Research progress on the role of gut microbiota dysregulation in the pathogenesis of diabetic nephropathy
2022-06-23HanWangDunFangWangHongXinSongXuRanMaJinXueMiaoJiaLiWeiPengYangHaiNanWang
Han Wang, Dun-Fang Wang, Hong-Xin Song, Xu-Ran Ma, Jin-Xue Miao, Jia Li,Wei-Peng Yang ✉ , Hai-Nan Wang
1. Department of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun 130117, China
2. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China
3. Hospital of Tsinghua University, Beijing 100084, China
4.National Medical Products Administration, Beijing 100053, China
ABSTRACT There are about over 100 trillion microbial cells in human gut, which affect the nutritional,metabolic, physiological and immune functions of the host. This paper reviews the differences in gut microbiota between patients with diabetic nephropathy (DN) and healthy people.These differences lead to the disorder of symbiotic relationship, which may have induced the progression of DN, as well as targeted interventions to reconstruct the symbiotic relationship.Recent studies have found that endotoxin from intestinal bacteria and a large number of toxic metabolites were produced by fermentation of gut microbiota, such as trimethylamine-N-oxide, indoxyl sulfate and p-cresol sulfate, leading to the disruption of intestinal barrier function. Endotoxin and bacterial metabolites, entering the systemic circulation, were involved in DN progression by mediating inflammatory responses, renin-angiotensin-system and vascular injury. The reduction of some beneficial bacterial metabolites in DN patients, such as short-chain fatty acids, would weak body energy metabolism and destroy glucose homeostasis.In addition, gut microbiota is essential for the conversion of bile acids, and plays an important role in the development of DN by synthesizing secondary bile acids and regulating glucose and metabolic balance through foresaid X receptor (FXR) and G protein-coupled bile acid receptor(TGR5). Animal and clinical studies have revealed that probiotics, prebiotics, fecal microbiota transplantation, and Chinese medicine intervention may have potential therapeutic effects in maintaining a metabolically balanced gut microbiota to reduce the progression of DN, endstage renal disease and cardiovascular complications.
Keywords:Diabetic nephropathy Gut microbiota Gut-kidney axis Lipopolysaccharide Inflammatory response
Diabetic nephropathy (DN) is chronic kidney disease (CKD)caused by diabetes. It is one of the main microvascular complications of diabetes and it is also end-stage renal disease(ESRD) worldwide.The most common cause[1]. Epidemiological studies have shown that there are 425 million people with diabetes in the world. Without intervention, the number of people with diabetes in the world will rise to 629 million by 2045[2]. With the prevalence of diabetes, the incidence of DN is increasing rapidly.About 30%-40% of diabetic patients develop DN, and one-third of patients further develop ESRD, which brings a huge economic burden to the society.
The pathogenesis of DN has not yet been fully elucidated. It is currently believed to be related to long-term hyperglycemia,glycation end products, oxidative stress, polyol pathway, protein kinase C activity increase and other pathways. At the same time,genetic susceptibility and environmental factors also play an important role, which ultimately leads to kidney structure. And the change of function causes the occurrence and development of DN. Recent studies have shown that imbalance of intestinal flora is a direct cause of various metabolic diseases such as obesity,insulin resistance, diabetes, intestinal diseases and cardiovascular diseases[3]. Recently, it has been recognized that changes in the intestinal flora may also play an important role in the occurrence and development of DN, but the specific mechanism is not yet clear, and the treatment of intestinal flora is considered a new way to prevent and treat DN. Therefore, exploring the role of intestinal flora in the pathogenesis of DN and exploring therapeutic strategies that target intestinal flora are of important scientific value.
1. Differences in intestinal flora between DN patients and healthy people
DN patients have intestinal flora imbalance[4,5], real-time fluorescent quantitative PCR, 16S rRNA gene high-throughput sequencing and other technologies can in-depth study of the intestinal flora structure of DN patients. Tao et al.[6] studied the biopsy-proven DN, type 2 diabetes mellitus (T2DM) without kidney disease and the intestinal flora of healthy patients, and found that the composition of the intestinal flora among the three, There is a significant difference in relative abundance. At the genus level, compared with DM patients,Escherichia-shigella in DN patients was significantly higher, and Prevotella was significantly lower. This proves that the intestinal flora may be involved in the development of DM to DN, and by detecting the ratio of Escherichia-shigella to Prevotella in feces,DN can be distinguished from DM patients, which may help the clinical diagnosis of DN[6]. Salguero et al. [7] used the same method to analyze the intestinal flora of patients with DN (stage 4 and stage 5). Compared with healthy control patients, the abundance of Gramnegative bacteria (Proteobacteria, Verrucomicrobia and Fusobacteria)in DN patients A significant increase. Researchers studied the stool samples of different DN animal models (rat, mice) and found that the ratio of Bacteriodetes/Firmicute increased [8,9], which is similar to the study data of type 1 diabetic children [10] and T2DM [11]patients. In addition, the increase in the abundance of Bacteriodetes and the decrease in the abundance of Firmicute have also been shown to be closely related to blood sugar and obesity [11,12], but this conclusion is controversial. The study found that the abundance of Firmicute in stool samples of DN patients was reduced, and the correlation analysis also showed that Firmicute was significantly negatively correlated with fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c) and urine albumin-creatinine ratio (UACR),but no Bacteriodetes abundance was found Significant increase [6]. In addition, some probiotics (Lactobacillus and Bifidobacterium) have a reduced relative abundance in the intestines of DN patients, and their relative abundance is negatively correlated with the severity of proteinuria [5].
In addition, a large number of harmful bacterial metabolites can be detected in patients with DN, while beneficial metabolites are reduced. It can be inferred from this that the change of the intestinal flora of patients with DN is the proliferation of harmful metabolic bacteria and the decrease of beneficial metabolic bacteria, which is specifically manifested by the reduction of short-chain fatty acids (SCFAs) production and the promotion of endotoxins. ,Trimethylamine-N-oxide (TMAO), indoxyl sulfate (IS) and p-cresol Sulfate (PCS) and other harmful substances to expand the group of bacteria[4] .
2. The mediating effect of intestinal flora on the occurrence and development of DN
The “microbe-gut-renal axis” theory clarifies the close relationship between the structure and function of the intestinal flora, the intestinal mucosal barrier and the kidney in substance metabolism,immunity and inflammatory reactions [13]. The composition and function of the intestinal flora of patients with DN have changed,destroying the intestinal epithelial barrier, and increasing toxic metabolites produced by intestinal microorganisms, causing chronic inflammation, changes in blood flow, energy imbalance, glucose and lipid metabolism disorders, and vascular endothelial damage.Aggravated the progression of kidney disease (Table 1). Due to the decreased glomerular filtration rate in patients with DN, a large amount of metabolic wastes in the body cannot be excreted by the kidneys and accumulate, and enter the intestinal cavity through the intestinal wall, resulting in changes in the intestinal environment and further aggravating the imbalance of intestinal flora [14]. Therefore,DN and the imbalance of intestinal flora affect and regulate each other, and this relationship puts the body in a vicious circle.
2.1 Endotoxin and chronic inflammation
Lipopolysaccharide (LPS), also known as endotoxin, is a component of the outer membrane of gram-negative bacteria and can stimulate a variety of inflammatory reactions in the body. Endotoxin can be detected in the blood of DN patients and animal models.LPS caused by “intestinal leakage” enters the blood circulation through the tight junctions of the small intestinal mucosa [23]. In the kidney, the regulation of cell surface pattern receptors Toll-like receptor 2 (TLR2) and TLR4 has been involved in the pathogenesis of inflammation in DN. More and more evidences indicate that the renal inflammation caused by LPS in the host is mediated through TLR2/4-related pathways [24,25] (Figure 1). The binding of LPS and TLRs receptors activates the MyD88/NF-κB signaling pathway and produces pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) to increase kidney tissue inflammation. The activation of TLRs also promotes the secretion of transforming growth factor-β (TGF-β), which mediates the proliferation of renal mesangial cells, glomerular sclerosis and interstitial fibrosis [26]. In the type 1 DN mouse model with TLR2 receptor gene defect, the kidney MyD88 signaling pathway is weakened, proteinuria, podocyte and renal tubular damage, macrophage accumulation, and pro-inflammatory cytokines are reduced [27]. Another study on type 1 DN showed that in mice lacking TLR4 receptors, renal NF-κB activation is reduced, which can protect against proteinuria, mesangial expansion, glomerular sclerosis, and loss of renal function [28] . Porphyromonas gingivalis(porphyromonas gingivalis) is the main microorganism that causes periodontitis. Its outer membrane component lipopolysaccharide(Pg-LPS) binds to TLRs receptors in glomerular endothelial cells,which can cause type 1 diabetic mice and hypertension Fat diet-induced T2DM mice develop DN, which causes glomerular type 1 collagen protein expression, proteinuria, SCr, and BUN increase,and reduces the survival rate of diabetic mice [26,29] . Therefore, LPS destroys the intestinal mucosal barrier and binds to kidney TLRs receptors to activate NF-κB signaling pathway is the inflammatory mechanism of intestinal flora mediated DN.
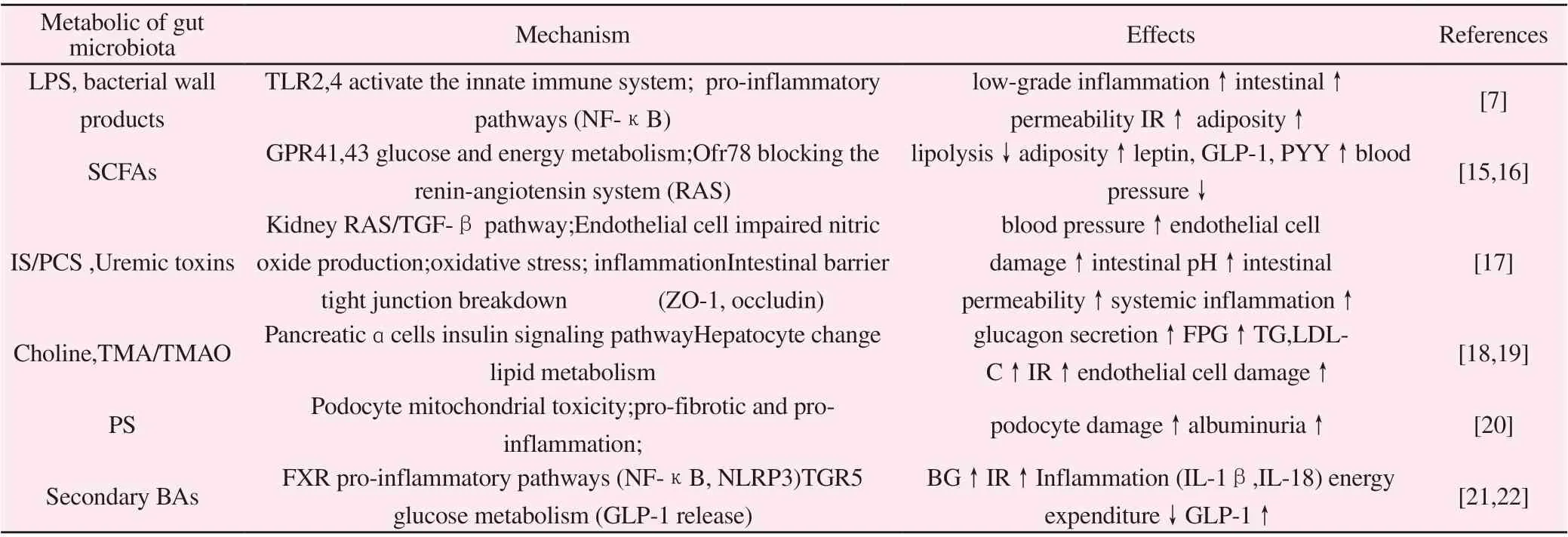
Table 1 The role of gut microbiota metabolism in the pathogenesis of DN
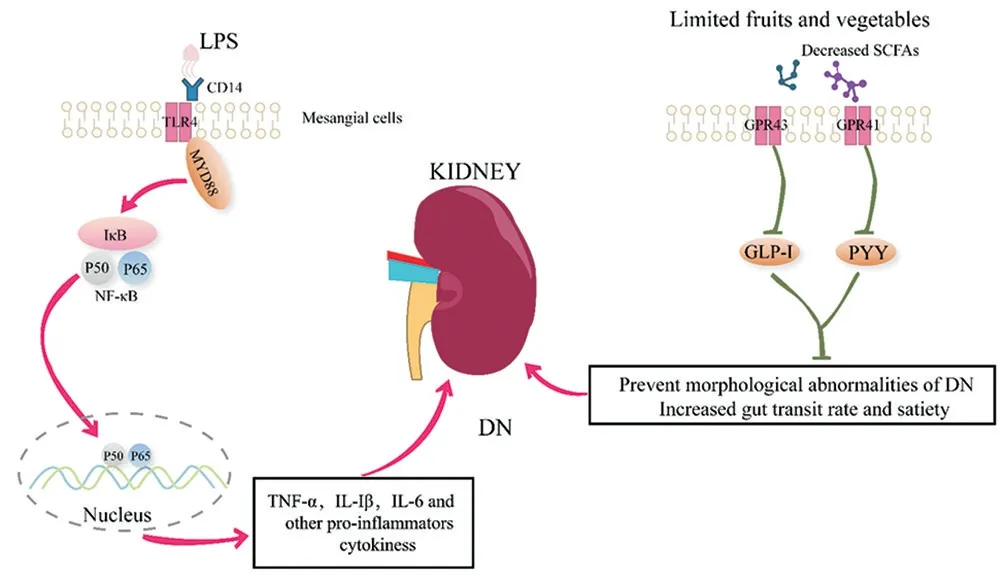
Figure 1 The inflammatory responses and the decreased SCFAs play critical role in the progression of DN
2.2 Short-chain fatty acids and energy balance
Short-chain fatty acids (SCFAs) are produced by the intestinal microbiota by fermenting dietary polysaccharides that the host cannot digest and absorb. They are not only a medium for the intestinal flora to maintain intestinal immunity, but also as signal molecules that can be used by free fatty acid receptor 2 ( free fatty acid receptor, FFAR2) (a G protein-coupled receptor, also known as GPR43) and free fatty acid receptor 3 (FFAR3, also known as GPR41) recognize and activate signaling pathways in the body,which are useful for regulating host energy metabolism and Glucose homeostasis plays an important role [15]. In order to prevent hyperglycemia and hyperkalemia, patients with DN have restricted their consumption of sugar-rich and potassium-rich foods, including fruits and vegetables, for a long time. Since fruits and vegetables are the main sources of fermented SCFAs, when the intake of these foods is restricted, the production of SCFAs is reduced, which also affects the progress of DN [27] (Figure 1). SCFAs can activate the GPR41 receptor of intestinal epithelial cells, leading to the release of the intestinal hormone peptide YY (peptide YY), increasing the intestinal transit rate and feeling of fullness. Activation of GPR43 receptor can reduce inflammation and stimulate L cells to release glucagon-like peptide-1 (glucagon-like peptide 1, GLP-1) [30].GLP-1 can regulate gastric emptying, control appetite, promote the survival and proliferation of pancreatic β cells, and stimulate insulin secretion. Clinical studies have shown that GLP-1 receptor agonists can improve islet function in patients with DN, and reduce blood pressure, blood lipids, inflammation and weight loss [31]. Incretin-based drug treatment can not only inhibit the reabsorption of renal tubular sodium in patients with DN, but also reduce glomerular hypertension and proteinuria, thereby preventing abnormal kidney morphology [32]. In addition, recent studies have found that SCFAs still have anti-inflammatory, anti-oxidant and protective effects on kidney damage. Huang et al. [33] observed the effects of SCFAs on glomerular mesangial cells (GMCs) induced by high glucose and LPS through in vitro experiments, and found that SCFAs can significantly inhibit the proliferation of GMCs, reduce the production of ROS, and increase MAD And the level of SOD reduces the release of ICAM-1, MCP-1 and IL-1β. The results also proved that SCFAs can inhibit the oxidative stress and inflammation of GMCs induced by high glucose and LPS.
2.3 Bile acid and glucose and lipid metabolism
Bile acids (BAs) are synthesized by a series of enzymatic reactions in the liver using cholesterol as a raw material. Intestinal flora is essential for the conversion of BAs. Under the action of the intestinal flora, the primary bile acid 7ɑ-hydroxyl deoxygenation produces secondary bile acids, namely deoxycholic acid and lithocholic acid [34]. The intestinal flora synthesizes and lipids in BAs through the BAs receptor farnesoid X receptor (FXR) and the G-proteincoupled bile acid receptor (Gpbar1, or TGR5) It plays an important regulatory role in metabolism and glucose homeostasis [35]. FXR has a high level of expression in the intestine and kidney, and can be activated by primary bile acids. Previous studies have proven that FXR can negatively regulate the occurrence and development of DN[36]. FXR agonists can reduce glomerular sclerosis, tubulointerstitial fibrosis and proteinuria induced by high fat in C57BL/6J mice. In glomerular mesangial cells, overexpression of FXR or treatment with FXR agonists can also inhibit sterol regulatory element-binding protein-1 (SREBP-1) and other fat-related genes, suggesting FXR may play a role in regulating kidney lipid metabolism, inflammation,and fibrosis, while FXR knockout mice show aggravated kidney damage [37]. Contrary to FXR, secondary bile acids such as lithocholic acid and deoxycholic acid are ligands of TGR5. They bind to the TGR5 of small intestinal L cells, activate the intracellular cAMP pathway, stimulate GLP-1 secretion, and promote glucose metabolism [38] . Thomas et al. [39] confirmed that mice lacking TGR5 receptors had impaired glucose tolerance, while mice overexpressing TGR5 had increased GLP-1 and insulin secretion and improved glucose tolerance. The administration of TGR5 agonists to obese mice can increase the secretion of GLP-1 and improve insulin sensitivity. Therefore, the intestinal flora regulates the occurrence and development of DN through BAs metabolism and FXR/TGR5 signal transduction pathways.
2.4 Short-chain fatty acids/indoxyl sulfate/p-cresol sulfate and renin-angiotensin system
Abnormal renal hemodynamics is an important feature in the early stage of DN, manifested as a state of glomerular hyperperfusion.In a diabetic environment, hyperglycemia may activate the reninangiotensin-system (RAS), leading to impaired glomerular microcirculation self-regulation, leading to increased renal blood flow and intraglomerular hypertension [40]. In this complicated process, the release of renin in the paraglomerular organs plays an important signaling mechanism. Pluznick et al. [16] found that the propionic acid in SCFAs can bind to the olfactory receptor 78(Olfr78) of the paraglomerular organs to reduce the secretion of renin and reduce blood pressure. After antibiotic treatment and Olfr78-/-small The mice found that the antihypertensive effect was weakened,which proved that the intestinal flora binds to the Olfr78 receptor through SCFAs to reduce the signal transduction mechanism of renin secretion. As the main effector of RAS, angiotensin-II (Ang II) is a potent vasoconstrictor formed by enzymatic hydrolysis of angiotensin I (Ang I) by angiotensin-converting enzyme (ACE). It is the main effector peptide of this system. Studies have shown that butyric acid, as an end product of complex carbohydrate fermentation by the intestinal microbiota, can reduce Ang II-induced hypertension in SD rats and at the same time improve Ang II-mediated kidney damage, including urine protein, glomerular sclerosis and renal fiber Chemical and inflammatory factors [41]. On the contrary, the uremic toxins IS and PCS fermented by the intestinal flora can act as stimulators of RAS, activating the RAS/TGF-β signaling pathway and leading to renal fibrosis. Animal experiments have shown that blocking RAS with Losartan can significantly reduce IS And the severity of glomerulosclerosis caused by PCS [42]. This study shows that the intestinal flora is involved in the activation of renal RAS,which leads to changes in renal hemodynamics and mediates the development of DN.
2.5 Trimethylamine oxide and vascular damage
Studies have confirmed that compared with diabetic patients without DN, DN patients have a higher mortality rate, and most of the deaths are due to cardiovascular disease (CVD) [43]. Vascular complications caused by diabetes, in addition to the classic mechanisms such as AGEs, oxidative stress, lipid metabolism disorders, and hypertension caused by high glucose, the effects of trimethylamine-N-oxide (TMAO) The importance has also received widespread attention [18]. Wang et al. [44] used metabolomics methods to screen >2000 compounds and found that TMAO, choline and betaine (a precursor of TMAO) were associated with CVD risk and the progression of atherosclerosis. Detection of plasma TMAO concentration can be used as a predictor of coronary atherosclerosis,CVD and mortality in CKD patients [45]. TMAO can increase the expression of scavenger receptor differentiation antigen 36 (CD36)and scavenger receptor A (SRA) before atherosclerosis, causing the accumulation of cholesterol in cells. At the same time, TMAO can reduce the expression of bile acid synthase CYP7A1 (cholesterol 7alpha-hydroxylase), thereby inhibiting the transport of cholesterol,causing the accumulation of cholesterol in cells and the formation of foam cells, thus becoming a risk factor for atherosclerosis and CVD[44]. In addition, TMAO can be cleared by the kidneys, and elevated serum TMAO concentrations have been detected in ESRD[46] and stage 3-4 CKD[47] patients. It has also recently been shown that TMAO is associated with renal function and inflammation in CKD patients [48]. Adding TMAO or its precursor choline component to the animal’s diet can increase the serum TMAO concentration, which can lead to tubular interstitial fibrosis, collagen deposition, Smad3 phosphorylation, and progressive damage to renal function[49].Transplanting stool samples from CKD patients into sterile mice also increased the concentration of serum TMAO, and antibiotic treatment of mice can prevent atherosclerosis caused by the increase in TMAO caused by choline [47]. The above studies have shown that the occurrence and development of atherosclerosis has a certain relationship with food-borne phosphatidylcholine that depends on the metabolism of intestinal flora, and it is involved in the progress of DN and CVD.
3. A new method to treat DN with intestinal flora as a target
The above summarized the important role of intestinal flora in the occurrence and development of DN. Taking the intestinal flora as the target, the use of probiotics, prebiotics or fecal bacteria transplantation for targeted regulation of the intestinal flora is expected to become an effective method for the prevention and treatment of DN.
3.1 Probiotics and prebiotics
Probiotics are live bacteria that have been proven to be beneficial to human health in clinical trials and used as food additives. Prebiotics refer to food ingredients that can be utilized by the intestinal flora in the human body to stimulate the growth and metabolism of beneficial bacteria (including probiotics). Abbasi et al. [50] found that probiotic soy milk supplemented with Lactobacillus plantarum A7 can improve renal function in patients with DN, including urine protein,SCr and eGFR, as well as new markers of DN (tumor necrosis factor receptor 1, cystatin C). The significant decrease may be caused by the probiotic soy milk which improved the oxidative stress of the kidney. In patients with stage 3-4 DN, treatment with mixed bacteria (Lactobacillus acidophilus KB27, Bifidobacterium KB31,and Streptococcus thermophilus KB19) for 6 months reduced the levels of BUN and UA [51]. The use of probiotics in ESRD patients also has certain benefits. Taking probiotics during hemodialysis in ESRD patients [52] or fiber-rich foods [53] can reduce the production of serum PCS. In a clinical trial of healthy volunteers, preliminary data has also proved that a functional food rich in prebiotic fiber(barley β-glucan) can regulate the composition and metabolism of the intestinal flora [54]. In addition, barley β-glucan can increase the level of fecal SCFAs and reduce the level of circulating PCS[55], which indicates that barley β-glucan can induce intestinal metabolism driven by glycolytic bacteria. These data indicate that the presence of probiotics and prebiotics changes the metabolism of intestinal bacteria, promotes the utilization and excretion of nitrogenous substances, thereby reducing the accumulation of uremic toxins. Treatments targeting probiotics and/or prebiotics may help delay the progression of DN.
3.2 Fecal bacteria transplantation
Fecal microbiota transplantation (FMT), as the most effective means to rebuild the intestinal flora, is considered a breakthrough medical advancement in recent years [56,57]. It has been proven that FMT can effectively treat relapsed and refractory C. difficile infections, and it can also be used in the treatment of metabolic disorders and other diseases [58]. At present, there are few studies on the correlation between FMT and DN, mostly focusing on obesity,diabetes, ESRD animal models and a small number of patients.The results of the study [59] showed that after FMT treatment of DN mice induced by streptozocin (STZ), the levels of SCFAs in the intestine increased, and the levels of 24h urine total protein (24-UP), SCr, and BUN were reduced , Suggesting that the intestinal flora has a regulatory effect on the kidney function of DN mice. The mechanism may be related to the increase of specific bacteria that produce SCFAs after FMT treatment. Hu et al. [60] proved through in vivo and in vitro experiments that FMT can effectively reduce the serum acetic acid level in DN model rats and reduce the disturbance of cholesterol homeostasis mediated by GPR43 activation, thereby reducing the interstitial damage of renal tubules and protecting the kidney. Wang et al. [61] used FMT to study STZ-induced T2DM model mice. After 8 weeks of treatment, the levels of FBG, HbA1c and the inflammatory factor IL-6 in the pancreas were significantly reduced, which improved pancreatic β-cell function and IR.According to the literature, the prevalence of T2DM among the Kazakh people in my country is 1.56%, which is significantly lower than that of other ethnic groups [62]. The researchers transplanted the intestinal flora of healthy Kazakh individuals into db/db mice. After continuous gavage for 2 weeks, fluorescence detection found that the flora of healthy Kazakh individuals had colonized the intestines of db/db mice. After 10 weeks of FMT treatment of db/db mice,the detection of FBG, 2hBG, TG and other metabolic indicators were improved. At the same time, the diabetes-related bacteria Desulfovibrio and Clostridium coccoides in the intestine of db/db mice The level was significantly reduced [63], suggesting that fecal bacteria from healthy Kazakh individuals may be used for clinical treatment of diabetes. The above research has opened a new window for FMT to treat DN. The key to its function is to rely on the overall flora.
4. Summary and outlook
With the rapid development of high-throughput sequencing technology and other systems biology technologies, it has become easier to obtain the whole genome sequence of microorganisms, and the relationship between DN and intestinal microorganisms has been continuously revealed. Regarding the mechanism of intestinal flora affecting DN, it is mainly derived from the cell wall component LPS and its metabolites SCFAs, TMAO, IS, PCS and so on. However,the role of changes in the structure and function of the intestinal flora in the pathogenesis of DN has not yet been fully clarified.The framework of “Hefa Principle” carries out “verification of the body”. At present, there are more and more researches on DM and ESRD and intestinal flora. DN, as the intermediate stage of the development of DM to ESRD, is often ignored and there are few studies. In the future, whether in animal studies or clinical trials, it is necessary to explore the specific changes and roles of intestinal flora in the development of DM-DN-ESRD, so as to optimize the treatment plan for the intestinal flora and prevent disease progression
Authors’ contribution
Wang Han is the main author of this article; Wang Dunfang and Song Hongxin are responsible for the literature review and part of the content writing; Ma Xuran and Miao Jinxue made drawings; Li Jia proposed amendments to the article; Yang Weipeng and Wang Hainan proposed the article Thoughts and reviewed and revised the article.
杂志排行
Journal of Hainan Medical College的其它文章
- Research on the effect of Bletilla striata and the mechanism of the treatment of bronchoplumonary inflammation based on network pharmacology
- Efficacy and safety of Nephritis rehabilitation tablet combined with Valsartan for chronic glomerulonephritis: A system review and metaanalysis
- Meta-analysis of efficacy and safety of Endostar combined with vinorelbine and cisplatin in the treatment of non-small cell lung cancer
- Clinical significance of the detection of Rh blood group antigens and irregular antibodies in pregnant women with a second pregnancy
- Randomized controlled trial of Qing Gan Huo Xue Prescription in the treatment of alcoholic liver cirrhosis
- Mechanism of Xingnaojing injection intervention in cerebral ischemiareperfusion rat model based on GC-MS metabolomics
