The levels of osteopontin in human milk of Chinese mothers and its associations with maternal body composition
2022-06-23HuijunRunQingyTngXunZhoYjieZhngXuelinZhoYiXingWeiGengYiFengWeiCi
Huijun Run, Qingy Tng, Xun Zho, Yjie Zhng, Xuelin Zho,Yi Xing, Wei Geng, Yi Feng, Wei Ci,c,*
a Department of Clinical Nutrition, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China
b Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition, Shanghai Institute of Pediatric Research,Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine Shanghai 200092, China
c Department of Pediatric Surgery, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China
Keywords:
Osteopontin
Human milk
Body composition
Breastfeeding
A B S T R A C T
Objective: This study explored the content and change trend of osteopontin (OPN) in breast milk and analyzed the relationship between OPN in breast milk and maternal body composition. Methods: Breast-feeding mothers were recruited in Xinhua Hospital to collect breast milk and provide pertinent information.The content of OPN in breast milk was determined by enzyme-linked immunosorbent assay (ELISA).Determination of protein content in human milk was performed via the BCA method. The maternal body composition was determined by the bioelectrical impedance method. Serum glucocorticoid receptor α,adiponectin, insulin, and leptin were determined by ELISA. SPSS 25 was used for statistical analysis.Results: In the longitudinal cohort, 106 mothers provided 318 milk samples at different lactation periods.The results indicate that the OPN showed dynamic changes. OPN levels were (343.2 ± 163.5) mg/L during 1-14 days postpartum, (228.4 ± 121.5) mg/L during 2-4 months postpartum, and (204.8 ± 100.6) mg/L during 5-7 months postpartum. The content of OPN was very high in the first 1-14 days postpartum and then decreased.Compared with early postpartum milk, the OPN content of mature milk showed a significant relationship with maternal weight-related parameters. Additional body composition analysis was performed in 88 women at the mature milk phase. The results show that the OPN in milk is related to the mother’s body composition,especially the content of skeletal muscle mass, but not to relevant humoral factors. Conclusions: The levels of OPN in human milk of Chinese mothers showed dynamic changes with the extension of lactation time. The OPN in human milk was related to the mother’s body composition but not related to related humoral factors.
1. Introduction
In addition to the nutrients necessary for the growth and development of infants, breast milk contains a large number of active proteins. The composition of breast milk varies with lactation time,race, diet, and other factors, which is why breast milk is the ideal food in the early life of infants [1]. Research on breast milk, especially active proteins, has grown significantly in recent years. Many studies have explored several common active proteins’ content and physiological functions, such as lactoferrin and sIgA (secretory immunoglobulin A),but only a few have focused on osteopontin (OPN).
OPN is a multifunctional bioactive protein that is implicated in numerous biological processes, such as bone remodeling, inhibition of ectopic calcification, and cellular adhesion and migration, as well as several immune functions [2-5]. It is present in different tissues, body fluids, and milk. Its primary structure includes an arginine-glycine-aspartic acid. OPN is a non-collagenous protein involved in the biomineralization of bone tissue. Beyond its role in biomineralization, OPN is essential to the quality of collagen fibrils in bones. OPN regulates type I collagen fibril formation in bone tissue [6].OPN has different physiological functions in different tissues. In cardiovascular disease research, OPN serum levels are associated with vascular functions and inflammation in coronary artery disease patients [7].Likewise, serum OPN levels are associated with arterial stiffness and the presence and severity of coronary artery disease [7,8]. OPN also plays a role in the occurrence and development of tumors [9-17]. Recent studies have found that OPN helps reduce liver damage caused by alcohol [18].
OPN widely exists in various human body tissues, and its content is the highest in milk. In recent years, human milk OPN has gradually become a research hotspot. Previous research works have provided some preliminary understanding of the content and functions of OPN in human milk [2,19-26]. Some studies explored the content of OPN in human milk, compared the content difference between human milk OPN and other body fluids, and explored the functions of OPN [4,5,18-20,23,24,26,27]. In 2018, a multi-center study found that the content of OPN in milk varies significantly in different countries and regions [23]. A longitudinal follow-up study of lactating women in the United States found that the content of OPN in their milk decreased rapidly at the early stage and remained stable in the late stage. This trend seems to be similar to lactoferrin and sIgA found in previous studies [28].
Previous studies have initially focused on analyzing the content and change trend of OPN in human milk. After comparing the OPN of breast milk in different countries and regions, Bruun found that the OPN of breast milk in Asian countries, especially China, is higher than that in other countries and regions [23]. However, the influencing factors of its content are still unclear. Although previous studies have discussed the relationship between serum OPN and serum indicators such as leptin, few have reported on milk OPN and serum indicators [29].Previous studies on the composition of breast milk have suggested longitudinal changes in breast milk during lactation, likely due to the immune, growth, and nutritional needs of offspring. But what changes will occur in the later stages of breastfeeding? What are the influencing factors of milk composition in the late stage of breastfeeding? To provide answers to these questions, we conducted a longitudinal observational study, describing the content and change trend of OPN in the milk of postpartum mothers at different lactation periods. We conducted a cross-sectional survey to explore the relationship between OPN and maternal body composition and related humoral factors.
2. Materials and methods
2.1 Objects of study
From December 2019 to December 2020, volunteer enlistment was conducted in Shanghai Xinhua Hospital to recruit Chinese women who have recently given birth and were breastfeeding. All the recruits were Chinese women who lived in Shanghai for at least half a year. The study consisted of two parts: longitudinal and cross-sectional.
The longitudinal cohort included mothers who entered the study process through recruitment in late pregnancy or early postpartum.Before the formal enlistment, the sample size was determined using the sample calculator based on previously published literature and pre-experiment results [30]. To account for a 20% attrition rate,we had to recruit at least 120 people. After appropriate expansion,we finally recruited 123 people. After obtaining written informed consent, milk collection was arranged in the corresponding time range after delivery, and the collection of milk three times was considered as the completion standard. Milk was collected in 1-14 days,2-4 and 5-7 months. Each time the milk was collected, relevant questionnaires were administered, and the contents were reviewed and collected. Personal information was collected through written questionnaires and Wechat interviews. Milk was transported via Cold Chain Express.
2.1.1 Inclusion criteria
Chinese mothers who were breastfeeding within 14 days postpartum or earlier time; living in Shanghai for more than 6 months
2.1.2 Exclusion criteria
Lack of breast milk leading to cessation of breastfeeding at the time of recruitment; inability or unwillingness to provide milk at the time of recruitment; unable to communicate due to language barriers or mental problems; had severe medical condition requiring medication such that the composition of milk may be affected; failure to collect or store breast milk as required
2.1.3 Study termination criteria
Lack of breast milk at follow-up resulted in cessation of breastfeeding; inability or unwillingness to provide milk within the specified follow-up duration; lost contact at follow-up.
The mothers in the cross-sectional cohort were all breastfeeding mothers at the mature milk period. Some were long-term lactating mothers from the longitudinal cohort, while some were recruited separately. After the signing of the written informed consent, the respondents went through face-to-face interviews. During the visit,the respondents were subjected to body composition analysis (BIA method), and some were asked to provide blood samples. The respondents were required to collect breast milk samples at home,which were then collected by the Cold Chain Express.
This study was approved by the Ethics Committee of Hospital with approval number XHEC-C-2020-081, and the necessary patients’written informed consents were obtained.
2.2 Clinical data collection
Interviews and questionnaires were used to collect the basic information of the mothers, including age, height, weight before pregnancy, disease history, family history, drug, and toxic and harmful food contact history, pregnancy, and delivery history. The birth status of infants, including gender, mode of delivery, pregnancy complications, birth weight, and length, were also recorded. The maternal body mass index (BMI) was calculated as weight/height2(kg/m2).The infant birth weight percentile (%) assessment was calculated according to the child’s gender, gestational age, and birth weight according to the Fenton Growth Chart reported in 2013 referred to previous studies [31-33]. In the longitudinal cohort, 123 mothers were initially enlisted, and 106 were able to complete three milk collections and provided complete information. From the longitudinal cohort,18 were included in the cross-sectional cohort, and 70 mothers were recruited for the cross-sectional study. Therefore, the clinical data of 176 mothers were collected.
2.3 Breast milk collection
After detailed communications with the respondents through in person or by WeChat, the mothers were asked to complete the breast milk collection at home. In the morning, before feeding their babies,the mothers were asked to use a device that pumped and emptied milk from one side of the breast. After mixing the milk, 15 mL was taken as a test sample and put into the unified breast milk collection bag,which was then stored at -20 °C for freezing and transferred through the cold chain. After each sample recovery, the milk properties were evaluated. If milk agglutination, stratification, odor, and other conditions were detected, the sample would have to be recollected.The collected and encapsulated milk was stored in a refrigerator at-80 °C until further detection. This process was performed for every breast milk collection.
2.4 Follow up of mothers and infants
The longitudinal cohort was followed up three times, while the cross-sectional cohort was followed up once. The follow-up time of the longitudinal cohort was 1-14 days, 60-120 days (equivalent to 2-4 months p.p), and 150-210 days (equivalent to 5-7 months p.p).The questionnaires were properly collected and included data on the weight change of the mother, feeding situation, and willingness to continue with the research. The flow chart of the longitudinal queue is shown in Fig. 1.

Fig. 1 Study design flow chart (longitudinal queue).
2.5 Determination of breast milk protein and OPN
In the longitudinal cohort, 318 breast milk samples were collected. Eighteen were re-recruited into the cross-sectional study,and 70 were enlisted into the cross-sectional study. A total of 388 milk samples were analyzed. The collected milk was analyzed for OPN with an ELISA kit according to the manufacturer’s instructions(R&D Systems, Inc., Minneapolis, MN, USA) and validated for the quantitative determination of OPN in human milk. The determination process can be found in the description of previous literature [23].Pre-experiments were carried out for each determination, and the results of each sample were tested by ELISA in duplicated wells.The protein of human milk was determined using the BCA method.The BCA Protein Assay Kit was used to determine protein in breast milk according to the manufacturer’s instructions (Shanghai Westang Bio-Tech Co., Ltd., China). The theoretical basis and experimental process are found in the description of previous literature [34]. The test results were then saved in a designated computer in Excel format.
2.6 Anthropometric measurements and body composition
Eighty-eight mothers from the cross-sectional cohort were measured for their body composition using unified equipment (InBody 720, Biospace Co., Ltd., Seoul, Korea) and were asked to fast in the morning. After defecating, their body composition was analyzed via the validated electrical impedance (BIA) method [35].
Height measurements were performed using Seca-217 telescopic height gauge. The ambient temperature was maintained at 25-28 °C.Body weight, BMI, body fat percentage, visceral fat area, skeletal muscle mass, and bone mineral content were measured, and the measurement results were stored in a printed report.
2.7 Collection and determination of blood samples
Blood samples were acquired from 37 mothers from the cross-sectional cohort, with each test subject providing additional written consent. Venous blood was collected by a qualified nurse who had worked for 13 years. The blood samples were let to stand before being placed into the centrifuge. Serum was then taken and stored at-20 °C until detection. The collected sera were analyzed for insulin,human glucocorticoid receptor α (GRα), adiponectin, and leptin with an ELISA kit according to the manufacturer’s instructions. The test results were saved in a designated computer in Excel format.
2.8 Statistical analysis
Statistical analysis was performed using SPSS Statistics version 25.0 statistical software (IBM Co., Armonk, NY, USA). Continuous variables were presented as mean ± SD. Pearson’s correlation analysis was used to assess the correlation between OPN and other indicators.Partial correlation analysis was used after controlling variables.One-way analysis of variance (ANOVA) was used to compare the difference, while the least square distance (LSD) method was used to compare data within groups. Multiple linear regression was used to analyze the relationship between OPN and human composition.P< 0.05 indicated statistical significance. R language and Prism 9.0 were used to make figures.
3. Results
3.1 Characteristics of all the subjects
A total of 123 women participated in the longitudinal study, with 106 were able to complete the study and 17 mothers withdrawing,equivalent to a completion rate of 86.2%. The main reasons for non-completion included: breastfeeding was stopped due to various reasons, including milk shortage, recurrent mastitis, infant diseases,and family accidents (16/17, 94.12%). One of the respondents lost contact (1/17, 5.88%). The descriptive statistics for the 106 pairs of maternal and infant participants are summarized in Table 1.
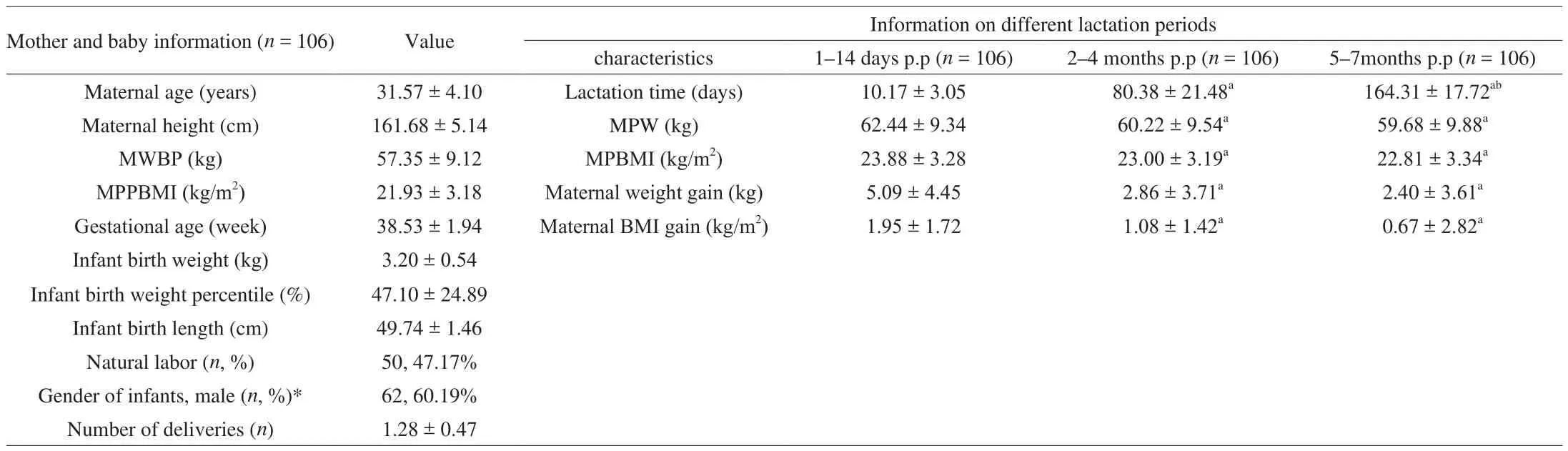
Table 1Characteristics of 106 subjects.
3.2 Content and change trend of OPN in breast milk at different lactation stages
In the longitudinal cohort, OPN, protein, and the OPN percentage in protein showed downward trends. OPN contents were (343.2 ±163.5) mg/L during 1-14 days postpartum, (228.4 ± 121.5) mg/L during 2-4 months postpartum, and (204.8 ± 100.6) mg/L during 5-7 months postpartum. The protein contents in mother’s milk were (2.3 ±0.7) g/dL during 1-14 days postpartum, (1.6 ± 0.4) g/dL during 2-4 months postpartum, and (1.6 ± 0.4) g/dL during 5-7 months postpartum. The OPN percentages in protein were (1.7 ± 0.9)% during 1-14 days postpartum, (1.4 ± 0.7)% during 2-4 months postpartum, and (1.3 ± 0.6)% during 5-7 months postpartum. The comparison results for the different time-points are shown in Fig. 2.The correlations between breast milk OPN level and the maternal and infant factors at different breastfeeding times are shown in Fig. 3.Compared with early postpartum milk, the OPN content in mature milk showed a significant relationship with maternal weight-related parameters.

Fig. 2 OPN, protein and OPN/pro in the breast milk of different lactation stages. p.p: postpartum; milk protein (g/dL): the protein determined by BCA method;OPN/pro (%): the proportion of OPN in milk protein.

Fig. 3 Partial correlation coefficients between analyzed variables. Only significant correlations are shown (P < 0.05) either in blue (positive) or in red (negative).Color intensity and size of the ellipse are proportional to the correlation coefficients. a: refers to the data of 1-14 days p.p; b: refers to the data of 2-4 months p.p;c: refers to the data of 5-7 months p.p; baby’s age: baby’s age of the collection day; milk protein: the protein was determined by BCA method; OPN/pro: the proportion of OPN in milk protein.
3.3 The relationship between the level of OPN in milk and the body composition of mothers
The body compositions of 88 mothers were analyzed in the cross-sectional cohort. Human body composition feature distribution in the pooled data was divided into quartiles (Groups 1-4) based on small to large values. The average lactation time for mothers was(209 ± 167) days, representing a longer breastfeeding period. When the lactation times were compared, the results showed no significant difference in the lactation time in each group. The results are shown in Fig. 4.
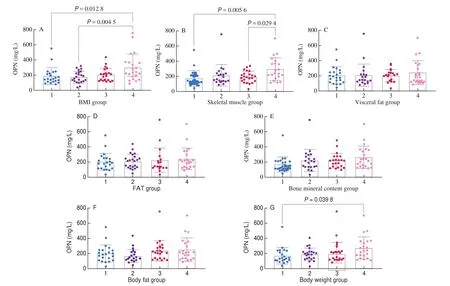
Fig. 4 The level of OPN under different human body composition and parameters grouping. The parameters of human body composition: body fat (kg), body fat percentage (FAT) (%), visceral fat area (cm2), and skeletal muscle mass (kg).
Correlation analysis showed that OPN was positively correlated with body weight (kg), bone mineral content (kg), skeletal muscle mass (kg), body fat (kg), BMI, and visceral fat area (cm2) (allP< 0.05).After controlling the lactation time, all positive correlations between the above indicators were found to be significant (allP< 0.05).
Multiple linear regression was used for further analysis.Collinearity diagnostics were conducted according to the variance inflation factor (VIF) [36]. To explore the relationship between different parameters in body composition and OPN, candidate indexes were incorporated into the multiple linear regression model.Single-factor analysis was performed. IfP< 0.05, the variable was included in the multivariate regression analysis as a candidate variable.After the correlation analysis and the collinearity diagnostics, the candidate parameters selected into the regression equation were bone mineral content (kg), skeletal muscle mass (kg), and body fat (kg), which reflect the leading indicators of human composition. The stepwise regression method was used, and the skeletal muscle mass was successfully entered into the model. The statistical results are listed in Table 2.

Table 2Statistics of multiple linear regression.
3.4 The relationship between the level of OPN in milk and serum indexes
Of the 88 respondents, 37 were tested for serum indicators. The relationship between the results and OPN is presented in Fig. 5. No significant linear relationship was found.

Fig. 5 The relationship between the serum indicators and OPN. LEP: leptin; INS: insulin.
4. Discussion
The present study investigated the content and trend of OPN in Chinese mothers’ milk and investigated the correlation between OPN and maternal body composition. The relationship between OPN and humoral factors related to glucose and lipid metabolism was also investigated.
In this study, we found that the content of OPN in colostrum and transition milk from Chinese mothers was highest and that the level of OPN in mature milk decreased significantly. There was no significant difference between the early and late stages of mature milk. The same trend was observed in the OPN percentage in protein, similar to the findings in previous studies [5,23]. The milk OPN level of Chinese mothers in our study is still very different from previous studies,which may be due to variations in genetic background, lactation periods, and other factors [5,23]. The current study found that OPN was related to fetal factors during different postpartum lactation periods. Similar to these results, a 2014 study investigated the content of OPN in umbilical cord blood and found that the level of OPN was related to gestational age [37]. These various findings support that OPN is closely related to the situation of infants from pregnancy to postpartum.
As reported in previous literature, OPN, as an active protein,plays various roles in the early life of infants, such as promoting development and regulating immunity [3,4,20,21,24,27,38]. In the early stage of postpartum lactation, to meet the needs of infant growth and development and promote immunity, the composition of breast milk keeps changing. In the present study, we found a dynamic relationship between the content of OPN and fetal factors. However,from 5-7 months postpartum, the findings suggest a relationship between the mother’s body weight and weight-related indicators.At the later stage of mature milk, the level of OPN in breast milk decreases until it reaches a stable state. This stable level reflects the characteristics of the mother’s own body weight and weight-related indicators in the present study. Similar to a recently published article,OPN does seem to be related to the weight-related parameters of lactating mothers [39]. This interesting phenomenon seems to embody the intelligent change in breast milk [28,40-42].
We further evaluated the body composition of women in the mature milk stage. The level of OPN was related to the body composition of the mother, especially skeletal muscle mass. Previous investigations have studied the relationship between serum OPN and human composition. In 2019, diabetes patients undergoing bariatric surgery were evaluated [43]. Baseline serum OPN and anthropometric and biochemical variables were assessed in 41 patients with type 2 diabetes who were treated with bariatric surgery and reevaluated one to three years after surgery. In the overall cohort of T2DM remission and non-remission patients, baseline circulating levels of OPN significantly correlated with reductions in body weight and BMI over time. This suggests that the OPN level could be used to predict diabetes remission in patients after bariatric surgery. In 2019,researchers studied serum OPN in patients with non-alcoholic fatty liver disease with type 2 diabetes mellitus and found that serum OPN levels were higher in the overweight/obese group than in the lean group [44].
The present study found a correlation between OPN content in mature milk and skeletal muscle, which had not been reported in previous literature. Similar to previous studies, we observed that OPN was associated with body weight in postpartum women. However,there were some contradictory findings. In 2020, by detecting the OPN level in the blood of children aged 0-18, scholars found that OPN plasma concentrations did not show any association with weight,height, BMI, and body surface area when tested by partial correlation while controlling for the effect of age [22]. Different findings may be due to variations in the characteristics of the study population. For example, the peak expression of OPN in newborns/postpartum may be due to increased bone turnover. Although OPN is a bone endocrine hormone, OPN in mother’s milk was not found to be related to bone mineral content in the present study. However, the results suggest a correlation between OPN and muscle content. The potential cause of our result may be that we used the BIA method in this study, which is not the gold standard for measuring SMM and bone content. Based on these preliminary findings, further accurate research is needed to explore the correlation mechanism between OPN content in breast milk and maternal composition.
We also collected blood and determined the humoral factors related to glucose and lipid metabolism [45-51]. Leptin is a hormone secreted by adipose tissue. Its content in serum is directly proportional to the size of animal adipose tissue. Adiponectin (ADP)is an endogenous bioactive polypeptide or protein secreted by adipocytes. Insulin is produced by the islets in the pancreas.It is secreted by cells stimulated by endogenous or exogenous substances (e.g., glucose, lactose, ribose, arginine, and glucagon).Insulin is an essential parameter of glucose metabolism. GRα is a particular type of receptor, which exists in human cells. Specific hormones can combine with it to perform its function. However, no relationship between OPN in milk and the levels of these factors was found in the present study. Similar to our results, Jeong et al. [29]measured leptin and adiponectin levels before and after weight loss to understand the effects of fat mass reduction on the serum level of adipokines mainly produced in adipose tissue. Serum leptin level decreased significantly and was closely related to the percentage of body fat. In addition, there was a negative correlation between serum adiponectin level and body fat percentage, but there was no significant change in serum adiponectin level. Serum OPN levels decreased significantly after this regimen. However, serum OPN levels were not correlated with the percentage of body fat and related factors [29]. The possible explanation is that serum osteopontin may be more affected by factors other than adipose tissue. However, this hypothesis needs further investigation and analysis.
We hypothesized that changes in OPN with lactation were associated with maternal weight. As a supplement and indirect test of this hypothesis, the correlation between OPN and humoral factors was also determined. Compared with previous investigations, the main strengths of this study are its use of a longitudinal design and a larger sample size. These allowed us to explore deeper and observe the relationship between OPN in breast milk and maternal body composition, which has not been previously reported. Our findings on OPN in human milk suggest a correlation between maternal factors (body weight, weight-related parameters and human composition) and composition for late mature milk. Our study concludes the following: if the mother is in a good condition (in our study, good condition is based on weight factors and human composition) while breastfeeding, the change of milk (in our study,it refers to OPN) will significantly benefit the child. Further research should explore how different maternal factors could affect the infant health and development through breastfeeding.
This study has several limitations. One major limitation is the short follow-up time. Due to various factors, the proportion of those breastfeeding for more than six months was small and would have to be followed up and reassessed in subsequent tests. Also, due to our recruitment method and the residential locations of participants, our respondents were mainly highly educated with a high breastfeeding awareness, which could have affected the results. Another shortcoming is that we analyzed human body composition based on the BIA method which is not necessarily the gold standard, SMM and bone mineral content are indirect indicators, although there have been previous studies supporting the use of the BIA method in evaluating human body composition [52,53].
5. Conclusions
The present study preliminarily described the content and change trend of OPN in milk through follow-up of the longitudinal mother-infant cohort. By exploring human body composition and related humoral factors, we found that OPN in milk is related to maternal body composition, especially skeletal muscle content. The results suggest more in-depth research to explore the correlation between breast milk composition and a mother’s personal factors.Subsequent tests should also explore the mechanism of dynamic changes in breast milk composition to provide a strong theoretical basis and support references for breastfeeding.
conflict of interest
The authors have no conflicts of interest relevant to this article.
Statement of ethics
This study was approved by the Ethics Committee of Xinhua Hospital with the approval number XHEC-C-2020-081 and with appropriate participants’ informed consent in compliance with the Helsinki Declaration. Written informed consents were obtained from the patients.
Acknowledgement
This study was supported by grants from the Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition(17dz2272000) and the National Natural Science Foundation of China-Key Program (81630039).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
