Patient-derived organoids for therapy personalization in inflammatory bowel diseases
2022-07-08MariannaLucafoAntonellaMuzzoMartinaMarcuzziLorenzoGiorioGiulianaDecortiGabrieleStocco
Marianna Lucafo,Antonella Muzzo, Martina Marcuzzi, Lorenzo Giorio, Giuliana Decorti, Gabriele Stocco
Abstract Inflammatory bowel diseases (IΒDs) are chronic inflammatory disorders of the intestinal tract that have emerged as a growing problem in industrialized countries. Knowledge of IΒD pathogenesis is still incomplete, and the most widely-accepted interpretation considers genetic factors, environmental stimuli,uncontrolled immune responses and altered intestinal microbiota composition as determinants of IΒD, leading to dysfunction of the intestinal epithelial functions.In vitro models commonly used to study the intestinal barrier do not fully reflect the proper intestinal architecture. An important innovation is represented by organoids, 3D in vitro cell structures derived from stem cells that can self-organize into functional organ-specific structures. Organoids may be generated from induced pluripotent stem cells or adult intestinal stem cells of IΒD patients and therefore retain their genetic and transcriptomic profile. These models are powerful pharmacological tools to better understand IΒD pathogenesis, to study the mechanisms of action on the epithelial barrier of drugs already used in the treatment of IΒD, and to evaluate novel target-directed molecules which could improve therapeutic strategies. The aim of this review is to illustrate the potential use of organoids for therapy personalization by focusing on the most significant advances in IΒD research achieved through the use of adult stem cells-derived intestinal organoids.
Key Words: Inflammatory bowel disease; Organoids; Intestinal epithelium; 3D cell cultures; Personalized medicine
INTRODUCTION
Inflammatory bowel diseases (IΒDs) are chronic inflammatory disorders of the gastrointestinal tract that have emerged as a growing problem in industrialized countries[1]. IΒDs include Crohn’s disease (CD)and ulcerative colitis (UC)[2], which can be distinguished by differences in genetic predisposition,clinical aspects, and risk factors[3], and by the location and nature of inflammation[4]. UC is restricted to the mucosal surface of the large intestine: It starts in the rectum and extends through the entire colon in a continuous manner[3]. The severity of symptoms depends on the intestinal areas involved in inflammation[4]: It usually occurs with bloody diarrhea with pus and mucus (often nocturnal and postprandial), fever, weight loss, abdominal pain, and signs of systemic inflammation[5]. CD is characterized by the involvement of both the small and large intestine; it most commonly affects the ileocecal region, the terminal ileum, the perianal area, and the colon, with segmental and transmural inflammation, fissuring ulcerations,non-caseating granulomas, strictures, abscesses, and fistulas[4,6,7]. The clinical presentation of CD depends on the location, extent, and severity of inflammation: Patients usually show postprandial abdominal pain, especially around the periumbilical area, bloody diarrhea,nausea, emesis, dysphagia, early satiety, and weight loss[7].
Although no curative treatment is available for these diseases, current therapeutic options aim at achieving an improvement in symptoms and can be divided in two different groups: The remission induction drugs, which are used to achieve clinical remission, and the maintenance drugs which sustain the clinical remission and prevent disease relapse[8]. Aminosalicylates are administered as first-line agents to treat mild to moderately active UC. They exert their anti-inflammatory activity by binding to the peroxisome proliferator-activated receptor[9]. Glucocorticoids are used for inducing remission;when bound to their receptor, they modulate the expression of inflammatory mediators and control inflammatory states[9,10]. The thiopurines azathioprine and mercaptopurine are efficacious for maintaining remission. Thiopurines are inactive prodrugs but their metabolites (thioguanine nucleotides) are responsible for causing immunosuppression, either by inhibiting thede novopurine synthesis or by incorporating into the DNA or RNA[9]. Infliximab and adalimumab, which bind the pro-inflammatory cytokine tumor necrosis factor (TNF), have been introduced both for inducing remission and for maintenance therapy[11]. Furthermore, other monoclonal antibodies such as vedolizumab, a selective α4β7 integrin inhibitor, and ustekinumab, an interleukin (IL)-12/23 p40 inhibitor, have been used as alternatives for patients who have failed or are intolerant to anti-TNF therapy[12]. Eventually, thalidomide, by selectively inhibiting TNF-α expression and suppressing TNF-α-induced nuclear factor-kappa Β (NF-κΒ) activation, can be employed when patients do not respond to the standard therapies[9,13]. Despite the multiple therapeutic options, high interindividual variability in response to these drugs is evident, and genetic and epigenetic factors could play a key role in these differences[14,15].
IΒDs are multi-etiological diseases: It is currently believed that uncontrolled immune response against intestinal microbiota, combined with environmental factors in genetically predisposed individuals, leads to bowel inflammation, perturbing the mucosal barrier and increasing intestinal epithelial cell (IEC) dysfunction[16,17]. However, even today, it is not completely understood which of these factors are the initiators of inflammation and which are contributors[18]. An altered intestinal architecture and abnormalities in the intestinal wall function have been associated with the onset of the disease[19].
The current models employed to study the intestinal barrierin vitroshow many weaknesses:Stabilized cell lines, such as the human epithelial colorectal adenocarcinoma cells Caco2 or HT-29, do not reproduce the heterogeneous intestinal cell types[20-22] and do not represent IΒD pathophysiology[23]. Animal models are highly costly, require probands to be raised for weeks[5], and may not reflect human pathobiology, which may contribute to drug failure in clinical therapy[21,24]. Consequently,new models are necessary to better define the pathogenesis underlying IΒDs and to find new clinical approaches in its treatment[25].
A major advancement in clinical research of human IECs is represented by the development of 3D organoid cultures, which re-create intestinal epithelial tissuein vitro[25] starting from adult stem cells(ASCs) or pluripotent stem cells (PSCs), such as embryonic stem cells or induced PSCs (iPSCs)[22].These cells, grown under appropriate conditions and stimuli, self-organize and show the specific tissue structures, properties and functionality of the gastrointestinal tissue, forming a spheroid-like structure[26].
INTESTINAL ORGANOIDS
ASCs are located at the base of the intestinal crypts, which can be isolated from intestinal biopsies[27].Intestinal crypts are embedded in a 3D membrane-like gel, such as matrigel, which mimics the extracellular matrix, and supplemented with an appropriate culture medium that is crucial for the development and expansion of ASCs, from which intestinal organoids can be obtained[5,28,29]. The organoids obtainedin vitrowith this method present only IECs (Paneth cells, goblet cells, enterocytes and enteroendocrine cells) derived from the crypt-based stem cells and are oriented with their apical side toward the lumen and their basolateral domain in contact with matrigel[25,28]. On the other hand,Spenceet al[30], have demonstrated that it is possible to successfully generate intestinal organoids starting from iPSCs with a process that mimics organogenesis, and that this type of organoids may present not only epithelial cells but also mesenchymal cells, including myofibroblasts, and smooth muscle cells, thus providing a more accurate model[30,31]. This method is advantageous when it is necessary to generate organoids starting from tissues not easily accessible, reprogramming peripheral blood mononuclear cells or fibroblasts to a pluripotent state, thus obtaining iPSCs that can be differentiated to the tissue of interest by using dedicated protocols[29]. The reprogramming phase is complex and can be achieved using different methods. These methods are chosen according to efficiency,footprint (integration of viral vector sequences into reprogrammed cell genome), and type of somatic cells to be reprogrammed[32]. Subsequently, in order to obtain the different types of cells constituting the organ of interest, a microenvironment that mimics embryogenesis is required[33]. However, iPSCderived intestinal organoids, unlike ASC-derived intestinal organoids, can acquire genetic and epigenetic variations. For example, mutations can occur during the reprogramming process or arise during the prolonged culture which can be conserved if they facilitate cell propagation[30,34,35].
After expansion, intestinal organoids can be employed in any experimental approach developed for cell lines, such as DNA-, RNA-, protein-based techniques, live imaging, drug screening and “omics”approaches (including single-cell RNA sequencing, MethylC sequencing, and mass spectrometry)[22].For example, Yinet al[36] have combined genome-wide analysis of open chromatin by Omni Assay for Transposase-Accessible Chromatin-seq with the transcriptome data from RNA-seq. Βy correlating gene expression with open chromatin peaks, intestine-specific processes were identified. These results confirm the relevance of intestinal organoids to better define the genetic characteristics and, at the same time, to investigate crucial physiological and biochemical processes in the gut epithelium[36].
Organoid cultures are also a powerful model for genetic manipulation tools, such as transposon mutagenesis, siRNAs, and CRISPR-Cas (e.g.,knock-down, knock-out, knock-in, or overexpression)[29].In particular CRISPR-Cas, along with transcription activator-like effector nuclease, and zinc finger nucleases technologies[37] allows the low frequency IΒD-associated mutations to be gradually introduced into organoids derived from healthy patients, providing a better understanding of their effect on the epithelial cell functions[22,29]. In this way, intestinal organoids can be useful to explain in a more specific manner the effect of known genetic variants identified by genome-wide association studies[38] and can become crucial tools for IΒD research, especially for the very early-onset IΒD (VEOIΒD).
In addition, ASC-derived organoids can undergo extensive expansion in culture and maintain genome stability, which makes them suitable for biobanking[39].
In this review, we focus on the most significant advances in IΒD research obtained using ASC-derived intestinal organoids (Figure 1).
Organoid models to study the genetic and epigenetic contribution on IBD epithelium
Several forms of IΒD are polygenic and multiple susceptibility loci have been correlated to IΒD onset[38]. Intestinal organoids retain the genetic and epigenetic signatures of the original tissue and can hence be employed for studying genetic and epigenetic-phenotypic profiles[22]. Moreover, the study of specific variants affecting the different cell types constituting the epithelium of IΒD patients could identify sub-phenotypes of the disease that could help to understand their behavior and outcome[40].

Figure 1 Intestinal organoid in inflammatory bowel disease research: The future of precision medicine. Patient-derived intestinal organoids are three-dimensional in vitro cell structures derived from stem cells that differentiate and self-organize into functional intestinal epithelium-specific cell types. For this reason, this model is suitable for different research approaches useful to inflammatory bowel disease (IBD) modelling and to study current and new therapeutic options. By retaining the disease-specific phenotypic defects, intestinal organoids can be employed to study epigenetic and transcriptomic profiles. In addition, the ability of intestinal organoids to differentiate into all the different cell types present in the intestinal epithelium makes this model useful to discover new cell-specific disease mechanisms. Furthermore, organoids can be used to study cytokine-induced apoptosis and to test currently used drugs to better understand their mechanisms. Co-culturing intestinal organoids with either microbiota components or immune cells helps to investigate IBD pathogenesis. Thanks to all these research approaches new pharmacogenomic biomarkers, new therapeutic targets and new drugs could be discovered, enabling the development of precision medicine for IBD patients. The image was created with https://biorender.com/.
Organoids also maintain the region-specific (duodenum/ileum/jejunum) gene expression of the gastrointestinal tract from which they derive[41]. Moreover, biopsies from IΒD patients and derived intestinal organoids show similar expression levels of several IΒD-marker genes. Dottiet al[42] have identified a set of genes, includingLYZ,CLDN18,andHYAL1,upregulated both in the mucosa of patients with active UC and in the derived organoids[42]. Accordingly, the expression levels of genes encoding junctional proteins, such asZO-1, also known astight junction protein 1,OCLNandCTNNB,were similar in intestinal crypts from CD-patients and derived organoids.
VEO-IΒDs are a subset of pediatric patients with IΒD diagnosed before the age of 6 years, and up to 15% of these patients may have a rare monogenic disorder. Several defects in genes responsible for the proper intestinal epithelial barrier functions have been discovered, includingNOX1[43] andTTC7A[44].To study the impact of these mutations on IECs, Schwerdet al[44] generated human colonic organoids of patients affected by IΒD carrying aNOX1p.N122H mutation[44]. These organoids show significantly less production of constitutive superoxide than organoids derived from healthy patients. This defect could favor the crypt colonization by luminal microorganisms since superoxide has an anti-adhesive or anti-invasive effect on bacteria. On the other hand, biallelic missense mutations in theTTC7Agene have been found in VEO-IΒD patients, resulting in the activation of RhoA-dependent proteins which regulate cytoskeletal architecture by altering cell polarization and proliferation, as demonstrated in organoid cultures[45].
Among the genetic variants associated with the risk of developing IΒD, polymorphisms inNOD-2,involved in the microbial pathogens recognition[46], andATG16L1[47] genes play an important role.However, their biological functions in the intestinal epithelium of IΒD patients remain unclear[47,48].
Organoids generated fromNOD2knockout (KO) mice show increased intracellularEscherichia coli(E.coli) following metabolic stress[48], while the number of Paneth cells, known to have antimicrobial activity in the intestinal epithelium, is not affected.Atg16L1-deficient intestinal organoids derived from mice present a higher activation of STING-dependent type I interferon (IFN) signaling after IL-22 stimulation, leading to transient endoplasmic reticulum (ER) stress[49]. No data on patient-derived organoids with variants already associated with the increased susceptibility to IΒD have been published yet.
Kelsenet al[50] reported transcriptomic differences from RNA sequencing between organoids derived from pediatric IΒD (VEO and older onset) and non-IΒD patients[50]. One of the most upregulated genes in organoids generated from pediatric IΒD patients isLYZ, encoding for the antimicrobial protein lysozyme, confirming previous results by Dottiet al[42] conducted on adult IΒD patient-derived organoids[42]. In addition, an upregulation of the antigen presentation genesHLA-DRB1andHLADRA,necessary for T-cell activation, was demonstrated[50]. Similar results have been obtained by McDonald and Jewell[51] in mucosal epithelial cells by histological and immunohistochemical analysis on colonic biopsy specimens[51]. It is therefore possible that, since these genes are upregulated, the continuous and pathological activation of T cells may lead to the development of IΒD[50]. Moreover, the same authors have found that the most downregulated gene in IΒD-derived organoids wasCD177[50], a glycosylphosphatidylinositol-anchored glycoprotein expressed in neutrophils and colon crypt cells.
Looking at the role of epigenetic modifications in IΒD, a recent study showed changes in DNA methylation profiles in purified IECs from IΒD, compared to non-IΒD pediatric patients, that were strictly correlated to IΒD pathogenesis[40]. To assess whether these alterations are also preserved in 3D models, intestinal organoids have been developed from children newly diagnosed with IΒD and from healthy controls. Differences in DNA methylation and transcription profiles have been found between pediatric patient-derived organoids and controls. This suggests that IΒD-associated intestinal epithelialspecific epigenetic alterations can be preserved in organoid cultures. Moreover, it has been observed that these epigenetic alterations are stable over time and therefore can contribute to chronic relapsing inflammation due to impaired IEC function[40].
These data suggest that intestinal organoids can be a promising tool to assess the molecular and functional effects on the intestinal epithelium of genetic and epigenetic variations related to IΒD.
Intestinal organoids to study the epithelial barrier
The intestinal epithelium, consisting of different cell types (enterocytes, enteroendocrine cells, goblet cells, and Paneth cells), plays a fundamental role in maintaining intestinal homeostasis, acting as a physical and chemical barrier against pathogens through the production of mucus and antimicrobial peptides. A defect of these activities is evident in IΒD patients, but it is still not clear whether these are primary defects or secondary effects of the inflammatory state[52].
In particular, reduced levels of trefoil factor 3 in goblet cells of IΒD patients have been observed,which results in a decreased viscosity of the intestinal mucus layer and, as a consequence, the invasion of several IΒD-inducing microorganisms. In IΒD patients, decreased goblet cells number, diminished mucin 2 (MUC2) production, reduced mucus sulfation, and decreased mucus barrier have been detected[53-55]. Moreover, a decreased expression of constitutive defensins by Paneth cells in IΒD patients has also been observed: A low expression of the constitutive human ß-defensin 1 has been demonstrated in patients with UC, while a low expression of human ß-defensin 2 and human ß-defensin 3 was demonstrated in those affected by CD, allowing bacteria to enter the intestinal mucosa[56].
Intestinal organoids can be manipulated to induce differentiation to definite lineages, allowing the study of cell-type-specific functions/dysfunctions in the context of the intestinal epithelium as described in the study conducted by Treveilet al[57]. Intestinal organoids can be specifically differentiated towards goblet cell or Paneth cell lineages by specific small molecules, with improved representation of these cell types within the entire organoid cell population[57]. In particular, organoids enriched in Paneth cells can be obtained with the addition of DAPT, a small molecule inhibitor of Notch signaling,whereas the differentiation towards the goblet cell lineage can be induced through the addition of DAPT and IWP-2, which inhibits Wnt signaling[58]. To characterize the effect of cell type enrichment and to predict key molecular regulators involved in Paneth cell and goblet cell specific functions,authors have used mRNA, miRNA, and lncRNA profiles[57]. Thanks to this approach, they predicted key factors involved in differentiation or maintenance of Paneth cells and goblet cells, such as Cebpa,Jun, Nr1d1, and Rxra specific to Paneth cells, Gfi1b and Myc specific for goblet cells, and Ets1, Nr3c1 and Vdr shared between them. Interestingly, several predicted regulators are associated with inflammation and IΒD, indicating that their dysregulation can contribute to IΒD pathogenesis[57].
Organoids derived from a mouse model of colitis were established to investigate the relationship between the differentiation state and cytokine secretion in the intestinal epithelium[59]. Undifferentiated mouse-derived intestinal organoids showed expression levels of pro-inflammatory cytokines and other inflammatory mediators higher than those differentiated into secretory or absorptive lineages.This suggests that the differentiation state of the intestinal epithelium, regardless of bacteria and immune cells, promotes the release of immunomodulatory factors and has a crucial role in maintaining intestinal homeostasis[59].
In IΒD, cytokines play an important role in the pathological process. In particular, TNF-α, which is produced by immune and non-immune cells, through the activation of different pathways including NF-kΒ, can stimulate the production of other pro-inflammatory cytokines by macrophages and induce the death of Paneth cells, epithelial cells, and T cells[60]. TNF-α has an accepted role in the continuous immune stimulation in intestinal organoids by triggering several signaling cascades which lead to cell activation, survival, and gene expression[61]. It has also been confirmed that high expression of the TNF-α induced protein 3 gene, which encodes A20, a ubiquitin-editing enzyme and negative feedback regulator of NF-kΒ, promotes TNF-α dependent IEC death[62]. Intestinal organoids from A20 transgenic mice, in which A20 expression is driven by the Villin promoter, were more susceptible to TNFdependent death. Moreover, after TNF-α addition, A20 transgenic organoids showed a strong caspase 3 and 8 expression whereas control organoids were unaffected, indicating that immune cells or gut microbiota are not necessary for TNF-induced apoptosis of A20-expressing cells[62]. The authors demonstrated that this effect depended on receptor-interacting protein kinase 1 with its proapoptotic activity, and A20 was found to associate with the death-inducing signaling ripoptosome complex,potentiating its ability to activate caspase-8[62].
Grabingeret al[63] have found that both TNF-α receptors, TNFR1 and TNFR2, are involved in cell death in intestinal organoid models, although TNFR1 is the predominant mediator[63]. Indeed, the authors showed an almost complete abrogation of TNF-induced cell death in TNFR1-deficient organoids while organoids from TNFR2-deficient mice showed reduced cell death following exposure to TNF[63].
TNF-α also contributes to barrier deterioration by activating the myosin light chain kinase, which alters tight-junctions (TJ), and, therefore, intestinal permeability[63]. TJ dislocation seems to be a crucial event in IΒD pathogenesis as confirmed by Hallet al[65]. These authors have demonstrated that the downregulation of the creatine transporter (SLC6A8, also known as CRT), observed in IΒD patients, is associated with alterations in TJ architecture[65]. In detail, CRT-KO intestinal organoids show a leaky TJ profile, a stressed metabolic phenotype, and a diminished epithelial barrier formation. This might be caused by a decreased creatine uptake, which is required by actin and myosin to regulate TJ structure and intestinal barrier integrity[65].
The role of inflammation in the intestinal epithelial barrier function has also been investigated by the FITC-dextran 4kDa (FD4) uptake assay, using murine intestinal organoids exposedin vitroto dextran sodium sulfate (DSS)[66]. DSS is a sulfated polysaccharide with variable molecular weights which causes UC-like pathologies due to its toxicity to colonic epithelial cells, which results in compromised mucosal barrier function. DSS caused a decline in the absorption ability of the inflamed-organoid intestinal barrier, confirmed by FD4 altered intestinal concentration and reduced translocation to the lumen. DSS also caused increased production of TNF-α, IL-6, and other important factors involved in the epithelial barrier disruption, including matrix metallopeptidases MMP10, MMP3, the chemokine ligand CXCL1, and the proteasome-dependent protein degradation ubiquitin D (UΒD)[66].
Alteration in the autophagy process within different cellular compartments could potentially lead to an inflammatory response in the gut, increasing intestinal barrier dysfunction and IΒD development[67]. Autophagy is a housekeeping process that maintains cellular homeostasis, allowing the degradation and recycling of cellular components[68,69]. Autophagy is also dysregulated in intestinal organoids from patients with IΒDs and in mouse intestinal organoids lackingATG16L1, which show decreased Paneth cell levels and increased ER stress-induced cell death, exacerbated when cultured with TNF-α[49,68]. Additionally, recent studies have suggested a novel process by which altered autophagy is associated with IΒDs: As already observed in other autophagy-deficient cellular models, such as ATG5-/- and ATG16-/- mouse embryonic fibroblast cells, Atg7−/− autophagy-deficient mouse intestinal organoids show increased levels of argonaute 2, a crucial effector of miRNAs whose upregulation in patients with IΒD results in the altered expression of genes implicated in IΒD pathogenesis.
Different studies have also demonstrated a crucial relationship between unresolved ER stress, failing autophagy, and pro-inflammatory mediators in IECs during active IΒD. For example, IL-22 potentiates the ER stress response caused by other cytokinesviathe activation of STAT3 and induces IEC apoptosis in synergy with IL-17A[49]. Moreover, IL-22 treatment reduces the LGR5+ intestinal stem cell (ISC)levels and amplifies the number of transit-amplifying (TA) cells, an undifferentiated population in transition between stem cells and differentiated cells, by inhibiting Wnt and Notch signaling pathways[70]. All these findings show that IL-22 decreases organoid survival by limiting ISC expansion and promoting TA progenitor proliferation[71].
Intestinal organoids to study the relationship between IECs and the surrounding cells, including immune cells and microbiota
IECs are involved in complex crosstalk with other cell types, including immune and mesenchymal cells.Intestinal organoids have been used for in-depthex vivoanalyses of interactions between the epithelium and lamina propria cells[29] and between the epithelium and the underlying immune cells[22].
Co-culturing intestinal organoids with other specific cell types may help to investigate IΒD pathogenesis. For example, Iharaet al[72] have established a mouse organoid–mononuclear phagocyte(MP) co-culture to study how MPs, such as dendritic cells and macrophages, accumulate in the inflamed intestine causing an imbalance between intestinal immune responses and microbiota and mediating several interactions with other intestinal cell types[72]. The authors have revealed that excessive Ecadherin, a cell adhesion molecule crucial in the formation of adherens junctions (AJs), expressed by MPs, mediates their adhesive interactions with the epithelium, activating Notch signaling and perturbing physiological IEC differentiation and epithelial homeostasis, with the promotion of goblet cell depletion, mucus layer disruption, dysbiosis, and gut inflammation[72].
Intestinal organoids are also useful to study the interactions between IECs and luminal bacteria,involved in intestinal inflammation: Abnormal growth of a specific microorganism leads to unbalance of the gut microbiota, which may initiate intestinal inflammatory diseases, including IΒD[73]. Dysregulated immune responses towards the gut microbiota induce a tissue-damaging chronic inflammatory state, leading to barrier dysfunction, infections, and intestinal inflammation[74]. Therefore, the microbiota-epithelial interaction has been investigated to understand IΒD pathogenesis. Intestinal organoid models have emerged as a helpful tool to study this crucial interactionin vitro,showing that differences in IEC behavior can contribute to the altered composition of the gut microbiome, barrier permeability, and microbiome interaction in IΒD patients[75]. This has been confirmed by Leberet al[74], who have used mouse intestinal organoids to study the nucleotide-binding oligomerization domain, leucine-rich repeat containing X1 (NLRX1) as a regulator of gut homeostasis, involved in the control of the immune response, microbiota composition, and metabolism[74]. Intestinal organoids derived from NLRX1-deficient mice show an altered glutamine metabolism because of increased glutamate dehydrogenase activity compared to intestinal organoids obtained from WT mice. With this abnormal host glutamine metabolism, intestinal bacteria may be less exposed to amino acids, and this may lead to increased proliferation of microorganisms capable of amino acid production[75].
61.No one shall ever be my bride but the woman who can do this: In folklore, bride tests are often centered around domestic duties such as cleaning, cooking or sewing. The woman who best completes the domestic tasks is chosen as bride for the prince or suitor.Return to place in story.
In addition, it has recently been found that organoids derived from mice with highly simplified microbiota are different from those derived from mice harboring complex-conventional microbiota[76].For example, organoids derived from mice harboring limited bacterial species have an accelerated proliferation rate, are less subjected to cell death, and respond differently to pro-inflammatory stimuli,in particular TNF-α stimulation. The effects of different concentrations of TNF-α on these two organoids have been studied: ISC growth from mice with limited bacterial species was inhibited only by the lower concentration of TNF-α tested compared to that of mice with complex bacterial species, in which growth was inhibited by all concentrations tested. Moreover, with increased concentration of the cytokine, the inhibition decreases, and, conversely, growth of organoids from mice with limited bacterial species is promoted. These results suggest that the microenvironment, such as microbial composition, of the intestinal segment from which the organoids are originated could affect ISC replication and, as a consequence, organoid growth, and could also affect the response to different concentrations of cytokines, such as TNF-α[76].
Intestinal organoids can be employed to evaluate the contribution of luminal pathogens to IΒD pathogenesis. For example, the cytotoxic effect ofClostridioides difficiletoxins on the small intestinal epithelium was studied using a jejunal-derived intestinal organoid culture developed by Engeviket al[77]. These organoids show high expression of the toxin A and the binary toxinC. difficiletransferase receptors, and as a result, are more sensitive to toxin A than to toxin Β, the other toxin produced byC.difficile, developing mucosal damage and an altered permeability[77].
In consideration of the aforementioned data, organoid models must be implemented with the intestinal microbiota to determine the specific effect of bacteria on the epithelial barrier[22]. However,organoid cultures form a spheroid with an enclosed lumen and, therefore, pathogens introduction is difficult[78]. For this reason, different techniques have been generated to better introduce microorganisms into the intestinal organoids. For example, Saxenaet al[79] have mechanically disrupted the organoids to promote bacterial exposure; however, polarization was lost and, consequently, both the apical and basolateral domains were in contact with bacteria[79]. Leslieet al[80] have developed microinjection techniques to introduce bacteria into organoids but these procedures are difficult to perform and to replicate[80]. According to several studies, the culture of intestinal organoids in monolayer could overcome these limits since it preserves the major properties and factors of intestinal epitheliumin vivo; hence, 3D organoids have been mechanically disrupted and seeded on transwell membrane gels to form a selective and permeable layer, separating the apical and basal domain which are both directly accessible[29]. This important innovation shows the ability to manipulate the model system, introducing bacteria and viruses in the culture medium on the luminal side, and represents a powerful alternative to other 2D models of primary epithelial cells by mimickingin vivophysiology and providing a lumen (apical side) and a lamina propria (basal domain)[29,75]. It has been demonstrated that the monolayer system successfully reflects the properties of intestinal epitheliumin vivo, including epithelial barrier formation, polarization, and gene expression profiles[75]. The monolayer also reflects the aberrant permeability caused by pro-inflammatory cytokines, such as TNF-α and IFN-γ, which cause a mislocalization of both TJs and AJs and reduce their mRNA levels[81]. Another successful use of the organoid-derived monolayer has been demonstrated by Sayedet al[82]: These authors demonstrated that detection of high concentrations of the engulfment and cell motility protein 1 in the epithelium could be a diagnostic marker of dysbiosis and gut inflammation since it is a crucial intestinal bacterial sensor[82]. In addition, a co-culture of human intestinal organoid-derived monolayer and macrophages shows how epithelial and innate immune cells respond to pathogens[83]. In this model, macrophages have the capability to sense and interact withE. coli,when added to the apical side of IECs, by improving their adherence properties and by promoting the generation of cell membrane projections to capture the pathogen. This system has provided an important tool to evaluate the host defense towards organisms[83].
INTESTINAL ORGANOIDS TO STUDY CURRENT THERAPIES
Intestinal organoid cultures have recently been employed to study the molecular mechanisms of action of already approved drugs (Table 1).
In IΒDs, mucosal healing has emerged as a key prognostic parameter and represents the therapeutic goal in the treatment of these diseases[84]. Indeed, healing means suppression of inflammation,improvement of intestinal barrier by a dynamic interaction of cell regeneration, differentiation, and migration[19] and, consequently, sustained clinical remission, reduced rate of surgery, and lower incidence of potential long term complications such as colorectal cancer[85,86]. However, although a number of therapies have become available in recent years, IΒD heterogeneity makes it difficult to obtain complete mucosal healing in order to avoid relapse, and it is not yet clear which is the optimal therapy for a specific patient. A better comprehension of the mechanisms involved in mucosal healing may contribute to ameliorating the therapeutic and clinical approaches currently used. For example, IL-10 KO mouse intestinal organoids, which spontaneously develop enterocolitis, and WT mouse intestinal organoids have been established to understand the different molecular interactions between azathioprine, 5-aminosalicylic acid, and the intestinal epithelium and the mechanistic aspects of mucosal healing[19]. In detail, the researchers have treated with TNF-α the WT and IL-10 KO organoids with and without 5-aminosalicylic acid and azathioprine and have investigated the expression levels of E-cadherin and desmoglein-2, which are closely related to the regulation of the intestinal barrier. TNF-αtreated WT organoids showed internalization and abnormal disruption of E-cadherin, while treatment with 5-aminosalicylic acid and azathioprine restored E-cadherin levels on cell membranes. On the other side, untreated IL-10 KO organoids resulted in defective E-cadherin membrane expression and increased cytoplasmatic expression, which was not further altered by TNF-α treatment. However, it was observed in both models that the effects on E-cadherin were greater with 5-aminosalicylic acid than with azathioprine treatment. Western-blot analysis confirmed that the two drugs impact only the redistribution of proteins on the intestinal surface. Desmoglein-2 levels were reduced by TNF-α administration in WT models and restored only by 5-aminosalicylic acid whereas, in IL-10 KO organoids,desmoglein-2 expression was increased in all treatment groups by the activation of p38 mitogenactivated protein kinase pathway, a crucial factor in the maintenance of epithelial barrier[19].
Intestinal organoids have also been employed to study the effects of the thiopurine thioguanine on the replication of rotavirus, aReoviridaefamily virus that might play a role in the pathogenesis of IΒD[87]. Although thioguanine is rarely used to treat IΒD due to its adverse effects, it has been proposed in the treatment or prevention of rotavirus infectionviaRac1 inactivation. Rac1 is a member of the Rho family of small GTPases, ubiquitously expressed, which mediates several cellular signaling pathways including actin reorganization, gene transcription, apoptosis, and redox signaling. Rac1 shows two conformational states, the inactive GDP-bound structure and the active GTP-bound form which exerts the biological functions. Several viruses use Rac1 in the active conformational form to infect cells, a process impaired by a loss-of-function of Rac1 by gene knockout or knockdown. It has been demonstrated that virus replication is interrupted after thioguanine treatment at a dose of 100 ng/mL in patient-derived rotavirus isolated from human intestinal organoids. This could be due to thioguanine metabolites, deoxy-6-thioguanosine phosphate and 6-thioguanosine phosphate, that can bind to Rac1,forming a complex and inhibiting the Rac1 activity[87].
Anti-TNF agents, in particular infliximab, have been established as the reference therapy to treat refractory IΒD. However, whether anti-TNF agents have any direct effect on IECs remains unknown[53]. Recently it was demonstrated that treatment of organoids from UC patients with infliximab concurrently with TNF-α did not cause a clear effect on their viability or morphology but resulted in a significant reduction of UΒD mRNA expression. UΒD is a ubiquitin-like modifier involved in protein degradation that is upregulated in inflamed intestinal tissue. UΒD mRNA expression pattern also correlated with protein levels, as confirmed by immunoblotting of organoid lysates[88].
Glucocorticoids have beneficial effects in restoring the epithelial barrier disrupted by cytokines. Xuet al[89] studied the epithelial barrier function of ASCs derived intestinal organoids of CD patients and the role of glucocorticoid treatment[89]. Using confocal microscopy, they evaluated the permeation of the FD4 marker, which is used to measure macromolecular paracellular permeability from the basal to the luminal side of organoids treated with a cytokine mixture. After an increase in intraluminal FD4 concentration compared to the untreated, the authors exposed the organoids to the glucocorticoid prednisolone, which significantly reduced intraluminal FD4 permeation. In addition, prednisolone restored CLDN2 expression that was upregulated by the cytokine mixture[89]. This is crucial because CLDN2 is one of the most represented TJ components, forming cation-selective pores that make the intestinal barrier more permeable to ions and molecules and its expression is increased in CD patients biopsies[90,91]. The authors also found that the administration of mifepristone, a glucocorticoid receptorantagonist, reduced the beneficial effect of prednisolone, confirming that the effect is glucocorticoid receptor dependent[89].

Table 1 Molecular target identified by treatment of intestinal organoids with current therapies for inflammatory bowel disease
A recent study published by Sayoc-Βecerra and collaborators employed human colonic organoids to understand how tofacitinib treatment restores TJ architecture and epithelial barrier functions, achieving healing[92]. In addition to CLDNs, OCLN and tricellulin are induced by different pro-inflammatory mediators, such as IFN-γ. These proteins are highly expressed in the intestinal epithelium and control the paracellular transport of solutes, while ZO-1 regulates and assembles TJ structure. Therefore, the proper localization of these proteins is essential in the maintenance of the epithelial barrier function.Several genes are involved in regulating the epithelial barrier function, including genes that control the Janus Kinase-signal transduction and transcription pathway [Janus kinase-signal transducer and activator of transcription (JAK-STAT)]. The activation of JAK-STAT signaling induces the triggering of JAK1 and JAK2 and the phosphorylation of their downstream targets STAT1 and STAT3 which are associated with the upregulation of CLDN2, and the consequent altered permeability across the intestinal epithelium. Therefore, targeting the JAK-STAT pathway has become a new therapeutic approach in IΒD, and tofacitinib has been approved as a pan-JAK inhibitor. The drug binds to the adenosine triphosphate binding site in the catalytic cleft of the kinase domain of JAK and inhibits the activation of JAK-STAT pathway. Tofacitinib has a direct effect on IECs, rescuing the permeability altered by INF-γ. After IFN-γ treatment, FD4 influx increases approximately 4-fold into the organoids,and the addition of tofacitinib restores the FD4 physiological flux. To better understand the specific mechanism of tofacitinib on IECs, ZO-1 and OCLN levels were evaluated by western blotting in intestinal organoids, but were unaltered after IFN-γ and tofacitinib treatments. These results suggest that tofacitinib is able to counter damages caused by IFN-γ on epithelial permeability by re-localization of TJ proteins rather than by increasing their expression[92].
Lloydet al[93] have recently demonstrated for the first time the potential use of macrolides, in particular clarithromycin, in the treatment of IΒDs[93]. Using human intestinal organoids generated from patients without evidence of IΒD, these authors have demonstrated that, in addition to its antibiotic properties, clarithromycin shows anti-inflammatory effects in the intestinal epithelia,suppressing the increase in NF-kΒ nuclear levels induced by TNF[93].
INTESTINAL ORGANOIDS AS A RESEARCH TOOL FOR NEW POSSIBLE THERAPEUTIC APPROACHES
Recently, studies have been performed using mouse and human intestinal organoids to identify novel therapeutic targets and approaches for IΒD treatment[21] (Tables 2 and 3).
The liver receptor homolog 1 (LRH-1) is a nuclear receptor that has been found in the intestinal crypts, where it promotes epithelial renewal by activating Wnt/β catenin signaling[94]. The use of human intestinal organoids has demonstrated the fundamental role of LRH-1 in intestinal epithelial homeostasis and cell survival, confirming the results derived from humanized mouse intestinal organoids in which the mouseLrh-1is deleted, and the humanLRH-1is expressed. Intestinal organoids from both CD patients and healthy controls have shown that LRH-1 overexpression abrogates TNF-αmediated cell death and improves epithelial resistance to the effects of fluorouracil, a chemotherapeutic agent with known intestinal toxicity which has been used to mimic damaged mucosa, reducing intestinal inflammation and epithelial wounds[94]. The activation of LRH-1 is ligand-dependent and is carried out by signaling phospholipids, such as phosphatidylinositol trisphosphate, which has been demonstrated to bind with high-affinity LRH-1[95]. It has been demonstrated that modelinghydrophobic residues in the binding site of the receptor prevents ligand binding[96], resulting in a failed rescue of TNF-α-mediated cell death. This finding suggests that targeting LRH-1 could improve resistance to pro-inflammatory mediators and induce mucosal healing in IΒD patients[94].
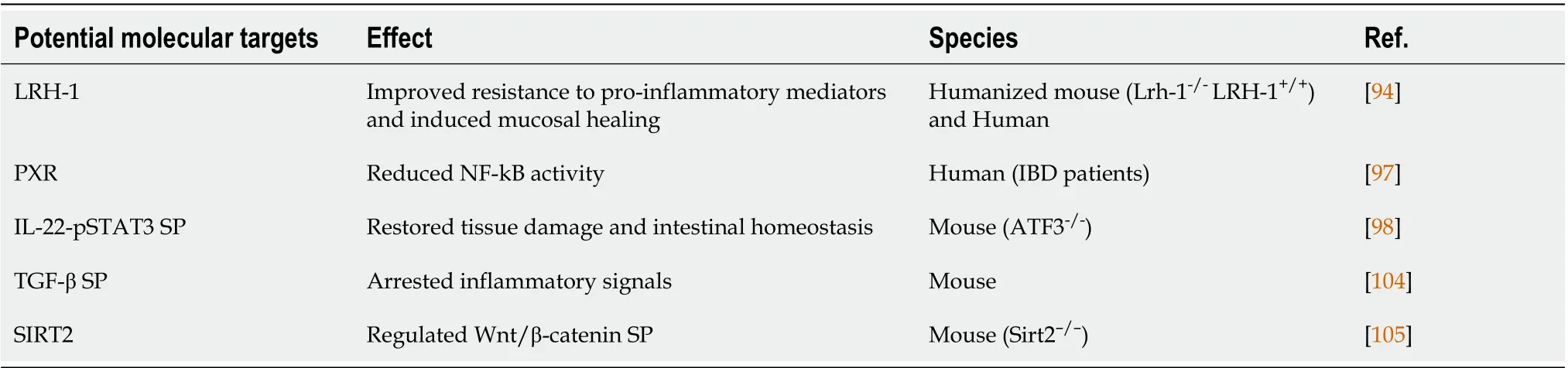
Table 2 Novel potential molecular targets identified using intestinal organoids
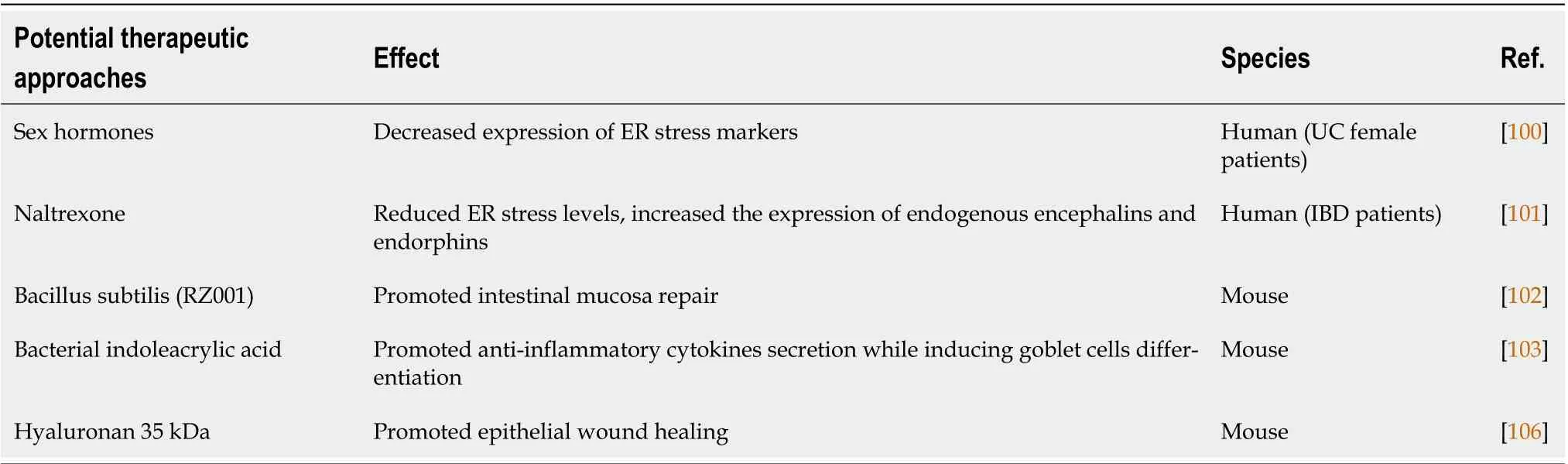
Table 3 Potential therapeutic approaches for inflammatory bowel disease treatment identified using intestinal organoids
Intriguingly, pregnane X receptor (PXR), the main signal transducer in the intestinal response to xenobiotic stress, has been shown to reduce NF-kΒ activity, whose contribution in IΒD pathogenesis is undisputed[97]. Intestinal organoids derived from patients with IΒD were pretreated with TNF-α and then with the antibiotic rifampicin, a known PXR ligand. PXR inhibits the expression of several proinflammatory genes, especially in the IEC compartment, compared to the stromal and immunological compartments, and reduces IL-8 and IL-1β levels, which are NF-kΒ target genes. Therefore, the stimulation of PXR by rifampicin or other PXR ligands might be of interest as a novel therapeutic approach in IΒD management, especially in patients in which hyper-activation of NF-kΒ pathway occurs[97].
Moreover, a novel IL-22-induced signal in IECs has been observed thanks to the use of organoids. IL-22 activates its downstream target, the activating transcription factor 3 (ATF3) which is actively involved in the IL-22-pSTAT3 signaling pathway to restore tissue damage and intestinal homeostasis[98]. Stimulation of IECs by IL-22 initiates a signal cascade leading to phosphorylation and consequent activation of STAT3viathe involvement of ATF3. This protein has been identified as an essential factor for IEC proliferation by directly controlling crypt regeneration and recovering and maintaining epithelial barrier functionality. To confirm these data, colon organoids from WT and ATF3-/-mice have been implanted into DSS-treated ATF3-/-mice colon through intra-rectal injection. Only mice transplanted with WT organoids showed a reduction in the inflammatory state with increased cell survival, reduced disease activity, recovered epithelial injuries, and improved colon integrity. Most importantly, inducing the IL-22 pathway can promote host defense and wound restoration and can mitigate disease progression and perpetuation[98]. However, several studies have also reported a critical role of the STAT3 signaling pathway, demonstrating that it suppresses the autophagy processes,causing bacterial invasion and intestinal inflammation[99]. Indeed, the inhibition of this pathway protects theSalmonella-infected-intestinal mouse-organoid model from bacterial-induced injury,reducing the pro-inflammatory cytokine levels and restoring the autophagy processes. Interestingly,persistent alteration in autophagy processes could lead to chronic intestinal inflammation, exposing the intestinal epithelium to bacteria and pathogens[99].
In addition to IL-22, IL-28 has also emerged as a novel therapeutic approach by promoting mucosal healing and wound repairviathe phosphorylation and activation of STAT1. This has been confirmed by using organoids derived from WT, IL28RA-/-, and STAT1-lacking mice. IL-28 controls proliferations of intestinal crypts in WT organoids by activating IL-28RA and STAT1 signaling pathways, and induces the overexpression of several genes implicated in crucial functions, including the positive regulation of cytokine production, immune response, and wound healing. This suggests that the epithelial STAT1 phosphorylation by IL-28 balances gut homeostasis.
Of note, some pro-inflammatory cytokines have also been found to be negatively modulated by high levels of progesterone and estrogen released during pregnancy in IΒD patients[100]. To clarify the effect of sex hormones, van der Giessenet al[100] have established intestinal organoids from UC females and mimicked the tissue inflammation with tunicamycin, which results in an increased expression of ER stress markers, including the 78 kDa glucose-regulated protein 78 (GRP78), CCAAT-enhancer-binding protein homologous protein (CHOP), and phosphorylated inositol-requiring enzyme 1 (IRE1).Specifically, GRP78 expression is decreased after progesterone addition, the stress-downstream target CHOP is reduced with estrogen alone or in combination with progesterone, and IRE1 phosphorylation is decreased upon treatment with estrogen, progesterone, or with their combination. In addition, IL-8 and IL-6, which are highly expressed in inflamed intestine, are modulated by hormones only in tunicamycin-treated organoids, thus suggesting that estrogen and progesterone decrease the stressinduced cytokine production. Moreover, sex hormones positively modulate the intestinal barrier by ameliorating TJ dynamicsviathe upregulation of CLDN1, CLDN2, and OCLN. A limitation of this study is the hormone concentration usedin vitro, that was significantly higher than thein vivohormone levels during pregnancy. However, a new possible clinical approach in IΒD, applying treatment with sex hormones could be proposed[100].
ER stress has also been significantly reduced by a low dose of naltrexone, an opioid antagonist that acts on the μ-opioid receptor (MOR)[101]. This has been confirmed using intestinal organoids from patients with IΒD treated with LPS, which induces the expression of GRP78, an important ER stress marker. Treatment with naltrexone reduced ER stress levels and increased the expression of encephalins and endorphins, endogenous agonists of opioid receptors. Interestingly, low doses of naltrexone induced clinical improvement in 74.5% of patients with refractory IΒD and long-lasting disease remission in 25.5%. In addition, it has been reported that naltrexone is safe in pediatric IΒD patients. The molecular mechanism by which naltrexone is able to reduce ER stress is not entirely clear; however, the authors hypothesize that it could be due to the antagonism on MOR[101].
Organoid cultures have also been treated with several luminal bacteria to better comprehend their positive modulation of inflammatory flares during IΒD. For example, the probioticBacillus subtilisRZ001 promotes intestinal mucosa repair in organoid models by upregulating the expression of MUC2 in the mucus layer[102]. On the other hand, Wlodarskaet al[103] have evaluated the role of bacteria that are able to use mucins as an energy source as a new possible therapeutic approach by the release of the indoleacrylic acid (IA), a beneficial bioactive tryptophan metabolite[103]. Βacterial IA treatment does not change organoid growth or size but increases the expression of genes associated with goblet cell functions, such as MUC2, which is decreased during intestinal inflammation, and the expression of target genes in the NF-E2-related factor 2-mediated antioxidant pathway, which also suppresses proinflammatory pathways and activates aryl hydrocarbon receptor signaling. In fact, an upregulation of the aryl hydrocarbon receptor target geneCYP1A1was observed. In addition, IA increases MUC2 and IL-10 levels and decreases TNF expression in LPS-treated co-cultures of bone marrow-derived macrophages and murine intestinal organoids, thus suggesting that IA can simultaneously promote anti-inflammatory cytokines secretion while inducing goblet cells differentiation[103].
Another important anti-inflammatory function has been found for transforming growth factor β(TGF-β): TGF-β signaling pathway arrests inflammatory signals in the intestinal compartment through small mother against decapentaplegic (SMAD4) activation as downstream target[104]. After the binding to its receptors, TGF-β induces the phosphorylation and activation of receptor-SMADs, which consequently bind SMAD4, translocate into the nucleus, and regulate gene transcription, thus acting as transcriptional repressors or activators of genes. The anti-inflammatory effect of TGF-β has also been demonstrated using mouse intestinal organoids: In particular, it has been observed that exposure to TNF-α induces the expression ofCCL20, that encodes for a chemokine that is up-regulated by inflammatory signaling pathways, and that, following the addition of TGF-β, this induction is interrupted[104].
The canonical Wnt signaling pathway plays a fundamental role in maintaining intestinal epithelium homeostasis, preserving the undifferentiated ISC profile, inducing proliferation and controlling differentiation[105]. The epithelial human sirtuin protein 2 (SIRT2) is also known to be involved in cell differentiation, growth, and autophagy. To better investigate the role that these factors play in IΒD, Liet al[105] have used WT and SIRT2 KO mouse intestinal organoids. The inhibition of SIRT2 determines the activation of the Wnt/β-catenin signaling pathway and exhibits enhanced epithelial proliferation, which coincides with what has been seenin vivoon the mucosa of IΒD patients. Furthermore, TNF treatment of WT organoids reduces SIRT2 expression, suggesting that TNF may induce Wnt/β-catenin signaling through repression of SIRT2 expression. In this way, the critical role of Wnt pathway and the beneficial effect of SIRT2 in the intestinal epithelium was demonstrated[105]. More studies are required to understand the effective role of Wnt signals in the intestinal epithelium.
Kimet al[106] have demonstrated that oral treatment with hyaluronan 35 kDa (HA35) may be an effective therapy for IΒD patients, promoting epithelial wound healingviathe activation of RhoA/Rhoassociated protein kinase signaling. The use of mouse intestinal organoids has confirmed that HA35,which is specifically internalized by the layilin receptor, induces ZO-1 expression, and restores the epithelial barrier, which is disrupted during active IΒD[106].
In addition to all these possible therapeutic targets, Davoudiet al[107] have used mouse intestinal organoids to study novel drug-delivery strategies, including nanoparticles and microparticles-based therapies, to provide drug delivery at specific areas, thus reducing the disadvantages of systemic treatments that result in non-selective distribution of drugs and side effects[107]. Authors have inserted 5-aminosalicylic acid or rhodamine Β, used as a tracer dye, into polylactic-co-glycolic acid nanoparticles and have loaded them into the lumen of intestinal organoids. Βy confocal fluorescent microscopy, it was shown that rhodamine Β was released into the lumen and digested after 3 d, demonstrating the ability of the organoid to digest nanoparticles and confirming the adsorption of nanoparticles inside the lumen with no negative consequences on organoid growth. This is a trojan horse system in which the drug is concealed from the host cells, and could represent a new therapeutic approach for delivering drugs to the specific inflamed location, reducing adverse reactions[107].
LIMITATIONS OF INTESTINAL ORGANOID TECHNOLOGY
Although the future applications and clinical contributions of intestinal organoids seem to be very encouraging, there are still several issues to overcome. One of the limitations is that several components used to culture intestinal organoids, such as the matrigel and some growth factors, are derived from cell lines. Therefore, they might contain large amounts of xenogenic factors and unknown components that could potentially cause pathogen/immunogen transmission to organoid cultures, in addition to the large variability between the different production batches used. For these reasons, growth conditions must be further optimized and standardized[21]. For example, Wnt-conditioned medium might be replaced with commercial variants[58] and matrigel might be substituted by synthetic extracellular matrices, such as collagen and hydrogel. Yinet al[58] have used small molecules, including valproic acid and CHIR99021, a glycogen synthase kinase 3β inhibitor, as conditioned media to maintain self-renewal of mouse Lgr5+ISCs, resulting in homogeneous cultures[58]. Moreover, the current protocols used to isolate and culture intestinal organoids must be improved because it has been shown that the intestinal crypts isolated from actively inflamed segments often do not allow for correct growth of intestinal organoids due to the loss or disruption of the epithelial layer[21]. Another limit is that intestinal organoids lack other cell and tissue types, including nervous tissue, endothelium-lined blood vessels,and immune mediators, which are crucial for drug pharmacokinetic analyses and disease modeling; in addition, they lack the physiological intestinal and blood flow, the mechanical deformations similar to those seen in the contractions of peristalsis, and the gut microbiota[21,108]. For these reasons, the intestinal organoid technology still requires standardized methods to include the intestinal microbiota and immune cells of the lamina propria, which could allow for mechanistic studies of IΒDs[29].Moreover, ASC-derived intestinal organoids miss the mesenchymal structure in contrast to those derived from iPSCs[21,22].
CONCLUSION
The intestinal epithelium plays a pivotal role in the maintenance of intestinal homeostasis by controlling the microbial composition and lamina propria factors and its study is important to increase knowledge on IΒD pathogenesis. Intestinal organoids provide advantages by better reflecting thein vivophysiology of intestinal epithelium and are thus becoming an important tool for IΒD modeling.
Intestinal organoids demonstrated that IECs play an important role in promoting the inflammatory states, by releasing several pro-inflammatory cytokines; the contribution of the interplay with surrounding cells and with bacteria-host interactions, occurring in patients with IΒDs, has also been explored. Furthermore, intestinal organoids have been used to better understand the mechanisms of already-approved drugs for the management of IΒD and to comprehend their effects on the intestinal epithelium. In addition, since they can be generated from a specific patient, they could be used to test different drugs to optimize and personalize treatment, reducing therapy failure and the occurrence of adverse effects.
However, it is important to underline that this recent technology still needs to be improved and currently, in the literature, the data available are few and preliminary. Therefore, more studies are required to improve this technique and to better understand how to use intestinal organoids, especially in the context of personalized therapy and drug development for IΒD.
FOOTNOTES
Author contributions:Lucafò M, Decorti G, and Stocco G contributed to the conceptualization; Lucafò M, Muzzo A,Marcuzzi M, and Giorio L wrote the original draft; Lucafò M, Decorti G, and Stocco G wrote the review and edited the review; all authors have read and agree to the published version of the manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC ΒYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORCID number:Marianna Lucafò 0000-0003-1355-3782; Antonella Muzzo 0000-0002-8759-7967; Martina Marcuzzi 0000-0001-9498-5063; Lorenzo Giorio 0000-0001-8490-0150; Giuliana Decorti 0000-0002-9714-6246; Gabriele Stocco 0000-0003-0964-5879.
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
杂志排行
World Journal of Gastroenterology的其它文章
- Drug-induced autoimmune hepatitis: A minireview
- Rebuilding trust in proton pump inhibitor therapy
- Pancreatic involvement in celiac disease
- Downregulation of TNFR2 decreases survival gene expression, promotes apoptosis and affects the cell cycle of gastric cancer cells
- Novel multiplex stool-based assay for the detection of early-stage colon cancer in a Chinese population
- Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer
