Pancreatic involvement in celiac disease
2022-07-08DanielVasileBalabanIuliaEnacheMarinaCiochinaAlinaPoppMarianaJinga
Daniel Vasile Balaban, Iulia Enache,Marina Ciochina,Alina Popp,Mariana Jinga
Abstract Celiac disease (CD) is well recognized as a systemic, chronic autoimmune disease mainly characterized by gluten-sensitive enteropathy in genetically predisposed individuals but with various extraintestinal features. One of the affected organs in CD is the pancreas, consisting of both endocrine and exocrine alterations. Over the last decades there has been increasing interest in the pancreatic changes in CD,and this has been reflected by a great number of publications looking at this extraintestinal involvement during the course of CD. While pancreatic endocrine changes in CD, focusing on type 1 diabetes mellitus, are well documented in the literature, the relationship with the exocrine pancreas has been less studied. This review summarizes currently available evidence with regard to pancreatic exocrine alterations in CD, focusing on risk of pancreatitis in CD patients,association with autoimmune pancreatitis, prevalence and outcomes of pancreatic exocrine insufficiency in newly diagnosed and gluten-free diet treated CD patients, and the link with cystic fibrosis. In addition, we discuss mechanisms behind the associated pancreatic exocrine impairment in CD and highlight the recommendations for clinical practice.
Key Words: Pancreas; Celiac disease; Autoimmune; Pancreatitis; Cystic fibrosis; Exocrine insufficiency
INTRODUCTION
Celiac disease (CD) is an immune-mediated enteropathy that occurs in genetically predisposed individuals upon ingestion of gluten. Initially considered a small bowel disease, now it is widely recognized as a systemic illness, which accounts for its many protean manifestations. The systemic character along with its various clinical presentations make CD a sometimes difficult to recognize clinical chameleon. The wide spectrum of presenting features, sometimes subclinical, hinders casefinding strategies and delays diagnosis, as CD is often not considered among the differential diagnoses[1,2]. Moreover, there is also the further drawback of poor awareness among different medical specialties[3].
Among the extraintestinal features of CD[4-6], pancreatic involvement has rarely been reported compared to other organ-specific manifestations such as cutaneous, hematologic, liver-related, rheumatologic, cardiovascular, or neurological impairments[7-13]. Moreover, while some of these systemic features have been incorporated into case-finding and screening strategies such as anemia, osteoporosis or chronic liver disease[14], pancreatic-associated involvement in CD is scarcely reported in currently available guidelines[15-17]. The association between the pancreas and CD covered in guidelines is limited to the need to consider pancreatic exocrine insufficiency (PEI) as an alternative diagnosis in nonresponsive CD and the fact that upper gastrointestinal surgery including pancreas-related indications may unmask subclinical CD[15,16]. This has also been highlighted by previous reports on the impact of CD on exocrine and endocrine pancreas, which also set the need for further research focused on this association[18]. Contrasting the well-documented pancreatic endocrine changes in CD, referring to type 1 diabetes mellitus in particular, the relationship with the exocrine pancreas has been less covered in the literature.
On the other hand, the wide spectrum of pancreatic diseases does not include CD as a risk factor or associated condition, except counting CD as a potential cause of PEI[19]. Pancreatic involvement can occur in patients with CD, either caused by the small-bowel disease or co-existing with it. The main mechanisms for this association are believed to be the impaired cholecystokinin (CCK) and secretin release, but also the chronic duodenal inflammation that can lead to secondary modifications of papillary mucosal area[20].
Our aim was to summarize currently available evidence with regard to exocrine pancreatic involvement in CD, looking at CD as a risk factor or associated condition with pathologies of the exocrine pancreas, and testing indications regarding the bidirectional association of the two diseases.
SEARCH STRATEGY
For this purpose, we searched PubMed in January 2022 for all publications on the association between pancreas and CD, using the medical subject headings (MeSH) terms–“pancreas” (ID: D010179) and“celiac disease” (ID: D002446), with the following search syntax (("pancreas" [Mesh]) OR (pancreas[Title/Abstract])) AND (("celiac disease" [Mesh]) OR (celiac disease [Title/Abstract])). We performed additional searches with pancreas-related terms, “Pancreas, exocrine” (ID: D046790), “pancreatitis” (ID:D010195), “pancreatitis, chronic” (ID: D050500), “autoimmune pancreatitis” (ID: D000081012) and“celiac disease” (ID: D002446). The extended search yielded a total of 889 results, of which 145 were duplicates. Search results were imported into Reference Citation Analysis (Βaishideng Publishing Group, Inc.), which was used for article processing and selection. We filtered the search for reviews,editorials, comments and opinion articles (n= 140). We excluded papers referring to the association of CD with diabetes or alterations of the endocrine pancreas (n= 66). The remaining titles and consecutive abstracts were screened for pertinence to the topic. We selected relevant articles on exocrine pancreatic involvement in CD for full-text analysis and summarized findings according to significant associations.References and citing articles of selected papers were also analyzed for potentially relevant articles that might have been missed in the initial search. The process of article selection is detailed in Figure 1.
CD AND RISK OF PANCREATITIS
CD patients are at risk both for acute pancreatitis (AP) and chronic pancreatitis (CP)[21-23]. While some have described worse outcomes and increased medical burden among CD individuals[21], others have found lower morbidity and mortality among this patient group[24] (Table 1).
Several pathogenic mechanisms have been theorized to account for the elevated pancreatitis risk in CD–malnutrition, papillary stenosis, and immune phenomena[25] (Figure 2). Severe malnutrition affects pancreatic secretion and can cause pancreatic atrophy[26]. Also, chronic inflammation of the duodenal mucosa in CD patients can also involve the papillary area and lead to papillary stenosis and consequent pancreatic disease[20]. Finally, autoimmune pancreatitis (AIP) or other autoimmune phenomena such as islet-specific autoantibodies in CD-associated type 1 diabetes mellitus can contribute to the link between pancreatitis and CD[27,28].
With regard to CP, results are also discordant using similar diagnostic criteria; while some authors have reported CP features to be common in CD patients undergoing endoscopic ultrasound (EUS)assessment[29], others did not reveal significant structural alterations in the pancreatic parenchyma of CD individuals[30]. In their study using EUS criteria and elastography, Ranaet al[30] concluded that PEI is functional and reversible after gluten-free diet (GFD). Supporting this finding, the pathognomonic pancreatic calcifications have been rarely reported in CD[31-35]. However, a biopsy-based study published as abstract has shown CD prevalence as high as 7.4% in established CP, recommending screening in this group[36].
Concerning CD screening in pancreatic diseases, there have been reports of asymptomatic hyperenzymemia, macrolipasemia, or macroamylasemia[37-42] in CD patients, but prevalence studies are missing. According to these reports, decrease or even resolution of macroamylasemia/macrolipasemia or elevated pancreatic enzyme levels can be seen on GFD. Of note, the occurrence of hyperenzymemia in CD can be a confounder for the reported elevated risk of AP associated with CD, by overdiagnosing AP in this scenario.
On the other hand, idiopathic recurrent pancreatitis and sphincter of Oddi dysfunction might be considered a testing indication for CD, given the mechanism of chronic papillitis[20,43]. Non-response to treatment in CP might warrant testing for CD, as suggested in some case reports[31].
EXOCRINE PANCREATIC INSUFFICIENCY AND CD
CD is a well-recognized less common etiology of PEI[19,44,45]. This is well reported in currently available guidelines[46]. EPI has been reported with variable frequency in CD patients, depending on the test used to diagnose it. Early studies were based on direct pancreatic function testing (pancreatic enzyme or bicarbonate secretions measurements) and found that PEI is common in classical CD, but non-severe[47]. Fecal elastase (FE) is recommended for detecting PEI in newly diagnosed CD[48].Impairment of pancreatic exocrine function can be seen both in newly diagnosed CD, in up to 80% of cases[19], and in treated CD, where it should be considered a cause of treatment failure in patients unresponsive to GFD[49,50]. In this latter group comprising GFD-treated CD patients with continuing diarrhea, EPI has been reported in 12%-18% of cases[51,52]. While CD patients improve with pancreatic enzyme replacement therapy (PERT), probably paralleling the restoring of mucosal architecture on GFD, some authors have reported that PERT could be discontinued in some patients who experience improvement in symptoms[53]. However, in CD patients with PEI, who report good adherence to GFD but experience continued malabsorption with adequate dosing PERT, additional pathogenic mechanisms such as enteric infections (e.g.,Giardia), small intestinal bacterial overgrowth, or complications such as refractory CD and enteropathy associated T-cell lymphoma should be sought[54].Moreover, gastroparesis in the setting of type 1 diabetes mellitus associated with CD could contribute to incomplete response to PERT. PEI should be readily recognized in slowly recovering children with CD on GFD, as it might accelerate weight increase with adequate enzyme supplementation[55].
Correlation of certain genetic polymorphisms with PEI has also been studied, but without conclusive associations in a small cohort of CD patients[56].
A concern with using FE is represented by the lower diagnostic accuracy compared to direct pancreatic function testing, variability among different tests and analytical processing of samples,taking into account the potential dilution due to diarrhea[57-59].
Despite the increased risk of pancreatitis in CD discussed above, changes in pancreatic enzyme secretion in these patients seen to be mainly functional, as reported by Ranaet al[30], who found no correlation with structural parenchymal alterations, assessed by EUS. Another study, based on magnetic resonance imaging assessment, did not reveal morphological changes in CD patients with PEI[60]. This impaired function of the exocrine pancreas is also supported by the inverse correlation between mucosal damage and pancreatic enzyme levels, with reversal of PEI on GFD[19,61]. However, proteinmalnutrition, also present in CD patients, has been shown to be associated with acinar cell atrophy and fibrosis[18].

Table 1 Summary of studies looking at acute pancreatitis and chronic pancreatitis prevalence among individuals with celiac disease

Figure 1 Flow diagram of the article selection process.

Figure 2 Mechanism behind the increased pancreatitis risk in celiac disease patients.
The main mechanism of PEI in CD patients is disruption of mucosal release of enteric hormones,mainly CCK and secretin, which represent a potent stimulus for pancreatic function. While exocrine pancreatic function is intrinsically normal, impaired CCK and secretin release from the atrophic mucosa leads to decreased secretion of enzymes into the duodenal lumen. The functional PEI that occurs in CD is reversible upon exogenous administration of CCK-pancreozymin[62]. Others have suggested that PEI can develop independent of this reduced entero-hormone release[63]. Another theorized mechanism is deficiency of amino acids, which leads to reduced protein synthesis for pancreatic enzymes. This mechanism is also backed up by the fact that correction of deficiencies alleviates PEI in CD patients[64].Another hypothesis, but probably of less significance, is that of structural changes in the pancreatic parenchyma (acinar atrophy, fibrosis) seen in advanced malnutrition[65]. Mechanisms behind EPI in CD are summarized in Table 2[18,47,50,66].
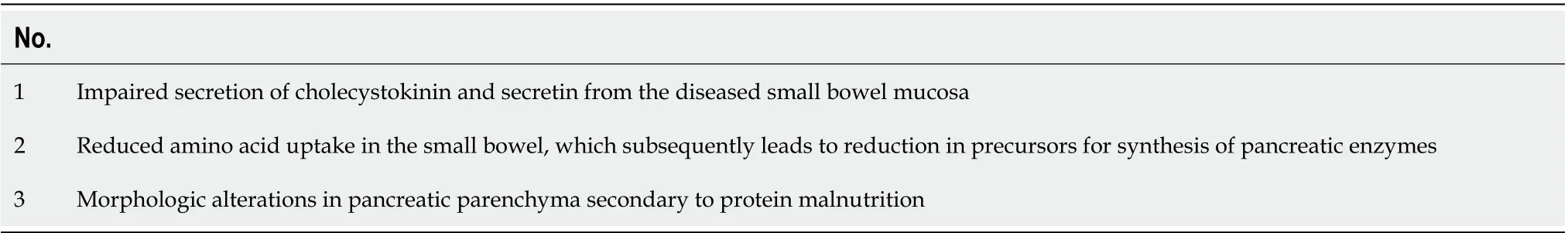
Table 2 Mechanisms of pancreatic exocrine insufficiency in celiac disease
CYSTIC FIBROSIS AND CD
Another underrecognized, pancreas-related association for CD is cystic fibrosis (CF), an autosomal recessive disease characterized by mutations in the CF transmembrane conductance regulator gene,which encodes a chloride and bicarbonate channel expressed in epithelial cells in multiple organs[67].While the pancreatic dysfunction in CF is well known, altered immune regulation has been described in these patients, which predisposes them to developing autoimmune phenomena. The systematic review and meta-analysis by Imreiet al[68] showed pooled prevalence of 1.8%-2.3% of biopsy-proven CD among CF patients, which is more than twice that of the general population[69]. Βased on this observation, the authors recommend routine screening for CD in CF patients. This recommendation is supported by another group[70], who suggested performing CD screening in CF with persistent gastrointestinal symptoms or malabsorption (including improper weight and/or height gain in pediatric patients), despite adequate enzyme replacement therapy.
AIP AND CD
AIP is a chronic fibroinflammatory disease of the pancreas, with a relapsing steroid-responsive pattern.Given the common immune dysregulation background of both AIP and CD, researchers have looked whether there is an association of the two. While some isolated reports have shown a potential link between AIP and CD[71,72], a study looking at CD frequency in a cohort of AIP patients did not show an increased CD prevalence among this group (1.4%) and concluded that serologic screening for CD is not supported[73]. However, a murine model of AIP has provided insight that gliadin sensitization and challenge can induce pancreatitis and extrapancreatic inflammation in HLA-DQ8 transgenic mice[74].On the other hand, immunoglobulin G4-positive cells, which have been searched for in duodenal/ampullary biopsies as an alternative to pancreatic biopsy for diagnosing AIP, have also been reported in 7 of 18 CD patients in the study by Cebeet al[75]. Given the scarce data on AIP and CD,further research is warranted to conclude if there is a link between the two beyond the plausible biologic mechanism. A recognized association is that of type 2 AIP and ulcerative colitis, and considering the also established connection between CD and inflammatory bowel diseases[76], linking the three diseases might provide some insight on the relationship between AIP and CD.
CONCLUSION
Pancreatic involvement, both endocrine and exocrine, is frequent in CD and should be searched for in the appropriate clinical setting. Conversely, certain pancreatic-related diseases should prompt looking for CD–CF with ongoing digestive symptoms, non-responsive CP, idiopathic recurrent pancreatitis.Concomitant pancreatic disease in a CD patient may contribute to aggravated malnutrition and should be readily recognized in order to improve nutritional status and prognosis. Also, there is increasing data that impaired pancreatic function in CD can improve on a GFD.
FOOTNOTES
Author contributions: Βalaban DV proposed the research idea; Βalaban DV, Enache I, and Ciochina M performed the literature search and article selection, and drafted the initial version of the manuscript; Popp A and Jinga M critically reviewed the manuscript and supervised the project; All authors contributed to drawing the figures and tables, and have read and approved the final version of the manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC ΒYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Romania
ORCID number:Daniel Vasile Balaban 0000-0003-3436-8041; Iulia Enache 0000-0001-6905-5008; Marina Ciochina 0000-0002-1573-6358; Alina Popp 0000-0002-7809-5762; Mariana Jinga 0000-0001-5826-0815.
Corresponding Author's Membership in Professional Societies:European Society for Gastrointestinal Endoscopy, No.45910264; Association for Pancreatic Pathology Romania; European Society for the Study of Coeliac Disease (ESsCD);Romanian Society of Gastroenterology and Hepatology; Romanian Society of Digestive Endoscopy; European Pancreatic Club;World Endoscopy Organization
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
杂志排行
World Journal of Gastroenterology的其它文章
- Patient-derived organoids for therapy personalization in inflammatory bowel diseases
- Drug-induced autoimmune hepatitis: A minireview
- Rebuilding trust in proton pump inhibitor therapy
- Downregulation of TNFR2 decreases survival gene expression, promotes apoptosis and affects the cell cycle of gastric cancer cells
- Novel multiplex stool-based assay for the detection of early-stage colon cancer in a Chinese population
- Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer
