Emerging curative-intent minimally-invasive therapies for hepatocellular carcinoma
2022-07-04KylieZanePaulNagibSajidJalilKhalidMumtazMinaMakary
Kylie E Zane,Paul B Nagib,Sajid Jalil,Khalid Mumtaz,Mina S Makary
Kylie E Zane,Paul Β Nagib,College of Medicine,The Ohio State University,Columbus,OH 43210,United States
Sajid Jalil,Khalid Mumtaz,Division of Gastroenterology,Hepatology and Nutrition,Department of Internal Medicine,The Ohio State University Wexner Medical Center,Columbus,OH 43210,United States
Mina S Makary,Division of Vascular and Interventional Radiology,Department of Radiology,The Ohio State University Wexner Medical Center,Columbus,OH 43210,United States
Abstract Hepatocellular carcinoma(HCC)is the most common cause of liver malignancy and the fourth leading cause of cancer deaths universally.Cure can be achieved for early stage HCC,which is defined as 3 or fewer lesions less than or equal to 3 cm in the setting of Child-Pugh A or B and an ECOG of 0.Patients outside of these criteria who can be down-staged with loco-regional therapies to resection or liver transplantation(LT)also achieve curative outcomes.Traditionally,surgical resection,LT,and ablation are considered curative therapies for early HCC.However,results from recently conducted LEGACY study and DOSISPHERE trial demonstrate that transarterial radio-embolization has curative outcomes for early HCC,leading to its recent incorporation into the Barcelona clinic liver criteria guidelines for early HCC.This review is based on current evidence for curativeintent loco-regional therapies including radioembolization for early-stage HCC.
Key Words: Hepatocellular carcinoma;Loco-regional therapy;Radiation segmentectomy;Transarterial radio-embolization;Ablation;Transarterial chemo-embolization;Curative intent
lNTRODUCTlON
Hepatocellular carcinoma(HCC)is the most common cause of liver malignancy and the fourth leading cause of cancer death across the globe[1].Curative outcomes can be achieved for early stage HCC,which is defined using the Barcelona clinic liver criteria(BCLC)as 3 or fewer lesions less than or equal to 3 cm in diameter with preserved liver function and functional status.Patients with intermediate or advanced HCC who can be down-staged with loco-regional therapies(LRT)to resection or transplantation can also achieve curative outcomes.Traditionally,surgical resection,liver transplantation(LT),and ablation are considered curative therapies for early HCC.
In early HCC,resection and LT are often preferred over ablation when possible.However,many patients are not surgical candidates,whether due to medical comorbidities,inability to tolerate anesthesia,or tumor location.Additional drawbacks to surgical approaches include elevated postoperative morbidity and mortality rates[2,3],life-long immunosuppression in the case of LT[4],and high recurrence rates with resection[5].Fortunately,loco-regional therapies such as radiofrequency ablation(RFA)make curative treatment possible for these patients.Studies demonstrating comparable overall survival and recurrence rates for ablation are now over a decade old,and ablation as a curative therapy has been present in the National Comprehensive Cancer Network(NCCN)guidelines since 2017[6].However,recent work demonstrates that transarterial radio-embolization(TARE)is an effective,safe,and curative treatment option for early HCC.
This review discusses the evidence for curative outcomes in early HCC using LRT including ablation,TARE,transarterial chemoembolization(TACE),and combination therapy.We further review the role of these therapies in down-staging and bridging with curative intent for LT or resection.
CONCEPT OF CURE
While cure is the ultimate goal in the treatment of HCC,it is not always apparent what defines a curative-intent therapy.Cure for HCC using various modalities including LT,resection and ablation is reported in the form of overall survival and recurrence.When curative LT outcomes are considered for HCC,overall survival is > 70% at 5 years[7],with a recurrence rate of 6%-15%[8].For resection,overall survival is 50%-70% at 5 years[3,9]and recurrent HCC is seen in > 60% of patients at 5 years[5,10].Notably,these outcomes may be influenced by differing criteria between surgical and locoregional therapy candidacy;for example,surgical candidates are typically without significant portal hypertension[11].For ablation,overall survival is around 60% at 5 years with a local recurrence rate of 3%-22% at 5 years in lesions up to 5 cm[12,13].All three therapies are considered potentially curative in appropriate patients,and thus establish a standard for outcomes necessary to be considered curative(Table 1).
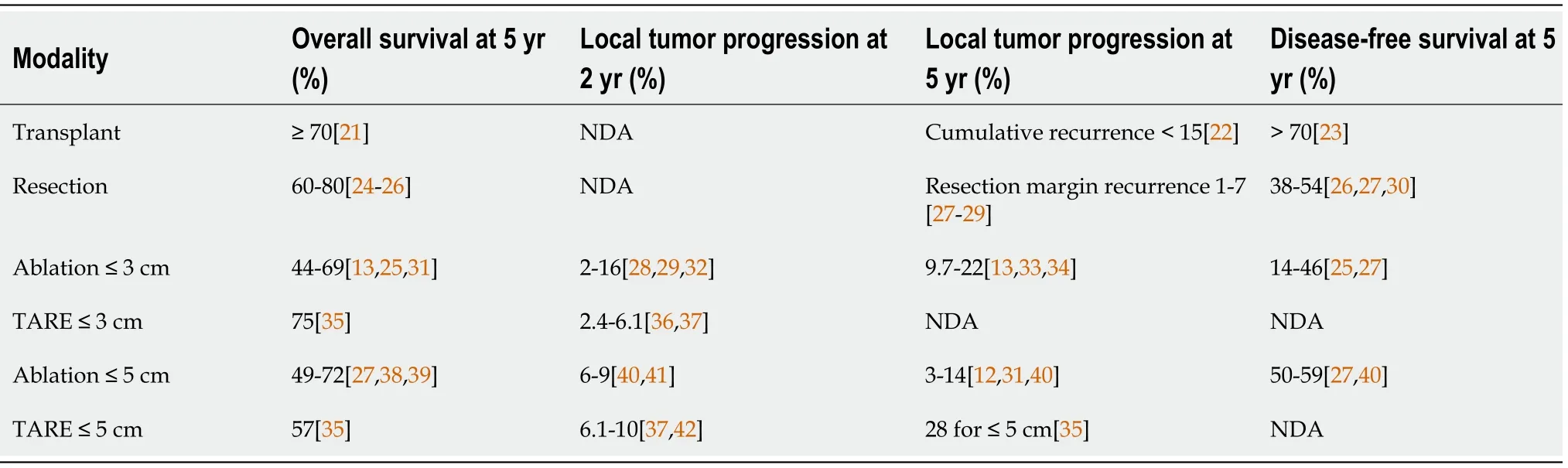
Table 1 Outcomes for curative-intent therapies in hepatocellular carcinoma within Milan criteria
Of note,the modified Response Evaluation Criteria in Solid Tumors(mRECIST)assessment of the radiologic response to LRT validates the use of tumor response rate as a surrogate outcome for survival[14].Generally speaking,tumor response rate utilizes established imaging criteria to group patients into non-responders,partial responders,and complete responders[15].The commonly reported objective response rate(ORR)is the combination of partial and complete responders over all subjects.As might be expected,complete response is associated with the greatest improvement in outcomes[16].
Loco-regional therapies also play a neo-adjuvant role in the treatment of HCC.They can be used to bridge patients to definitive therapy with LT or can down-stage HCC to meet transplant criteria.Using LRT,down-staging is successful in approximately half of patients,regardless of whether TARE or TACE is used[17].Importantly,bridging and down-staging do not worsen LT outcomes in terms of overall survival or recurrence[18-20].
AΒLATlON
The most common ablation techniques for HCC are radiofrequency(RFA),microwave ablation(MWA),and cryoablation.In early stage HCC with preserved liver functions(Child-Pugh A/B),this is a potentially curative modality for patients who are not candidates for surgery or resection.In radiofrequency(RFA)and microwave ablation(MWA),probes are placed percutaneously into the tumor so that thermal energy may be used to directly induce tumor necrosis.In cryoablation,cold gas is delivered through hollow needles into the tumor tissue and frozen,inducing cell death[43].For very early(BCLC 0)and early-stage(BCLC A)HCC patients with preserved liver function(Child-Pugh A/B),RFA and MWA offers survival outcomes comparable to resection despite lower baseline liver function[44-46].These early-stage HCC patients are often disqualified from surgery by significant comorbidities,portal hypertension,poor hepatic function,intolerance to general anesthesia,or high-risk lesion location[47].The use of ablation is limited by tumor location near central biliary structures,gallbladder,stomach,or sub-diaphragmatically given risk of unintended damage to these structures,as well as concern for the rare possibility of tumor tract seeding in case of sub-capsular tumors[35].Additionally,ablation near large vascular structures decreases the ablative power as a heat sink effect from fluid flow draws thermal energy away from the target area[16].A minority of patients experience a self-limiting postablation syndrome(PAS)characterized by fever,malaise,and chills in the first week[48].Less than 4% of patients experience serious complications such as bleeding,abscess formation,liver failure,and damage to surrounding structures[49].
Ablation has been considered a curative treatment for early HCC ≤ 3 cm by the NCCN since 2017[39,44].A study including 120 patients with HCC ≤ 3 cm were randomized into either RFA or resection treatment groups.Results showed insignificant differences in the disease-free and overall survival rates at 1,2,and 3 years.However,the RFA group exhibited meaningfully better hepatic function a week post-treatment,fewer incidences of postoperative complications,and shorter hospital stays[44].Another study of RFA efficacy in 218 patients demonstrated complete response for lesions < 2 cm in more than 90% of the cases,with a local recurrence rate of < 1% and no mortality[16].Studies have demonstrated that ablation of lesions up to 5 cm carries a 5-year overall survival rate of 60% with low rates of local recurrence[12].
RFA and MWA can also be considered in intermediate and advanced stage patients(BCLC B/C)for down-staging to transplantation,with demonstrable success when combined with TACE[50].Additionally,no significant differences in overall survival were noted between patients who were down-stagedviaablation to within Milan criteria before being transplanted,vstransplanted patients who defaulted within the Milan criteria[51].Ablation can also be considered in patients within the Milan criteria for bridging to transplantation,with RFA leading to a lower waitlist dropout rate than bridging with TACE[52].
Future work should be focused on the role for new or less commonly used ablative modalities in early HCC,including high-intensity focused ultrasound[53],laser ablation[54],and irreversible electroporation[53](Figure 1).

Figure 1 Βarcelona clinic liver criteria guidelines for hepatocellular carcinoma treatment.
TARE
TARE,also called selective internal radiotherapy(SIRT),is the administration of glass or resin microbeads coated in Yttrium-90(90Y)viaa catheter into the hepatic artery supplying the tumor of interest[55].This therapy targets tumors by taking advantage of the fact that they are preferentially supplied by hepatic arteries due to neo-angiogenesis,while liver parenchyma is supplied primarily by the portal vein[56].Unlike for ablation therapy,tumor location in relation to other important structures is less of a concern for catheter-directed therapies;it is more important that the vascular supply to the tumor can be identified and accessed with an intravascular approach.
As a result of the studies reviewed below,TARE has recently been incorporated into the BCLC guidelines as a second-line therapy for early stage HCC.Contraindications include any shunting to the GI tract,excessive lung shunting,complete portal vein occlusion,severe liver dysfunction.Recent efforts to determine both optimal procedural approach and maximum tolerable radiation dose have led to data that suggest a curative role for TARE in early HCC[37,57].Regarding the optimal procedural approach,there has been a trend towards increasingly selective TARE.In the past,where lobar or even whole liver radiation may have been administered,a segmental approach is now preferred[58].Regarding radiation dose,recent work suggests targeted doses of 400 Gy or greater are well tolerated and demonstrate complete pathologic necrosis in all patients compared to prior thresholds of 190 Gy(complete response of 100%vs65% respectively)[59].
Using these techniques,three studies demonstrated the potentially curative role for TARE in early HCC.In 2018,Lewandowskiet al[35]published the results of a retrospective study on 70 patients with HCC who were treated with TARE alone.For patients with a single lesion ≤ 5 cm and preserved liver function(Child-Pugh A)who were not candidates for surgery or ablation,overall survival was(comparable to surgical resection)98%,66% and 57% at 1-,3-,5-year respectively.They also reported median overall survival(OS)of 6.7 years.This cohort with a single lesion ≤ 3 cm had 1-,3-,5-year overall survival rates of 100%,82% and 75% respectively.In addition,this study reported 42.7%(100/234)patients with solitary HCC ≤ 5 cm were successfully down-staged to resection(n= 9)or LT(n= 91)after TARE.
In 2021,Salemet al[37]published the results of the retrospective LEGACY study which included 162 patients with solitary HCC up to 8cm(average size 2.7 cm),preserved liver function(Child-Pugh A),and preserved functional status(ECOG 0-1)who were treated with selective TARE.Patients with prior LRT,LT,resection,or systemic therapy were excluded,as were patients with vascular or extrahepatic disease or significant ascites or encephalopathy.In this study,overall survival at 3-years was 86.6% for patients treated with TARE alone(median dose 410 Gy),and 92.8% for patients who underwent TARE and successfully down-staged to LT(21%;34/162)or resection(6.8%;11/162).Local recurrence rate was reported in only 5.6%.Despite the segmental delivery of radiation doses exceeding 400 Gy,there were no cases of REILD,and severe adverse events potentially related to treatment occurred in 5.6% of patients.Of note,analysis of patients who were bridged or down-staged to transplant after treatment with TARE shows similar outcomes to typical liver transplant recipients in terms of overall survival[60,61].
Garinet al[57]further published the DOSISPHERE trial in 2021 on role of TARE.This is a phase 2 multicenter trial comparing lobar TARE using a 120 Gy radiation dose("standard dosimetry")to delivery of a radiation dose of > 205 Gy to the tumor itself("personalized dosimetry").Included patients had local,unresectable advanced disease with at least one lesion ≥ 7 cm.Patients with micro-aggregate albumin(MAA)studies demonstrating poor targeting of the tumor were excluded from the study.Personalized dosimetry was associated with significantly better response rates,defined as partial and complete responders at 3 mo using the European Association for the Study of the Liver(EASL)criteria.Partial and complete responders at 3 mo in the largest lesion were significantly higher in the personalized dosimetry group compared to the standard group: 71%(20/28)vs36%(10/28).TARE achieved down-staging to resection in 36%(10/28)patients in the personalized dosimetry group and 3.5%(1/28)in the standard dosimetry group,including patients with portal vein tumor thrombus.Overall survival was improved in the personalized dosimetry group(26.6 movs10.7 mo).This trial,while not attempting to demonstrate cure,is significant for its rigorous design,inclusion of patients with larger lesions and more advanced disease(including portal vein thrombus)and remarkable outcomes.Using more selective approaches and higher radiation doses,these studies demonstrates that the best possible outcomes for TARE are yet to come.
While the aforementioned studies used glass beads,future work will clarify appropriate dosing with resin beads.The ongoing DOORwaY90 trial will provide data on overall response and duration of response for resin beads,as well as data on safety,quality of life,bridging and down-staging(NCT04736121)(Table 2).

Table 2 Comparing key features of the LEGACY study and DOSlSPHERE trial
TACE
TACE refers to the delivery of chemotherapyviaa catheter within the hepatic artery supplying the tumor.Chemotherapy can be delivered as a liquid solution followed by embolics or as drug-eluting beads.To date,TACE is not generally considered a curative-intent therapy.However,it may be offered to patients with early HCC as second-line therapy for those who are not candidates for surgical approaches or ablation in a process that has been termed stage migration.Typical contraindications include extrahepatic disease,main or lobar portal vein thrombus,and poor liver function(Child-Pugh C).Relative contraindications include elevated total bilirubin(≥ 2-3 mg/dL),as this increases the risk for radio-embolization-induced liver disease(REILD)in TARE and liver failure in TACE.
Novel technical approaches that have demonstrated improved survival are reviewed here.Selective TACE,defined as administration to a segmental artery,and super-selective TACE,defined as administration at the distal portion of a sub-segmental hepatic artery,both improve survival compared to lobar administration[62].Another described technique is ultra-selective TACE,in which lipoidal is administered to the hepatic artery until opacification of the tumor's portal venous supply is seen[62,63].In theory,this prevents post-procedural compensatory increase in portal venous supply to the tumor ensuring complete tumor ischemia and multiple studies have demonstrated improved local tumor response using this method[64,65].While outcomes for selective and super-selective TACE include complete response rates around 40%-50%[62,66]and 5-year overall survival around 20%-35%[67],it is important to consider that TACE has primarily been studied in intermediate and advanced HCC,as opposed to early HCC.In fact,a small prospective study of selective TACE in early HCC(BCLC 0 or A)demonstrated a 3-year survival of 80%[68].Patients most likely to benefit from TACE include those with fewer lesions and preserved liver function(BCLC A)[63,69].Additional developments include the use of modified techniques including balloon-occlusion(B-TACE)and microvalve infusion catheters.These have demonstrated improved tumor targeting and greater rates of tumor necrosis but have not yet demonstrated improved clinical outcomes[70,71].In the case of B-TACE,higher rates of complete response have been observed when compared to conventional TACE[70,72].Furthermore,there is wellestablished evidence for the use of TACE in bridging and down-staging to transplantation[73,74].
Barriers to improved outcomes in TACE include lack of technical standards,specific chemotherapeutic agents,and the embolic effect of therapy,which prevents treatment of distal vessels.Thus,future work for curative TACE will require the development of technical standards and improved chemotherapeutics that are both tolerable and effective.
CHOOSlNG TACE VS TARE
In accordance with recent updates to the BCLC guidelines,TACE and TARE are now both acceptable second-line therapies for early stage HCC.TARE is indicated for single lesions less than 8 cm,whereasTACE is recommended for multifocal disease.Overall survival for TACEvsTARE in HCC has not been directly compared in an RCT.However,a small randomized study demonstrated that TARE led to significantly increased time to progression(> 26 movs6.8 mo)compared to TACE[75].Other work has suggested an increased time to progression and higher quality of life for TARE compared to TACE[76].Between TACE and TARE,the 2021 MERITS-LT trial demonstrated no differences in the rate of or time to successful downstaging to LT when either LRT was the initial downstaging treatment[77].
Considerations for choosing one over the other may be guided by patient characteristics.For example,patients with prior biliary instrumentation are higher risk for the development of hepatic abscess after TACE[78].Despite its name,the ischemic effect of TARE is minimal compared to TACE.As a result,TARE is generally preferred for patients with significant portal vein tumor thrombus,given concerns for excessive ischemia using TACE[79-81].
COMΒlNATlON THERAPY
In solitary HCC lesions up to 7 cm in size,combination therapy with ablation and TACE improves outcomes compared to ablation alone[82].In 2021,Zhanget al[82]in a RCT of 189 patients,demonstrated superior overall survival for RFA plus TACE compared with RFA alone for early HCC < 7 cm(5-year and 7-year OS of 52% and 36%vs43% and 19%,respectively).The benefit was particularly pronounced in tumors > 3 cm.This is consistent with prior studies demonstrating the benefit of combination ablation plus embolization in 3-5 cm tumors[83,84].
Given the high rates of recurrence after resection and ablation,the phase 3 STORM trial examined whether the addition of adjuvant sorafenib could reduce the recurrence rate after curative-intent treatment compared to active surveillance,but was unable to demonstrate benefit[85].Ongoing trials explore the potential for other systemic therapies as adjuvants,including pembrolizumab monotherapy(NCT03867084),nivolumab monotherapy(NCT03383458),atezolizumab plus bevacizumab(NCT04102098),and durvalumab plus bevacizumab(NCT03847428).
The recent addition of TARE as an acceptable stage migration treatment modality for early HCC[86]suggests that future trials on combination treatments for early HCC may increasingly incorporate TARE as a treatment modality.
CONCLUSlON
The treatment of early HCC is evolving,with improved outcomes for transplant,resection,and ablation.Most recently,evidence demonstrates curative outcomes for catheter-directed transarterial therapies for early HCC and suggest that a fourth modality may soon join the list of curative options.Longer follow up periods,technique standardization,and larger randomized controlled trials comparing loco-regional therapies to other curative modalities and defining patients who are most likely to benefit from TARE are needed to confirm these findings.
ACKNOWLEDGEMENTS
Zane KE would like to thank Drs.Osman Ahmed,Edward Kim,and Joe Massa for illuminating conversations on transarterial chemoembolization.
FOOTNOTES
Author contributions:All authors contributed to the preparation of the manuscript.
Conflict-of-interest statement:The authors declare they have no conflicts of interest
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORClD number:Kylie E Zane 0000-0002-0914-839X;Paul B Nagib 0000-0001-8538-3116;Sajid Jalil 0000-0001-6123-153X;Khalid Mumtaz 0000-0001-7868-6514;Mina S Makary 0000-0002-2498-7132.
S-Editor:Ma YJ
L-Editor:A
P-Editor:Ma YJ
杂志排行
World Journal of Hepatology的其它文章
- Role of hepatitis Β virus in development of hepatocellular carcinoma:Focus on covalently closed circular DNA
- Saving time and effort: Βest practice for adapting existing patientreported outcome measures in hepatology
- Loco-regional treatment of hepatocellular carcinoma:Role of contrast-enhanced ultrasonography
- Βenign focal liver lesions:The role of magnetic resonance imaging
- Pediatric acute viral hepatitis with atypical variants:Clinical dilemmas and natural history
- Functions of three ubiquitin-conjugating enzyme 2 genes in hepatocellular carcinoma diagnosis and prognosis
