1 (S307, B) and AKT (S473, C).*P < 0.05, **P < 0.01, ***P < 0.001 iglycin treated group compared with HFD group.
3.4 Glucose uptake
It is well known that insulin decreases the levels of blood glucose via promoting glucose uptake into adipocytes and muscle cells. To investigate the underlying mechanism of iglycin improving insulin sensitivity and glucose homeostasis, the effect of iglycin on insulin-stimulated glucose uptake into adipocyte under insulin resistance condition was explored. As shown in Fig. 4, there was less glucose remained in medium of normal adipocytes compared with that remained in medium of insulin-resistant adipocytes induced by dexamethasone. While iglycin improved insulin-stimulated glucose uptake in adipocytes under insulin resistance condition, which may explain that iglycin normalized fasting blood glucose levels in C57BL/6J mice.
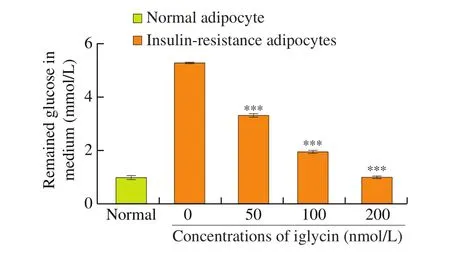
Fig. 4 Glucose consumption in 3T3L1 adipocytes. 3T3L1 adipocytes were exposed to 0, 50, 100 or 200 nmol/L iglycin in DMEM containing 10% fetal bovine serum with 100 nmol/L insulin stimulation. The concentration of glucose in the culture medium was analyzed with the glucose oxidase method. Data were the mean ± SEM of 3 independent experiments. ***P < 0.001 iglycin treated group compared with 0 nmol/L iglycin treated group under the same condition.
3.5 GLUT4 translocation and insulin signaling activation in adipocytes
Under basal condition, about 95% of GLUT4 protein is localized in the cell, while the remaining 5% protein is at the cell surface [32].By contrast, insulin provokes the translocation of GLUT4 to the plasma membrane, which leads to glucose uptake. It is worthy to note that impaired GLUT4 translocation to the plasma membrane has been considered as one of the earliest defects leading to insulin resistance and type 2 diabetes [33]. In the present study, translocation of GLUT4 to the plasma membrane and phosphorylation of IRS1 and AKT in adipocytes were investigated by Western blot in Fig. 5A. Iglycin up-regulated the level of GLUT4 on the plasma membrane (as shown in Fig. 5B) in a dose dependent manner, which enhanced glucose uptake into adipocyte. Furthermore, in accordance with the observation in adipose tissue, iglycin also induced insulin-stimulated phosphorylation of IRS1 (Fig. 5C) and AKT (Fig. 5D). Improved GLUT4 translocation and insulin signaling activation by iglycin may contribute to reconstruction of insulin-stimulated glucose uptake in adipocyte.

Fig. 5 The in fluence of iglycin on IRS1, AKT phosphorylation and insulin-responsive GLUT4 translocation to plasma membrane. Insulin-resistant 3T3L1 adipocytes were incubated with 0, 50, 100 or 200 nmol/L iglycin in DMEM containing 10% fetal bovine serum for 48 h. (A) Western blot. The levels of membrane GLUT4 (B) and phosphorylated IRS1(C), Akt (D). Data were the mean ± SEM of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, iglycin treated group compared with 0 nmol/L iglycin treated group.

Fig. 6 Oxidative stress in 3T3L1 adipocytes. Insulin-resistant 3T3L1 adipocytes were treated with 0, 50, 100 or 200 nmol/L iglycin in DMEM containing 10% fetal bovine serum for 48 h. (A) The reactive oxygen species production was measured with cellular reactive oxygen species detection assay kit. (B) Lipid peroxidation was assessed with lipid peroxidation assay kit. Data were the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001,iglycin treated group compared with 0 nmol/L iglycin treated group.
3.6 Oxidative stress
Oxidative stress is defined as an imbalance between the production of reactive oxygen species and antioxidant defenses.Recent evidence indicates a central role for oxidative stress in obesity, insulin resistance, diabetes and its complications [34]. In this study, iglycin suppressed reactive oxygen species production at the concentration of 50, 100 and 200 nmol/L (Fig. 6A). Thereafter,it reduced lipid peroxidation in a concentration dependent manner (Fig. 6B), which may lead to ameliorated oxidative stress and consequent restored insulin sensitivity in adipocytes.
3.7 Adipocyte apoptosis
Oxidative stress is a main cause of cell apoptosis. Previous report proposed adipocyte apoptosis were markedly increased in adipose tissue from both mice with diet-induced obesity and obese humans.Furthermore, inhibition of adipocyte apoptosis protected against the development of systemic insulin resistance [35]. In our study,compared with the insulin resistance group, iglycin reduced the percentages of apoptotic cells at the concentration of 50, 100, and 200 nmol/L measured by flow cytometry (as shown in Figs. 7A-D),and the average ratio of apoptotic cells in three independent experiments was calculated in Fig. 7E. Therefore, iglycin significantly inhibited adipocyte apoptosis, especially early apoptosis (the cell percentage in lower and right area).
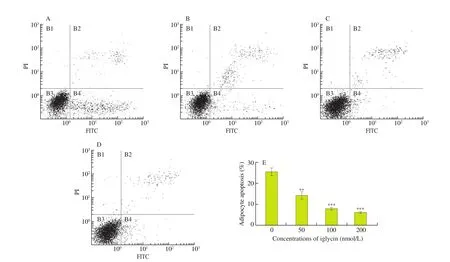
Fig. 7 Apoptosis of 3T3L1 adipocytes. Insulin-resistant 3T3L1 adipocytes were treated with (A) 0, (B) 50, (C) 100 or (D) 200 nmol/L iglycin in DMEM containing 10% fetal bovine serum for 48 h. (E) Cell apoptosis was analyzed using an annexin V-FITC/PI apoptosis assay kit. The cell percentage of different apoptotic stages was determined by flow cytometer. Data were the mean ± SEM of three independent experiments. **P < 0.01, ***P < 0.001, iglycin treated group compared with 0 nmol/L iglycin treated group.

Fig. 8 Mitochondrial succinate dehydrogenase activity, mitochondrial membrane potential and intracellular ATP content assessment in 3T3L1 adipocytes.Insulin-resistant 3T3L1 adipocytes were treated with 0, 50, 100 or 200 nmol/L iglycin in DMEM containing 10% fetal bovine serum for 48 h. (A) Activity of mitochondrial succinate dehydrogenase activity measured using MTT assay. (B) Mitochondrial membrane potential was estimated using fluorescent probe JC-1.(C) Intracellular ATP concentrations assayed with an ATP assay kit. Data were the mean ± SEM of three independent experiments. ***P < 0.001, iglycin treated group compared with 0 nmol/L iglycin treated group.
3.8 Mitochondrial function
In the previous description, adipocyte apoptosis was associated with insulin resistance via activation of both the extrinsic, death receptor-mediated, and intrinsic, mitochondrial-mediated pathways of apoptosis [35]. In addition, reactive oxygen species were mainly generated inside mitochondria, mitochondrial dysfunction contributes to ROS production and cell apoptosis [36]. Moreover, mitochondrial dysfunction also induced insulin resistance [37]. It was not surprising that iglycin increased succinate dehydrogenase activity (Fig. 8A). At the same time, it enhanced mitochondrial membrane potential (Fig. 8B),which led to up-regulated intracellular ATP content (Fig. 8C).There data indicated iglycin improved mitochondrial function via rebuilding mitochondrial membrane potential. We previously identified voltage-dependent anion-selective channel protein 1 (VDAC-1) on the outer mitochondrial membrane as an interacting protein of aglycin, the analog of iglycin [25]. VDAC-1 is a key structural component of the mitochondrial permeability transition pore [38]. The mitochondrial permeability transition pore opening directly impairs mitochondrial cellular integrity, mitochondrial membrane potential and ATP stores [39]. Here, iglycin inhibited cell apoptosis and improved mitochondrial function, which might be associated with VDAC-1.
4. Conclusion
In conclusion, a novel soybean peptide iglycin was isolated and it normalized the levels of fasting blood glucose, improved insulin tolerance and oral glucose tolerance in high fat diet fed C57BL/6J mice with activated insulin signaling in adipose tissue. Furthermore,it reversed insulin resistance in 3T3L1 adipocytes via improvement of insulin signaling and ameliorated oxidative stress, adipocyte apoptosis and mitochondrial dysfunction.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China (31400794), Sichuan Province Science Fund s for Distinguished Young Scholar (2019JDJQ0017), Young and middle-aged Talents Project of the National People’s Commission(2799300127) and Fundamental Research Funds for the Central Universities, Southwest Minzu University (2019XMJXPY08).
杂志排行
食品科学与人类健康(英文)的其它文章
- Production of antihypertensive and antidiabetic peptide fractions from quinoa (Chenopodium quinoa Willd.) by electrodialysis with ultrafiltration membranes
- Identification and characterization of a novel tetrapeptide from enzymatic hydrolysates of Baijiu byproduct
- Effects of phosvitin phosphopeptide-Ca complex prepared by efficient enzymatic hydrolysis on calcium absorption and bone deposition of mice
- Structural requirements and interaction mechanisms of ACE inhibitory peptides: molecular simulation and thermodynamics studies on LAPYK and its modified peptides
- Anti-diabetic and anti-hyperlipidemic effects of sea cucumber(Cucumaria frondosa) gonad hydrolysates in type II diabetic rats
- Antibacterial and antibiofilm activity of peptide PvGBP2 against pathogenic bacteria that contaminate Auricularia auricular culture bags
