Yogurt-derived Lactobacillus plantarum Q16 alleviated high-fat diet-induced non-alcoholic fatty liver disease in mice
2022-06-23ChoTngWeiweiZhouMengyunShnZhoxinLuYingjinLu
Cho Tng, Weiwei Zhou, Mengyun Shn, Zhoxin Lu,*, Yingjin Lu*
a College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
b College of Food Science and Engineering, Nanjing University of Finance and Economics, Nanjing 210023, China
Keywords:
Lactobacillus plantarum Q16
Non-alcoholic fatty acid liver disease
Lipid metabolism
Energy metabolism
Colonic microbiota
A B S T R A C T
Accumulating evidence revealed that some probiotics regulated lipid metabolism and alleviated diet-induced non-alcoholic fatty liver disease (NAFLD). This study mainly explored whether yogurt-derived Lactobacillus plantarum Q16 modulated lipid and energy metabolism, and suppressed microbial dysbiosis in high-fat diet (HFD)-fed mice. Results showed that oral administration of L. plantarum Q16 improved serum and hepatic lipid profile. Protein analysis showed that L. plantarum Q16 could reduce hepatic lipid content by reducing the expression of FAS, ACC, SCD-1, Srebp-1c and ATGL, but increasing expression levels of CPT-1α,PPAR-α and ATGL. Meanwhile, L. plantarum Q16 also improved hepatic energy metabolism by regulating FGF21/adiponectin/AMPKα/PGC-1α signaling pathway. Metagenomic analysis also discovered that L. plantarum Q16 increased species diversity and richness of intestinal microbiota, promoted proliferation of beneficial commensals and suppressed the growth of endotoxin-producing microorganisms in the colon of HFD-fed mice. Overall, L. plantarum Q16 protected against HFD-induced NAFLD by improving hepatic profile and regulating colonic microbiota composition.
1. Introduction
Although the development of modern medicine has efficaciously attenuated some diseases, long-term unhealthy dietary patterns including high-fat diet (HFD), high-sugar diet and high-calorie diet still cause metabolic diseases [1]. Non-alcoholic fatty acid liver disease (NAFLD) is one type of serious metabolic diseases and is characterized by abnormal fat accumulation in the liver. Meanwhile,NAFLD often contributes to the pathogenesis of other metabolic disorders, such as obesity and type 2 diabetes mellitus (T2DM) [2].Thus, the development of NAFLD poses a threat to host health. In the past, it is well recognized that lipid metabolism disorder is the key factor to induce the progression of NAFLD. However, the pathogenic mechanism of NAFLD is very complicated [3]. It is necessary to explore an effective therapy for alleviating NAFLD.
Till now, many kinds of natural products and extracts including polyphenols, yeast-fermented wall-broken bee pollen, polysaccharides and ginkgolide C have been determined to efficaciously attenuate diet-induced NAFLD [4-7]. Recently, the development of next-generation sequencing technology has revealed that specific alteration in intestinal microbiota composition was closely associated with the pathogenesis of NAFLD [8]. Therefore, improvement of gut microbiota composition has been considered as a promising strategy for alleviating diet-induced NAFLD [9]. As one type of commensals,probiotics have been reported to improve intestinal microbiota composition. In recent years, many investigators have discovered that some probiotics includingLactobacillus sakeiO67 andBifidobacteria adolescentisZ25 could effectively alleviate diet-induced NAFLD by suppressing microbial dysbiosis in the intestine [10,11].
L. plantarum, one kind of lactic acid bacteria, also possesses probiotic functions and widely used in the functional foods [12]. Many strains belonging toL. plantarumhave abilities to reduce cholesterol concentration [13], restore gastrointestinal diseases [14]and alleviate inflammatory bowel disease [15]. A recent study has revealed thatL. plantarumZJUFT17 alleviated glycerol monolaurate (GML)-induced metabolic syndrome by reducing body weight, improving serum lipid profile and suppressing low-grade inflammation [12]. In our previous trial, we found thatL. plantarumQ16 possessed ability to reduce body weight and liver weight. However, no study has reported the lipid-lowering effects and mechanism ofL. plantarumQ16 on NAFLD. Thus, this study was to explore protective effects ofL. plantarumQ16 against HFD-induced NAFLD. Firstly, serum and hepatic lipid profile was detected to explore whetherL. plantarumQ16 could alleviate HFD-induced NAFLD. Then, the key proteins involved in lipid and energy metabolism were evaluated to determine the lipid-lowering mechanism ofL. plantarumQ16. Finally,alpha diversity, beta diversity and taxonomic differences among these groups were compared to elucidate the regulatory effects ofL. plantarumQ16 on gut microbiota composition.
2. Methods and materials
2.1 Materials and reagents
L. plantarumQ16, isolated from yogurt in Qinghai Province (China),was obtained from the College of Food Science and Technology of Nanjing Agricultural University (Nanjing, China). The commercial kits for triglyceride, total cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST),high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-1β, tumor necrosis factor (TNF)-α, leptin and adiponectin were also purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).The kit for glycerol level was obtained from Applygen Technologies Inc. (Beijing, China), whereas the kit for acetyl-CoA was purchased from Feiya Biotechnology Co., Ltd. (Jiangsu, China). The primary antibodies against carnitine palmitoyltransferase-1α (CPT-1α),peroxisome proliferator activated receptor-α (PPAR-α), sterol regulatory element-binding protein-1c (Srebp-1c), acetyl-CoA carboxylase (ACC), stearyl CoA dehydrogenase-1 (SCD-1), adipose triglyceride lipase (ATGL), diacylglycerol acyltransferase 1 (DGAT1)and fibroblast growth factor (FGF21) were purchased from Affinity Biosciences (Changzhou, China). The primary antibody against fatty acid synthase (FAS) was obtained from Cell Signaling Technology (Massachusetts, USA). The primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peroxisome proliferator-activated receptor-γ-coactivator-1 alpha (PGC-1α),sirtuin 1 (SIRT1), phosphor-AMP-activated protein kinase alpha (p-AMPKα), AMPKα and nuclear respiratory factor 1 (NRF1)were purchased from Beyotime Institute of Biotechnology (Shanghai,China). The secondary antibody was purchased from Boster Biological Technology Co., Ltd. (Wuhan, China). Other analytical reagents were obtained from Sinopharm Chemical Reagent Co.,Ltd. (Shanghai, China).
2.2 Animal experimental design
All animal experiments were strictly carried out according to the Laboratory Animal-Standards of Welfare and Ethics (DB32/T-2911-2016) and approved by the Institutional Animal Care and Use Committee at Nanjing Agricultural University (SYXK-2017-0027). All specific pathogen-free (SPF) and 4-week-old male mice were obtained from Comparative Medical Center of Yangzhou University (Jiangsu,China). All mice were kept in a SPF and temperature-controlled environment with free access to drinking water and food (Xietong Co., Nanjing, China).
After one-week adaption, all mice were randomly divided into three groups and treated for 8 weeks [16]. One group (normal control group, NC) was fed with normal diet (10% energy from fat). One group (high-fat diet group, HFD) was fed with high-fat diet (60% energy from fat), while another group (PL) was fed with high-fat diet and orally administered withL. plantarumQ16 at the dose of 109CFU/mL. Furthermore, the mice in both NC and HFD group were also orally administered with equal volume of sterile saline. At the end of experiment, all mice were fasted for 12 h and sacrificed after collecting peripheral blood sample. The liver samples and colonic content were collected and stored at -80 °C for the following experiments.The peripheral blood samples were centrifuged at 8 000 ×gfor 10 min at 4 °C and the supernatant serum was collected [17].
2.3 Determination of serum parameters
The levels of triglyceride, total cholesterol, AST, ALT, HDL-C,LDL-C, leptin adiponectin, TNF-α and IL-1β in the serum were detected according to manufacturers’ protocols.
2.4 Determination of hepatic parameters
The livers were homogenized in sterile saline and centrifuged at 2 000 ×gfor 10 min at 4 °C [18]. Then, the supernatant was collected for the further experiments. The concentration of triglyceride, total cholesterol HDL-C, LDL-C, glycerol and acetyl-CoA in the hepatic homogenates was determined according to manufacturers’ protocols.The protein content of hepatic homogenates was also detected using BCA assay.
2.5 Histological analysis of liver samples
Histological analysis of liver was performed according to previous literature [19]. The liver samples were kept in 10% formalin (pH 7.0)and washed with normal saline. After embedding in paraffin wax, all samples were sliced into 5 μm section. Subsequently, all slices were stained by hematoxylin and eosin (H&E) and oil O red. Histological images were observed using an optical microscope (Nikon Eclipse E100, Nikon, Co., Japan).
2.6 Western blot analysis
Total protein in the liver was extracted using radioimmunoprecipitation assay (RIPA) buffer supplemented with 1 mmol/L phenylmethylsulfonyl fluoride. The protein concentration of protein extracts was determined using BCA kit. The protein extracts were separated by 6%-15% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred to nitrocellulose (NC)filter membrane (GE Healthcare Life Science, Pittsburgh, USA).After blocking in Tris-buffered saline and 0.1% Tween 20 (TBST)buffer supplemented with 5% skim milk for 2 h, the membranes were incubated with diluent primary antibodies for 12 h at 4 °C.After washing with TBST solution for 3 times, the membranes were incubated with HRP-linked secondary antibody anti-rabbit IgG (Boster Biological Technology Co., Ltd., Wuhan, China). At last, all membranes were scanned using ECL plus solution (Affinity Biosciences, Changzhou, China). Target band density was quantified using Image J software.
2.7 Colonic microbiota analysis
Total bacterial DNA in colonic content was extracted using a TIANamp Stool DNA kit (Tiangen Biotech Co., Ltd., Beijing,China). The extracted DNA and 16S rRNA primers, including 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R(5’-GGACTACHVGGGTWTCTAAT-3’), were used to amplify the sequence from V3-V4 region of microbial 16S rRNA. The amplified gene products were sequenced by Personal Bio-technology Co.,Ltd. (Shanghai, China).
2.8 Statistical analysis
All experimental data were expressed as mean ± standard derivation (SD). Difference among different groups was determined by one-way analysis of variance with Tukey’s test using SPSS 17.0 software. Difference was statistically significant ifP< 0.05.
3. Results
3.1 Effects of L. plantarum Q16 on body weight, fat mass and liver weight of HFD-fed mice
As reported, long-term high-fat diet (HFD) may give rise to lipid accumulation in the body and eventually increased body weight [20].As shown in Fig. 1, HFD treatment significantly increased body weight, fat mass and liver weight as compared to those in the NC group (P< 0.05). By contrast,L. plantarumQ16 could obviously reduce body weight, fat mass and liver weight of HFD-fed mice when compared to those in the HFD group (P< 0.05). These data suggested thatL. plantarumQ16 had a lipid-lowering effect.
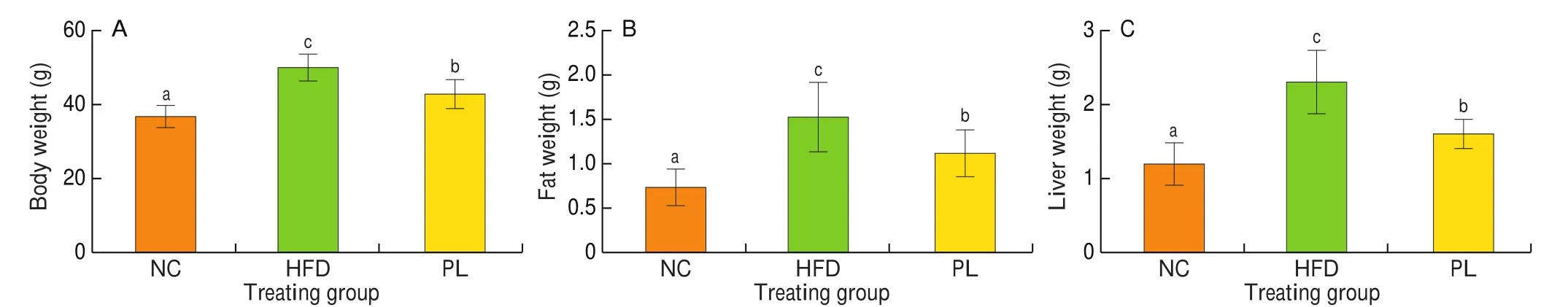
Fig. 1 Effects of L. plantarum Q16 on body weight, fat weight and liver weight in HFD-fed obese mice. Data are presented as mean ± SD (n = 8–10). Different lowercase alphabet letters were significantly different at the level of P < 0.05.
3.2 Effects of L. plantarum Q16 on lipid profile and pro-inflammatory cytokines in the serum of HFD-fed mice
As shown in Fig. 2, HFD treatment notably increased levels of triglyceride, total cholesterol, AST, ALT, LDL-C and leptin in the serum, but decreased serum adiponectin content as compared to NC group (P< 0.05), suggesting that HFD led to lipid metabolism disorder, cholesterol accumulation and hepatic injury in mice. In contrast to HFD group,L. plantarumQ16 significantly decreased triglyceride, total cholesterol, ALT, AST and leptin, but augmented the concentration of HDL-C and adiponectin in the serum (P< 0.05).These results also confirmed thatL. plantarumQ16 effectively improved serum lipid profile.

Fig. 2 Effects of L. plantarum Q16 on serum parameters in HFD-fed obese mice. Data are presented as mean ± SD (n = 8–10). Different lowercase alphabet letters were significantly different at the level of P < 0.05.
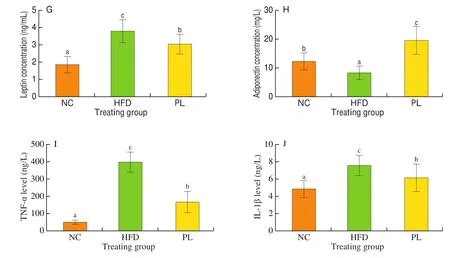
Fig. 2 (Continued)
To determine systemic inflammation status in mice, two major inflammatory cytokines including TNF-α and IL-1β in the serum were detected. As shown in Fig. 2, the concentration of TNF-α and IL-1β in the serum of HFD-fed mice was higher than those in the NC and PL groups (P< 0.05).
3.3 Effects of L. plantarum Q16 on lipid profile in the liver of HFD-fed mice
In order to determine whether HFD contributed to the pathogenesis of non-alcoholic fatty liver disease (NAFLD) andL. plantarumQ16 could ameliorate HFD-induced NAFLD, the content of triglyceride, total cholesterol, HDL-C, LDL-C, glycerol and acetyl-CoA in the liver was detected. As shown in Fig. 3, hepatic triglyceride and LDL-C content in the HFD group was higher than those in the NC group (P< 0.05), suggesting that HFD resulted in lipid accumulation in the liver. Compared to HFD group, lower concentration of triglyceride and LDL-C as well as higher levels of glycerol and acetyl-CoA were observed in the PL group (P< 0.05).As known, glycerol is a main product of triglyceride hydrolysis [21],whereas acetyl-CoA is an end product of fatty acid oxidation [22].Thus, we speculated thatL. plantarumQ16 suppressed hepatic lipid accumulation possibly by promoting fatty acid oxidation and triglyceride hydrolysis.
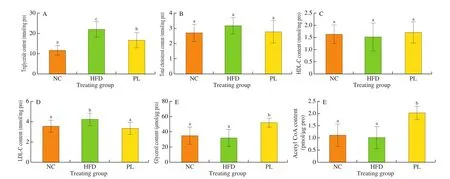
Fig. 3 Effects of L. plantarum Q16 on hepatic parameters in HFD-fed obese mice. Data are presented as mean ± SD (n = 8–10). Different lowercase alphabet letters were significantly different at the level of P < 0.05.
3.4 Histological examination of liver
H&E staining was performed to evaluate the effect ofL. plantarumQ16 on the morphology of liver in HFD-fed mice. As shown in Fig. 4, H&E staining analysis exhibited that the hepatocytes in NC group were uniformly sized, clearly bordered and neatly arranged.By contrast, the liver sample in HFD group displayed differently sized hepatocytes and was filled with lipid droplets. To further con firm this observation, oil O red staining analysis was carried out. In contrast to the NC group, the liver in the HFD group exhibited many red lipid droplets. These results further con firmed that HFD contributed to the development of NAFLD. Meanwhile, biochemical, H&E and oil O red analysis revealed thatL. plantarumQ16 could ameliorate HFD-induced NAFLD in HFD-fed mice.
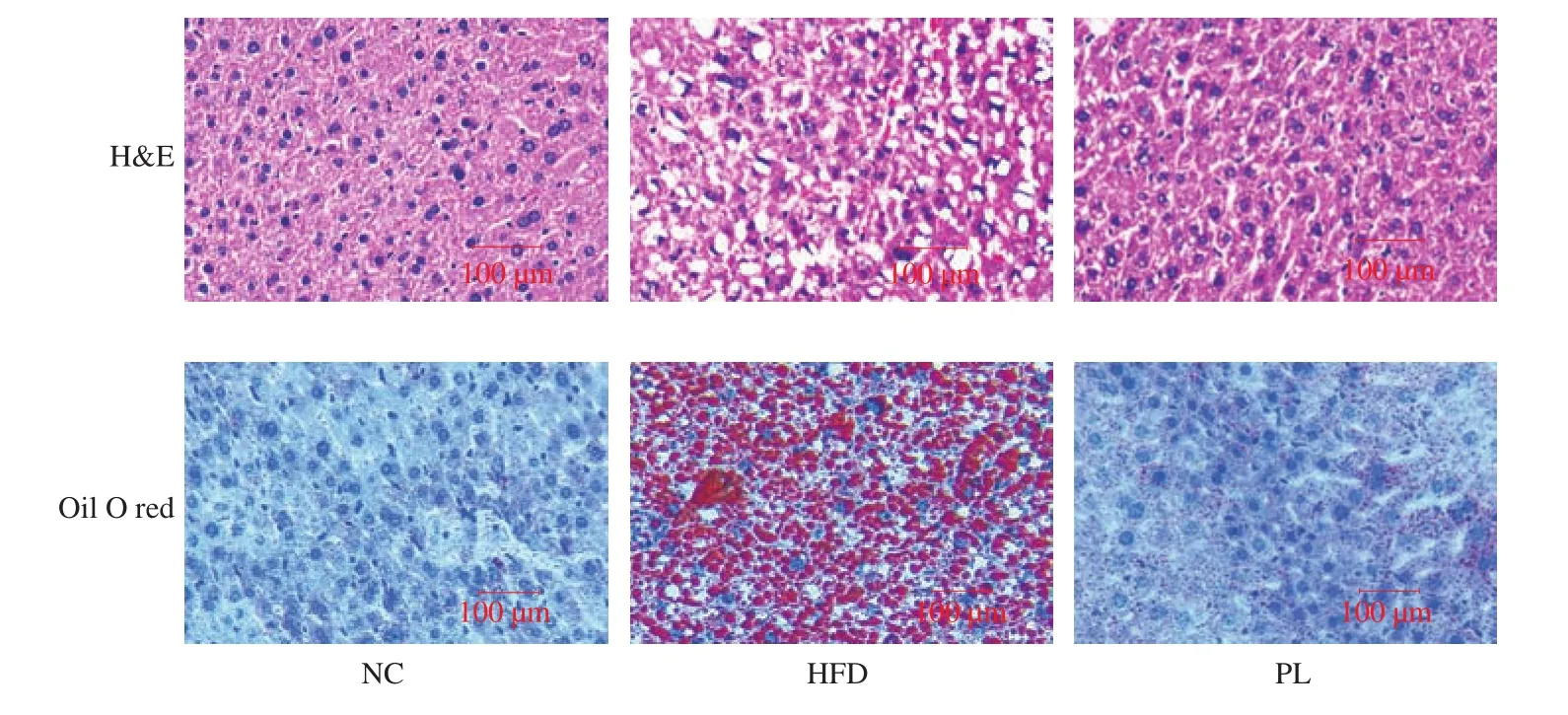
Fig. 4 Histological images of liver.
3.5 Effects of L. plantarum Q16 on key proteins involved in lipid metabolism in the liver of HFD-fed mice
To further determine the regulatory effects ofL. plantarumQ16 on lipid metabolism, the translational levels of key proteins involved inde novolipogenesis, fatty acid oxidation, triglyceride synthesis and triglyceride hydrolysis were detected. As shown in Fig. 5, HFD treatment notably increased protein levels of ACC, SCD-1, Srebp-1c and DGAT1, but reduced the expression of CPT-1α, PPAR-α and ATGL in the liver when compared to the NC group (P< 0.05). In contrast to HFD group,L. plantarumQ16 decreased expression levels of FAS, ACC, SCD-1, Srebp-1c and DGAT1, but enhanced protein expression of CPT-1α, PPAR-α and ATGL in the liver of HFD-fed mice (P< 0.05). Considering that FAS, ACC, SCD-1 and Srebp-1c participate inde novolipogenesis, while CPT-1α and PPAR-α are involved in mitochondrial fatty acid oxidation. Meanwhile, ATGL and DGAT1 mediate triglyceride hydrolysis and synthesis, respectively.These data indicated thatL. plantarumQ16 improved hepatic lipid metabolism by suppressingde novolipogenesis and triglyceride synthesis, and promoting fatty acid oxidation and triglyceride hydrolysis.

Fig. 5 Effects of L. plantarum Q16 on key proteins involved in hepatic lipid metabolism in HFD-fed obese mice. Data are presented as mean ± SD (n = 6).Different lowercase alphabet letters were significantly different at the level of P < 0.05.
3.6 Effects of L. plantarum Q16 on key proteins involved in energy metabolism in the liver of HFD-fed mice
Disregulation of energy metabolism in the liver is closely associated with the pathogenesis of NAFLD. As depicted in Fig. 6,lower expression levels of PGC-1α, p-AMPKα, FGF21 and SIRT1 were observed in the HFD group as compared to the NC group (P< 0.05). In contrast to HFD group,L. plantarumQ16 significantly increased the protein levels of PGC-1α, p-AMPKα and FGF21 in the liver of HFD-fed mice (P< 0.05), suggesting thatL. plantarumQ16 promoted mitochondrial biogenesis and energy expenditure.
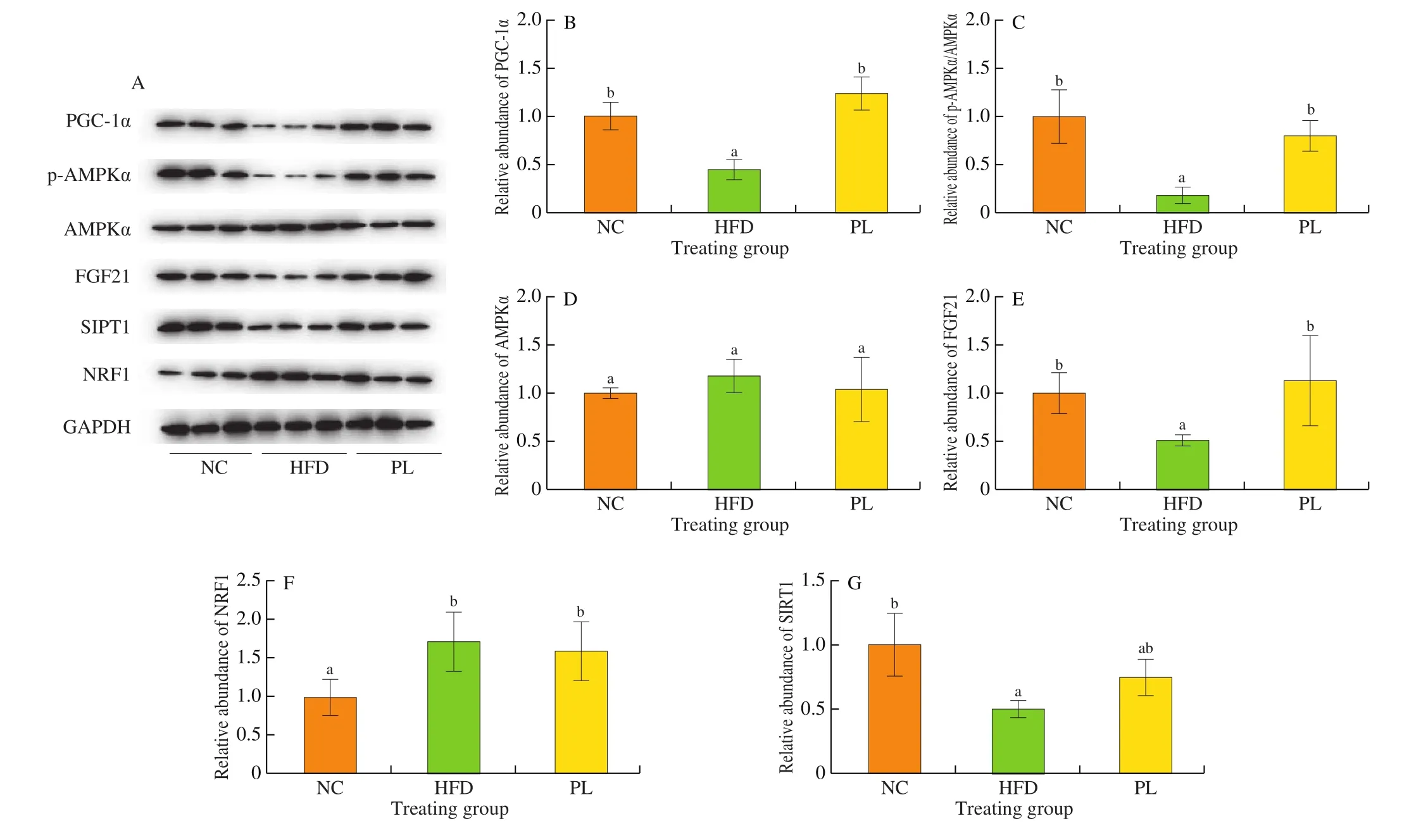
Fig. 6 Effects of L. plantarum Q16 on key proteins involved in hepatic energy metabolism in HFD-fed obese mice. Data are presented as mean ± SD (n = 6).Different lowercase alphabet letters were significantly different at the level of P < 0.05.
3.7 Effects of L. plantarum Q16 on colonic microbiota composition in HFD-fed mice
As previously reported, the pathogenesis of metabolic diseases was closely associated with specific alteration in intestinal microbiota composition [23]. To better determine lipid-lowering mechanism ofL. plantarumQ16, the alteration in colonic microbiota composition among these groups was detected. The Unweighted UniFrac principle coordinate analysis (PCoA) and non-metric multidimensional scaling (NMDS) analysis showed that colonic microbiota composition in these groups was obviously different (Figs. 7c and 7d). The rank abundance curve exhibited that microbial evenness in the HFD group was relatively lower than other two groups (Fig. 7a). The Venn diagram showed that these groups only shared 1 115 operational taxonomic units (OTUs) within colonic microbiota (Fig. 7b), further suggesting that colonic microbiota composition in these groups was viable. Moreover, alpha diversity analysis including Chao1, Shannon and Simpson index was carried out to determine the diversity and richness of colonic microbiota. As shown in Fig. 8, HFD treatment did not change Chao1 index and observed species (P> 0.05), but strikingly decreased Shannon and Simpson index (P< 0.05). In contrast to HFD group, Chao1 index, observed species, Shannon index and Simpson index were notably increased (P< 0.05). As reported, Chao1 index represented species richness, while Shannon and Simpson index indicated species diversity. Therefore, these results suggested thatL. plantarumQ16 could increase species richness and diversity of colonic microbiota.

Fig. 7 The (a) rank abundance curve, (b) Venn diagram, (c) PCoA and (d) NMDS analysis of colonic microbiota in each group.
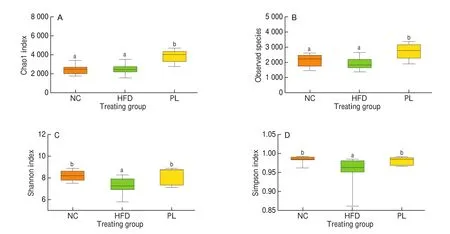
Fig. 8 Effects of L. plantarum Q16 on alpha diversity index within colonic microbiota in HFD-fed obese mice. Data are presented as mean ± SD (n = 6–10).Different lowercase alphabet letters were significantly different at the level of P < 0.05.
At the phylum level, the 4 dominant microorganisms in these groups were Bacteroidetes, Firmicutes, Proteobacteria and Actinobacteria (Fig. 9). As compared to the NC group, HFD treatment reduced the relative abundance of Verrucomicrobia and Actinobacteria,but increased the ratio of Firmicutes to Bacteroidetes (P< 0.05).In contrast to the HFD group,L. plantarumQ16 significantly decreased Proteobacteria abundance and the ratio of Firmicutes to Bacteroidetes (P< 0.05).
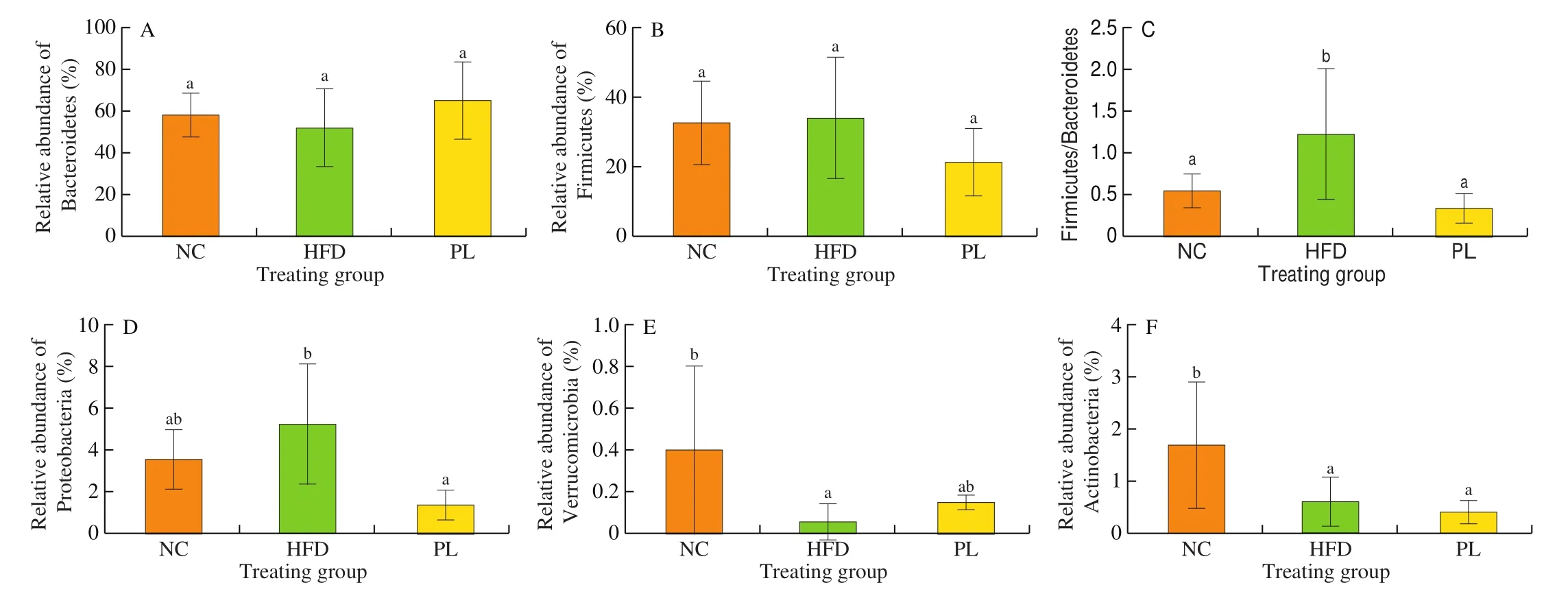
Fig. 9 Effects of L. plantarum Q16 on colonic microbiota at the phylum level in HFD-fed obese mice. Data are presented as mean ± SD (n = 6–10). Different lowercase alphabet letters were significantly different at the level of P < 0.05.
At the family level, 9 predominant families were analyzed (Fig. 10).As compared to the NC group, HFD treatment increased the abundance of Rikenellaceae, Lachnospiraceae, Paraprevotellaceae,Odoribacteraceae and Desulfovibrionaceae, but decreased Lactobacillaceae and S24-7 population in the colon (P< 0.05).In contrast to the HFD group,L. plantarumQ16 augmented the relative abundance of S24-7 and Lactobacillaceae, and inhibited the proliferation of Rikenellaceae, Lachnospiraceae, Odoribacteraceae and Desulfovibrionaceae in the colon (P< 0.05).
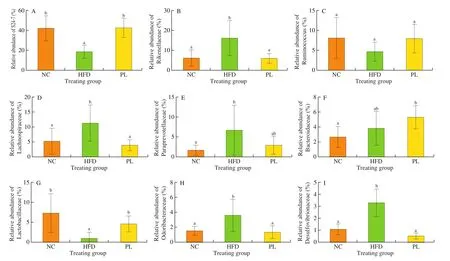
Fig. 10 Effects of L. plantarum Q16 on colonic microbiota at the family level in HFD-fed obese mice. Data are presented as mean ± SD (n = 6–10). Different lowercase alphabet letters were significantly different at the level of P < 0.05.
At the genus level, HFD group displayed higher levels ofOscillospira,Alistipes,Odoribacter,Akkermansia,Desulfovibrio,Allobaculum,HelicobacterandMucispirillum, and lower proportion ofLactobacillus,RikenellaandAdlercreutziathan those in the NC group (P< 0.05) (Fig. 11). As compared to the HFD group,L. plantarumQ16 consumption notably increased the relative proportion ofPrevotella,Lactobacillus,RikenellaandParabacteroides, but decreased relative abundance ofOscillospira,Alistipes,Odoribacter,Akkermansia,Desulfovibrio,Allobaculum,HelicobacterandMucispirillumin the colon (P< 0.05). These results con firmed thatL. plantarumQ16 regulated colonic microbiota at the phylum, family and genus level.
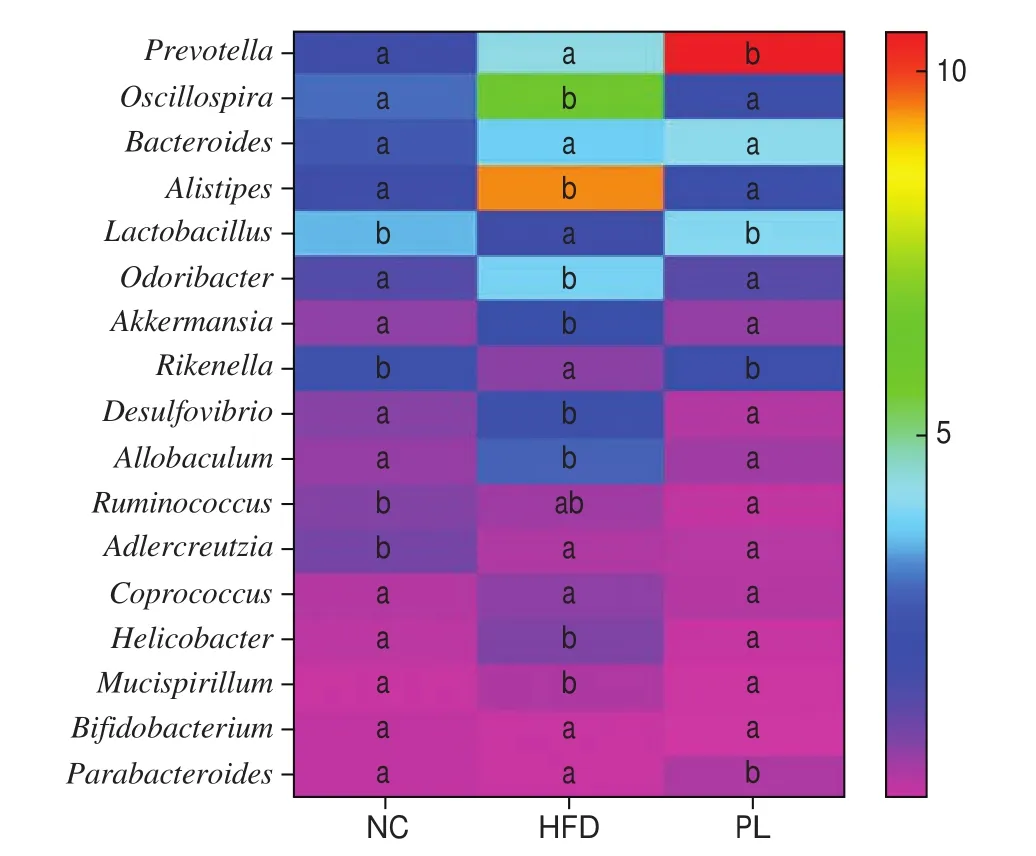
Fig. 11 Effects of L. plantarum Q16 on colonic microbiota at the genus level in HFD-fed obese mice. Data are presented as mean ± SD (n = 6–10).Different lowercase alphabet letters were significantly different at the level of P < 0.05.
4. Discussion
Given that the specific alteration in intestinal microbiota composition is a key factor to induce the pathogenesis of NAFLD,probiotics have been considered as a promising strategy for ameliorating NAFLD. In our study, we have discovered thatL. plantarumQ16 reduced the weight of the body, liver and adipose tissue, and also improved lipid profile in the serum and liver of HFD-fed mice (Figs. 1, 2 and 3). In addition to the improvement of lipid profile,L. plantarumQ16 also reduced leptin concentration in the serum (Fig. 2). Similar to insulin, leptin mainly regulates food intake and energy expenditure, further suppressing lipid accumulation and reducing body weight [24]. However, high concentration of leptin in the blood may trigger leptin resistance and further aggravates metabolic abnormality and contributes to the development of NAFLD [25].Therefore, we speculated thatL. plantarumQ16 may improve hepatic and circulating lipid profile partly by inhibiting leptin resistance. Our future investigation will explore whetherL. plantarumQ16 could regulate leptin signaling pathway.
As known, disregulation of lipid metabolism contributes to lipid accumulation in the body. Under normal circumstance, hepatic lipid metabolism mainly consists of fatty acid oxidation, triglyceride hydrolysis, triglyceride synthesis andde novolipogenesis. When the levels of triglyceride synthesis andde novolipogenesis exceed the rate of triglyceride hydrolysis and fatty acid oxidation, fat droplets would be accumulated in the liver. H&E and oil O red staining analysis also confirmed that HFD treatment contributed to hepatic fat accumulation (Fig. 4). Hepatic lipid metabolism is regulated by numerous genes and proteins. ACC is a rate-limiting enzyme that catalyzes acetyl-CoA into malonyl-CoA, which inhibits the expression of carnitine palmitoyltransferase-1 (CPT1) and consequently blocks mitochondrial fatty acid oxidation. SCD-1 is a rate-limiting enzyme and participates in fatty acid synthesis [26]. As reported, elevated SCD-1 expression suppressed fatty acid oxidation and promoted triglyceride synthesis [27].Thus, ACC, SCD-1 and FAS are three important proteins involved in de novo lipogenesis. Srebp-1c is a main regulator of lipogenesis and also promotes the expression of other lipogenic genes, includingFASandSCD-1[28]. As known, CPT1 is a rate-limiting enzyme that controls mitochondrial fatty acid oxidation by regulating the entry of long chain fatty acid into the mitochondria [29]. It is also reported that high CPT1 expression contributes to high ratio of fatty acid oxidation and suppressed triglyceride accumulation in the hepatic cells [30]. PPAR-α, a nuclear hormone receptor, also enhances mitochondrial fatty acid oxidation by increasing the expression of CPT-1α [21]. The main function of ATGL is to transform triglyceride into free fatty acids and glycerol. Previous studies also revealed that ATGL knockout mice exhibited lower level of lipolysis and higher fat mass than those in wild counterparts [31,32]. Similarly,another report also discovered that knockdown of ATGL in the liver triggered hepatic steatosis and reduced triglyceride hydrolysis rate in the mice [33]. Meanwhile, ATGL deficiency also reduced the gene expression of PPAR-α, leading to impairment of mitochondrial respiration and fatty acid oxidation [21]. All these results highlighted the important role of ATGL in lipolysis. DGAT1 is an important enzyme that synthesizes triglyceride [26]. Our results showed thatL. plantarumQ16 improved hepatic lipid metabolism and alleviated NAFLD by increasing the expression levels of PPAR-α, CPT-1α and ATGL, and reducing the expression of FAS, ACC, SCD-1,Srebp-1c and DGAT1 (Fig. 5), suggesting thatL. plantarumQ16 promoted triglyceride hydrolysis and mitochondrial fatty acid oxidation as well as suppressed triglyceride synthesis andde novolipogenesis. Consistent with our results, other reports also found that some probiotics includingL. plantarumHAC01,L. sakiO67 andL. ke firiDH5 could regulate genes responsible for lipid metabolism in the liver and adipose tissue of HFD-fed mice, further reducing fat mass in the target organs and tissues [10,34,35].
Mitochondrial dysfunction and disregulation of energy metabolism also trigger the pathogenesis of metabolic disorders.For example, lipid accumulation-induced mitochondrial dysfunction resulted in reactive oxygen species (ROS) overproduction and impaired mitochondrial fatty acid oxidation [36]. It is well known that mitochondria are the main organelle for energy production [37].Mitochondrial biogenesis is a biological process that mainly increases mitochondrial mass to promote ATP production [38]. PGC-1α is a critical protein that regulates mitochondrial biogenesis. The activation of PGC-1α enhances mitochondrial biogenesis by coordinating with nuclear respiratory factor (NRF) 1 and 2. Subsequently,the coordination of PGC-1α with NRF1 activates mitochondrial transcription factor A (TFAM), which consequently stimulates the transcription and replication of mitochondrial DNA [39]. SIRT1 can deacetylate PGC-1α and indirectly promotes mitochondrial biogenesis [38]. AMPK, mainly expressed in the liver and muscle,is the key factor that regulates lipid and energy metabolism. On the one hand, AMPK improves lipid metabolism by downregulating the expression of ACC and Srebp-1c, and increasing CPT1 expression [40]. On the other hand, previous study revealed that FGF21 activation could regulate adiponectin and adiponectin also induced AMPK phosphorylation, which further activated PGC-1α and promoted mitochondrial biogenesis [41,42]. In our study,L. plantarumQ16 was determined to increase serum adiponectin, and the protein levels of PGC-1α, p-AMPKα and FGF21 in the liver (Figs. 2 and 6), suggesting thatL. plantarumQ16 may improve hepatic energy metabolism via regulating FGF21/adiponectin/AMPKα/PGC-1α signaling pathway. Similarly, Chen et al. [43]also discovered thatL. reuteri263 increased energy expenditure in the adipose tissue by increasing the expression of CPT-1, PGC-1α and PRDM16.
Apart from the regulation of energy metabolism, SIRT1/AMPK also inhibits NF-κB activation by deacetylating p65 subunit Lys310 [44,45]. Meanwhile, the changes in adipokines also initiate metabolic inflammation. As leptin deficiency polarizes macrophages and mast cells into anti-inflammatory phenotypes [46], leptin has been considered as a pro-inflammatory adipokine. By contrast, adiponectin possesses abilities to suppress toll-like receptor (TLR) 4-mediated NF-κB activation and shift macrophages towards anti-inflammatory M2 phenotype [47]. Thus,L. plantarumQ16 reduced TNF-α and IL-1β content in the serum partly by activating SIRT/AMPK pathway as well as suppressing adipokine imbalance.
It is well known that the pathogenesis of NAFLD is accompanied by the alteration in intestinal microbiota composition. Similar to other inflammatory diseases, intestinal microorganisms in NAFLD patients are characterized by increased pathogens, reduced commensals and less microbial diversity. In our study, HFD treatment decreased species diversity and increased the ratio of Firmicutes and Bacteroidetes (Figs. 8 and 9), which was consistent with other studies [19,48].Verrumicrobia, colonized in the human intestine, is a beneficial bacterium that can inhibit chronic low-grade inflammation [49].By contrast, Proteobacteria contains numerous opportunistic pathogens and its proliferation is a potential signature of bacterial dysbiosis [50], which subsequently impairs gut barrier and causes endotoxin translocation into the circulation. S24-7 produces butyrate and is beneficial to intestinal epithelial health [51]. As previously reported, HFD increased the proportion of Rikenellaceae and Mucispirillum, both of which were involved in the development of NAFLD. Meanwhile, pathogen Mucispirillum could colonize in the mucosal barrier and triggers gut inflammation [52]. Similarly,Desulfovibrionaceae produces endotoxin and further impairs gut barrier integrity [48]. In addition to inducing intestinal inflammation,Desulfovibrionaceae also contributes to lipid accumulation in the body by upregulating CD36 expression and increased uptake of long-chain fatty acids [53]. Additionally, previous study revealed that the relative abundance ofAlistipeswas positively correlated with increased levels of lipid profile and inflammatory cytokines,such as body weight, serum triglyceride and IL-6 expression [54].Human investigation also confirmed these results [55]. The correlation analysis exhibited thatAllobaculumwas positively associated with body weight, liver weight, fat mass and serum triglyceride concentration [56], whereas the relative abundance ofParabacteroideswas negatively correlated with the pathogenesis of obesity [57]. Owing to its abilities to degrade human mucin O-glycans and utilizes liberated non-terminal monosaccharides,A. mucolyticumbelonging toAllobaculumgenus could destroy intestinal epithelium and has been thought as one type of inflammatory bowel disease-associated species [58]. AlthoughAkkermansiahas anti-obesity effect in humans and animals,Akkermansiaalso utilizes mucus in the gut and causes intestinal inflammation [59], suggesting the controversial roles ofAkkermansiain human health. The sulfate-reducing bacteriumDesulfovibriopossesses ability to convert sulfate into hydrogen sulfide and high content of hydrogen sulfide consequently impairs intestinal mucosal barrier, leading to intestinal and systemic inflammation [60]. Similarly, harmful microorganismHelicobacteralso disrupts intestinal micro-environment and causes gut inflammation [61]. Our findings showed thatL. plantarumQ16 increased the relative abundance of S24-7, Lactobacillaceae,Lactobacillus and Parabateroides, but reduced the proportion of Rikenellaceae, Lachnospiraceae, Desulfovibrionaceae,Alistipes,Odoribacter,Akkermansia,Desulfovibrio,Allobaculum,HelicobacterandMucispirillumin the colon of HFD-fed mice (Figs. 9-11),indicating thatL. plantarumQ16 promoted the growth of commensals and inhibited the proliferation of opportunistic microorganisms. This may be due to the colonization ofL. plantarumQ16 in the colon andL. plantarumQ16-produced antimicrobial molecules against pathogens. Meanwhile, decreased relative abundance of pathogenic microorganisms byL. plantarumQ16 also reduced endotoxin content in the blood and alleviated chronic low-grade inflammation. Our future investigation will explore anti-inflammatory mechanism ofL. plantarumQ16.
5. Conclusion
Our study found thatL. plantarumQ16 had protective effects against HFD-induced NAFLD. Biochemical and molecular analysis elucidated thatL. plantarumQ16 alleviated NAFLD by improving hepatic lipid and energy metabolism, and regulating gut microbiota composition. In detail,L. plantarumQ16 could suppress hepatic lipid accumulation by inhibitingde novolipogenesis and triglyceride synthesis, but promoted triglyceride hydrolysis and mitochondrial fatty acid oxidation. In addition,L. plantarumQ16 improved energy metabolism via FGF21/adiponectin/AMPK/PGC-1α signaling pathway (Fig. 12). Metagenomic analysis also discovered thatL. plantarumQ16 increased species diversity and richness of intestinal microbiota, promoted proliferation of beneficial commensals and suppressed the growth of endotoxin-producing microorganisms in the colon of HFD-fed mice. Our findings showed thatL. plantarumQ16 had potential applications in the field of functional foods owing to its lipid-lowering property.
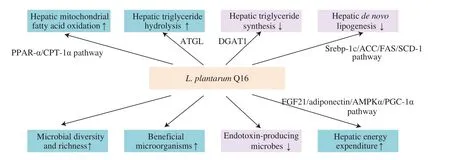
Fig. 12 Possible protective mechanism of L. plantarum Q16 against HFD-induced NAFLD.
conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors acknowledge the financial support for this work provided by the National Natural Science Foundation of China(31771948 & 32072182).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
