Characteristic and effect analysis of protein and peptide in Cantonese cured meat processing
2022-06-23ZhiQuChunqinFengRuilingLiNnLiuShnqingZheng
Zhi Qu, Chunqin Feng, Ruiling Li,*, Nn Liu,b,c,*, Shnqing Zheng*
a Institute of Chronic Disease Risks Assessment, School of Nursing and Health, Henan University, Kaifeng 475004, China
b College of Public Health, Zhengzhou University, Zhengzhou 450001, China
c Institute of Environment and Health, Pinghu Hospital, Shenzhen University, Shenzhen 518116, China
d School of Basic Medical Sciences, Henan University, Kaifeng 475004, China
Keywords:
Cantonese cured meat
Antioxidant activity
Sarcoplasmic proteins
Myo fibrillar proteins
A B S T R A C T
The aim of this work was to explore the physicochemical and structural properties, lipid oxidation and antioxidant capacity of the peptides extracted from Cantonese cured meat and as well as to investigate the effect of drying time on the sarcoplasmic and myofibrillar proteins of Cantonese cured meat. The results suggested that salting out, protein oxidation and heat treatment were closely related to surface hydrophobicity and the secondary structure of peptides was changed by processing. And the peroxide value and the value of tributyltin compounds were different in evaluating the degree of lipid oxidation. Glu and His were the major amino acid. The approximate molecular weights of the sarcoplasmic proteins and myo fibrillar proteins ranged from 31 kDa to 50 kDa and 66 kDa, respectively. The results indicated that reducing the levels of protein oxidation and improvement of the antioxidant properties should be of great interest to preserve the nutritional quality of meat products and prolong preservation period.
1. Introduction
Cured meat is a product has gained much popularity over the world because of its unique qualities. This product consists of raw meat that is pickled in salt, nitrate or nitrite, sugar or spices, and then subjected to a drying process [1]. For example, dry-cured ham and dry-cured loin are welcomed by European consumers partly because of their typical color, texture, taste and flavor, and occupy a larger share of the market [2,3]. Proteins in the raw meat can form network structure and interact with other ingredients. Thus, composition and physicochemical properties of protein during processing can change and influence on the textural, sensory and nutritional quality of meat product [4]. During meat processing, radical oxygen species from lipid peroxidation leads to the accumulation of oxidative damage in proteins [5]. Proteins can be decomposed into various amino acids (AAs) and then converted into carbonyl derivatives during oxidation process [6]. The interaction between protein oxidation and proteolysis has been investigated with surface hydrophobicity,carbonyl content and secondary structure [7]. The cured meat was fermented and dried at 50 °C for 3 days. Heat treatment is able to reduce the number of microorganisms and inhibit their growth.At the same time, phase, physics, chemistry, physicochemical and biochemistry of the cured meat change which demonstrate specific color, flavor and structure of products [8]. Moderate fat decomposition and lipid oxidation contribute to the formation of flavor substances during drying [9]. Zhang et al. [10]found that heat treatment can effectively promote adipose decompose and lipid oxidation, thus contributing to the formation of flavor of dry-cured hams. Jiang et al. [11]reported that high temperatures accelerated lipid oxidation, protein oxidation, and proteolysis. Heat-induced unfolding of native proteins generally results in the formation of cross-linked protein aggregates [5].These changes in tissue characteristics and sensory quality are due to the thermal changes in muscle myofibrillar which is an important salt-soluble protein in the biological function of muscle,mainly composed of myosin, actin, regulatory proteins, and the gelatinization functional characteristics of heat-induced meat products such as hardness, water retention and texture, etc. [12]. Myofibrillar denaturation and aggregation during heating can result in a series of physical and chemical changes in the product. The biological function of proteins depends on the specific three-dimensional structure (secondary structure). In the formation process of myofibrillar thermal irreversible gel, proteins begin to be denaturated and aggregated due to the damage or destroying of the secondary bond of the protein, and further resulted in the structural disintegration of the natural protein.
Lipid oxidation usually occurs during the processing of dry cured meat products. It was reported that butylated hydroxytoluene containing in apple polyphenols has the antioxidant effects and is able to extend the shelf life of meat for one month [13]. However, the use of synthetic antioxidants will cause potential health risks. Currently,natural substances with antioxidant activities have been widely studied as food preservatives such as flavonoids, phenolic acids, organic acids,and carotenoids, which reduce the lipid oxidation by scavenging free radicals, chelating metal ions, or quenching oxygen radicals, etc. [13].In recent years, the study of peptides with antioxidant activity has attracted attentions. One of the main biochemical mechanisms in the dry-cured meat processing is proteolysis, which is mainly produced by endogenous sarcopeptidase, in which the sarcoplasmic protein and myo fibrillary protein are hydrolyzed by endopeptidase, resulting in the formation of longer polypeptides and the change of texture of dry-cured meat [14]. Therefore, the control of proteolysis is essential for the production of stable, routine and higher quality products [14].Proteolysis plays an important role in the maturation of dry cured meat products by reducing high molecular weight proteins to polypeptides and free amino acids. These compounds have gustatory activity and may also have a strong effect on the final taste. It has been found that proteinase-derived proteins, with good antioxidant activity [15,16].The antioxidant properties of these hydrolysates largely depend on the properties of the releasing peptide, such as molecular weight (MW),AA composition, and its sequence [17]. To ensure safety, antioxidant peptides are required to reduce the lipid oxidation during bacon processing and prolong the storage life.
However, study on the physical/chemical changes in myoglobin and myo fibrillar and the change of peptide during the drying process of Cantonese cured meat and peptide relationship with lipid oxidation characteristics are still limited. The purpose of this study was to explore and understand the mechanism of thermal denaturation, texture characteristics of proteins and lipid oxidation process of Cantonese cured meat. The changes of AA composition and nitrite content during drying were also explored. During the heating process, investigation of the physical/chemical properties changes of the proteins helps us to understand the mechanism of the thermal denaturation of protein. It provides ideas and supports for screening new food preservatives.
2. Materials and methods
2.1 Samples and reagents
Lean pork was obtained from a local supermarket (Kaifeng,Henan Province, China). Sodium hydroxide, formaldehyde, ethyl ether, trichloroacetic acid, sulfuric acid,β-mercaptoethanol, SDS and Tris-HCl were obtained from Takara Biomedical Technology (Beijing)Co., Ltd. Others chemicals and reagents were obtained from Qiyun BiotechnologyCo. Ltd. (China). Ultra-purified water was prepared by the Milli-Q system (Millipore, Bedford, MA, USA) with a minimum resistivity of 18 Ω·cm. Cantonese cured meat was prepared according to the following formulation: pork (1 000 g), salt (20 g),sugar (40 g), wine (20 g), sodium nitrite (0.1 g) and soy sauce (40 g).The meat was cut as 36 cm × 2 cm, about 200 g per piece. They were mixed together and pickled for 3 h, then oven-dried for 72 h at 50 °C.During the drying process, samples were periodically taken at 0, 6,18, 36, 54 and 72 h for analyzing and kept at -18 °C for further use.
2.2 Determination of peptides
2.2.1 Analysis physicochemical, lipid decomposition and lipid oxidation of Cantonese cured meat during drying time
Moisture content (%,m/m) was determined in accordance with the method of Johansson [18]. The pH value was determined according to Yasosky’s work [19]. Salt content was determined by the titrimetric method of Careri et al. [20]. Texture analysis was performed by a texture analyzer (TA-XT. PLUS, Stable Micro System, London, UK).The following tests were in accordance with Wu et al. methods [21].The acidity value was monitored according to Fanco et al. [22]for assessing lipolysis of Cantonese cured meat. Lipid oxidation was evaluated by values of peroxide and 2-thiobarbituric acid-reactive substances (TBARS) which were determined by the methods reported by Visessanguan et al. [23], respectively. The level of nitrite was determined by colorimetric method [24].
2.2.2 Extraction of peptides
The extent of proteolysis was measured according to the method of Sun et al. [25]with some modification. Peptides were fractionated by micromembrane ultra filtration and the membrane with a cut-off MW of 5 kDa regenerated cellulose (PLCCC, Millipore, Billerica, USA)to obtain two fractions of peptide with MW of > 5 kDa and < 5 kDa(P1 and P2).
2.2.3 Analysis of AA composition of peptide
AA composition of peptides was determined by automatic AA analyzer (L-8900, Hitachi, Japan). Peptides were hydrolyzed at 110 °C for 22 h with 6 mol/L hydrochloric acid. Analysis conditions were 38 °C, at a flow rate of 1.0 mL/min, and the detection wavelength at 254 nm. The AA content was calculated using the equation (1).

WhereXrepresented AA content (g/100 g),Cwas the standard content of AA (mmol/50 μL),Fwas dilution multiple,Vwas solution constant volume after hydrolysis (mL),Mwas the MW of AA,mwas the weight of sample (g), 0.02 was AA content of sample (μmol/L),109was the coefficient of sample content by (g) conversion (ng).
2.2.4 Analysis of DPPH and OH radical scavenging activity (RSA)
The 1,1-diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) and 1,10-phenanthroline monohydrate (OH) RSA were analyzed by the modified method of Shimada et al. [26].
2.3 Determination of proteins
2.3.1 Extracted of sarcoplasmic and myo fibrillarproteins
Sarcoplasmic and myofibrillar proteins of Cantonese cured meat were extracted according to the methods in references [27,28],respectively.
2.3.2 Determination of proteins
The levels of SH group and S-S group were determined according to the method of Beveridge et al. [29]. Carbonyl groups were analyzed by the method of Oliver et al. [30]. Surface hydrophobicity of sarcoplasmic protein was determined by the methods described by Haskard and Li [31].
2.3.3 Circular dichroism (CD) spectroscopy of sarcoplasmic protein
CD spectra were obtained by applied photophysics spectropolarimeter (Chirascan, Britain). The protein concentration in the sample around 0.1 mg/mL was diluted by using 10 mmol/L phosphate buffer (pH 7.0) and samples were scanned from 190 nm to 260 nm. A mean value of 110 for the AA residue was assumed in all calculations. CONN CD spectra deconvolution software was used to crunch data, and molecular mass was 4 000 Da, number of AAs was 36.
2.4 Statistical analysis
Data were expressed as means ± standard deviations of 3 replicated determinations. Statistical calculation was investigated by the statistical package SPSS Statistics Version 25 (SPSS, Inc.,Chicago, Illinois, USA). Data from the samples and from panel assessors was analyzed variance (ANOVA) with different time points (0, 6, 18, 36, 54 and 72 h). Differences were considered significant atP< 0.05.
3. Results and discussion
3.1 Analysis of peptide determination results
3.1.1 Physical and chemical analysis
The physicochemical characteristics of the samples analyzed were exhibited in Table 1. The moisture content for 0 h was 38.01%,which decreased to 14.25% in the finished products. It also could be observed that pH values of Cantonese cured meat were stable. Results showed a sodium chloride content in the range of (2.45 ± 1.21)% at the beginning. Salt content (%) found in Cantonese cured meat after 72 h of drying increased to a value of (6.53 ± 0.23)% (Table 1).For residual nitrite content, it decreased gradually. In a mild acid environment (pH 5.5–6.0), nitrite could turn into nitrous acid, which was easily to be decomposed, following with released nitroso.Sequentially, nitroso reacted with myohemoglobin and formed nitrosomyoglobin with releasing of sulfhydrl group under the heating condition. The color of the meat would turn into rose by naked eyes [25].
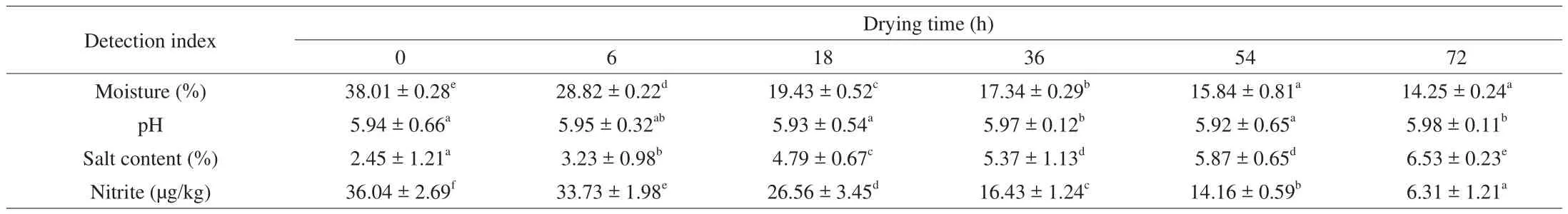
Table 1Changes in physicochemical characteristics of Cantonese cured meat during drying time.
3.1.2 Texture analysis
Textural properties of Cantonese cured meat were determined and the results were presented in Table 2. It exhibited a marked change inall the textural characteristics in the Cantonese cured meat. Hardness increased during the whole process and other textural properties were up to the maximum value at 18 h. Chewiness is calculated as the product of all the other textural traits and therefore represents an overall assessment of the product texture. Hardness, chewiness and viscosity were statistically significant (P< 0.05). Hardness of Cantonese cured meat is an index of maturation, resulting from denaturation and gelation of muscle proteins and the loss of water [32].Gumminess is defined as the product of hardness × cohesiveness, and chewiness as the product of hardness × cohesiveness × springiness.Springiness represents the extent of recovery of Cantonese cured meat height and sometimes is referred to as “elasticity” [33]. Long-term drying and quick evaporation rate of water will prevent the structural change of protein, and thereby limiting its network formation in the Cantonese cured meat system. Cohesiveness is considered as an index of the degree by breaking down of the internal structure of the Cantonese cured meat.
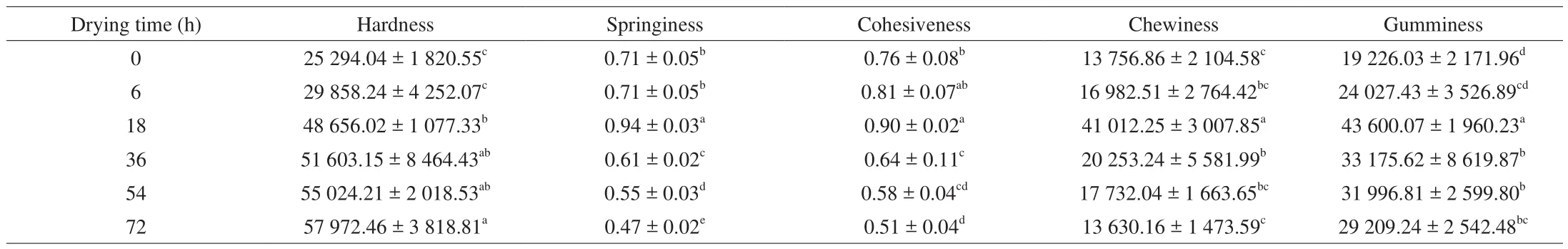
Table 2Changes in textural parameters of Cantonese cured meat during drying time.
3.1.3 Lipolysis and lipid oxidation analysis
The changes in lipolysis of Cantonese cured meat during the process were shown in Fig. 1. The results suggested that the lipid oxidation increased with the extending of drying time. The acidity value was used to represent an index of free fatty acids. We found that the acidity value of Cantonese cured meat was 1.37 mg KOH/g at 0 h, then increased to 1.96 mg KOH/g after 72 h of drying (P< 0.05).From 6–18 h, it was observed that the acidity value was on the rise without signification difference (P> 0.05). Due to the high drying temperature, it promoted the fat hydrolysis which was in agreement with Fanco et al. [22]. Wang [34]also investigated the increasing trend of free fatty acid content of dry cured ham during maturation.

Fig. 1 Changes in acidity value, peroxide value and TBARS during the drying of Cantonese cured meat. The values in a column followed by different letters are significantly different (P < 0.05).
Peroxide value measures the formation of peroxide or hydroperoxide groups that are initial products of lipid oxidation [35].The peroxide values of Cantonese cured meat were displayed in Fig. 1.It could be observed that they increased from 0.28 g/kg to 0.96 g/kg fat during 0–54 h (P< 0.05); while in 18-36 h, it increased rapidly from 0.48 g/kg to 0.90 g/kg which illustrated that the lipid in the interim (P< 0.05), especially most of the free fatty acid was oxidized and peroxide was accumulated rapidly. Latterly, the peroxide value has a downward tendency which attributed to the fact that the chemical instability of peroxide, and easily to be decomposed into small molecular substances.
Studies demonstrated that when the MDA content of meat reached to 5 mg/100 g, there will be appeared peculiar smelling after heating,so-called hot smell. As shown in Fig. 1, the TBARS value increased during 0–18 h (P< 0.05), and then there was a rapid increase during 36–54 h (0.83–1.17 mg MDA/100 g) (P< 0.05). This result was similar with the former study [36]. However, TBARS value declined during 18–36 h (P <0.05), it might be the product, i.e., 1-amino 3-amino propylene by MDA interaction with reactive amino group of the protein. The studies on Corsica and Jinhua ham found that the changes of TBARS value was not stable, fat oxidation produced aldehydes, and the subsequent reaction of small molecular aldehyde was also very fast [37].
3.1.4 DPPH and OH RSA of peptides
With the extending of the drying time, lipid oxidation increased,so the viable and safety of the antioxidant peptides were required. It was employed for evaluation by DPPH and OH. The DPPH RSA of peptides (peptides with MW of > 5 kDa was P1 and MW of < 5 kDa was P2)during drying was demonstrated in Fig. 2a. P1 was increased from 59.85% to 84.78% (P< 0.05) during 0–18 h of drying, followed by a slight decrease to 75.65% (P< 0.05) after 72 h. P2 was gradually increased throughout drying from 20.78% to 61.85% (P< 0.05).However, it was found no significant (P> 0.05) differences during the period of 0–6 and 6–18 h. Rapid increase (P< 0.05) was observed at the rest stage. The structural change of peptides, such as AA composition,should be responsible for the change in DPPH RSA of peptides degraded from Cantonese cured meat proteins during drying [24].
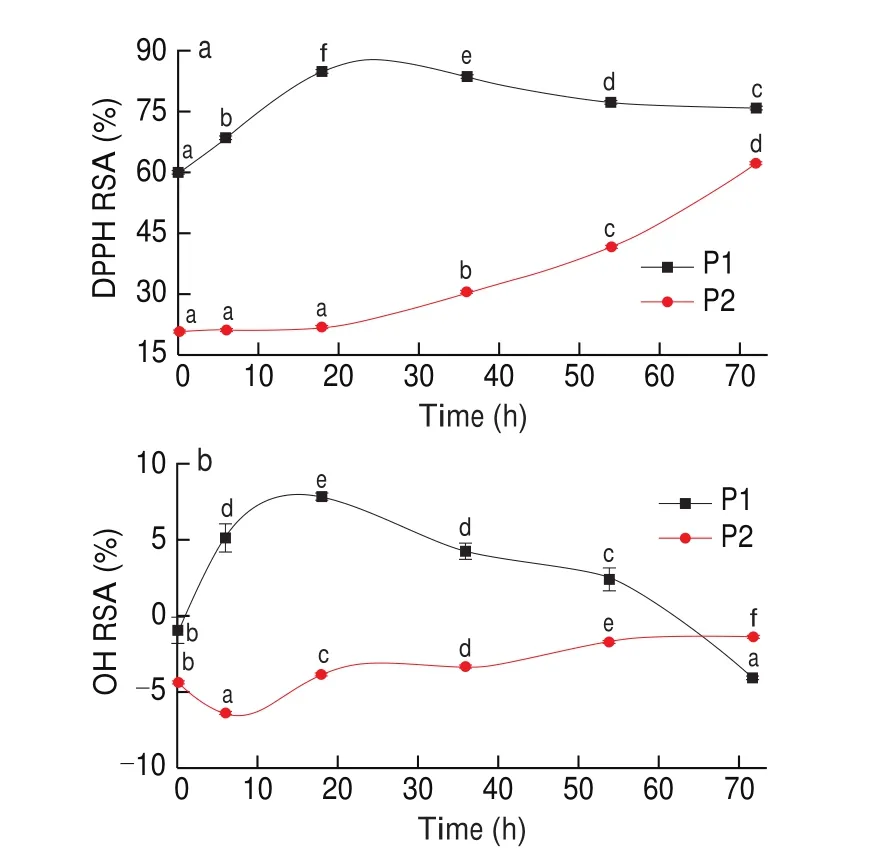
Fig. 2 Evolution of DPPH (a) and OH (b) RSA (%) during drying of Cantonese cured meat for peptides. The values in a column followed by different letters are significantly different (P < 0.05).
The OH RSA of peptides during drying was monitored, and the results were shown in Fig. 2b. The trend of the change was consistent with DPPH RSA. The numerical difference was mainly due to the different measuring methods. The study on EC50of glutathione was found that different methods for determination could obtain different results, the OH RSA of EC50for glutathione was 4.7 mg/mL, but EC50of DPPH was 0.77 mg/mL. The OH RSA of P1 increased to 53.58% (P< 0.05) after 18 h of drying, followed by a significant decrease to 17.98% (P< 0.05) at the 72 h. For P2, its OH RSA decreased primarily during the first 6 h (P< 0.05), then gradually increased throughout drying (P< 0.05, 25.95% in the end of the test).
The DPPH and OH RSA of P1 were higher than that of P2 (P< 0.05). The trends of DPPH and OH of P1were reached the maximum at 18 h. To sum up, it suggested that P1 of extracted from Cantonese cured meat drying processing at 18 h was the optimum antioxidant peptides [38-40].
3.1.5 Changes nitrogen contents of water-soluble nitrogen (WSN) and peptides
The nitrogen contents of WSN and peptide during the drying were displayed in Fig. 3. At the first 6 h, the WSN content decreased (P< 0.05) and increase up to 195.17 mg/100 g at 36 h (P< 0.05)and keeping constantly till the 54 h, and then it was sharply decreased during 54–72 h (P< 0.05). The decreasing of WSN of drying might be attributed to the denaturation of soluble proteins due to the heating treatment [24].
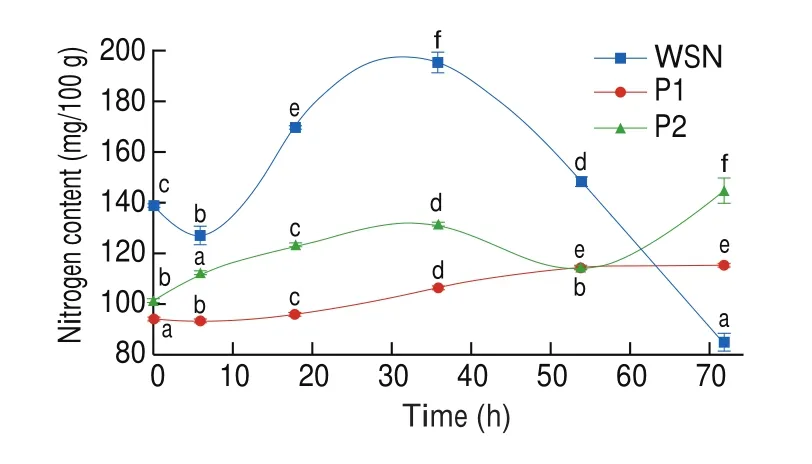
Fig. 3 Changes nitrogen contents in WSN and peptides of Cantonese cured meat during drying time. The values in a column followed by different letters are significantly different (P < 0.05).
The peptide content of P1 was observed significant difference (P< 0.05)during the first 18 h. It was increased from 95.66 mg/100 g to 106.13 mg/100 g during 18–36 h (P< 0.05), and thereafter remained constantly. For the peptide content of P2, it increased from 101.20 mg/100 g to 144.56 mg/100 g (P< 0.05) during 0–72 h. The results were in agreement with the previous study [24].
3.1.6 AA profiles of peptides analysis
AA composition and their sequences of the peptides played important roles for the antioxidant activity [41]. It reported that on the Iberian ham, the drying period was the free AAs in mass production period, the amino nitrogen content increased rapidly during this period, and it became the main component of nonprotein nitrogen [42].AAs of the two peptides during drying of Cantonese cured meat were evaluated (Tables 3 and 4). Glu and His were the major AAs in both peptide fractions. The special structural characteristics of His are very important in the RSA. The soy protein hydrolysate with Leu-Leu-Pro-His-His sequence possesses a good antioxidant activity [41,43]. Saito have screened 40 peptides structurally related to the above-mentioned sequence, and Pro-His-His was identified as the active center [44].In addition, Gly, Val, Phe were considered as the important AAs in both peptide fractions. Other peptides without Glu and His have also been confirmed to have antioxidant activity. Li et al. [45]isolated an antioxidant peptide from the collagen hydrolysates of porcine skin with a primary structure of Gln-Gly-Ala-Arg. Therefore, the antioxidant activities of P1 and P2 might be the contribution of Glu,His and other important constituents, such as Gly, Val and Phe, etc.
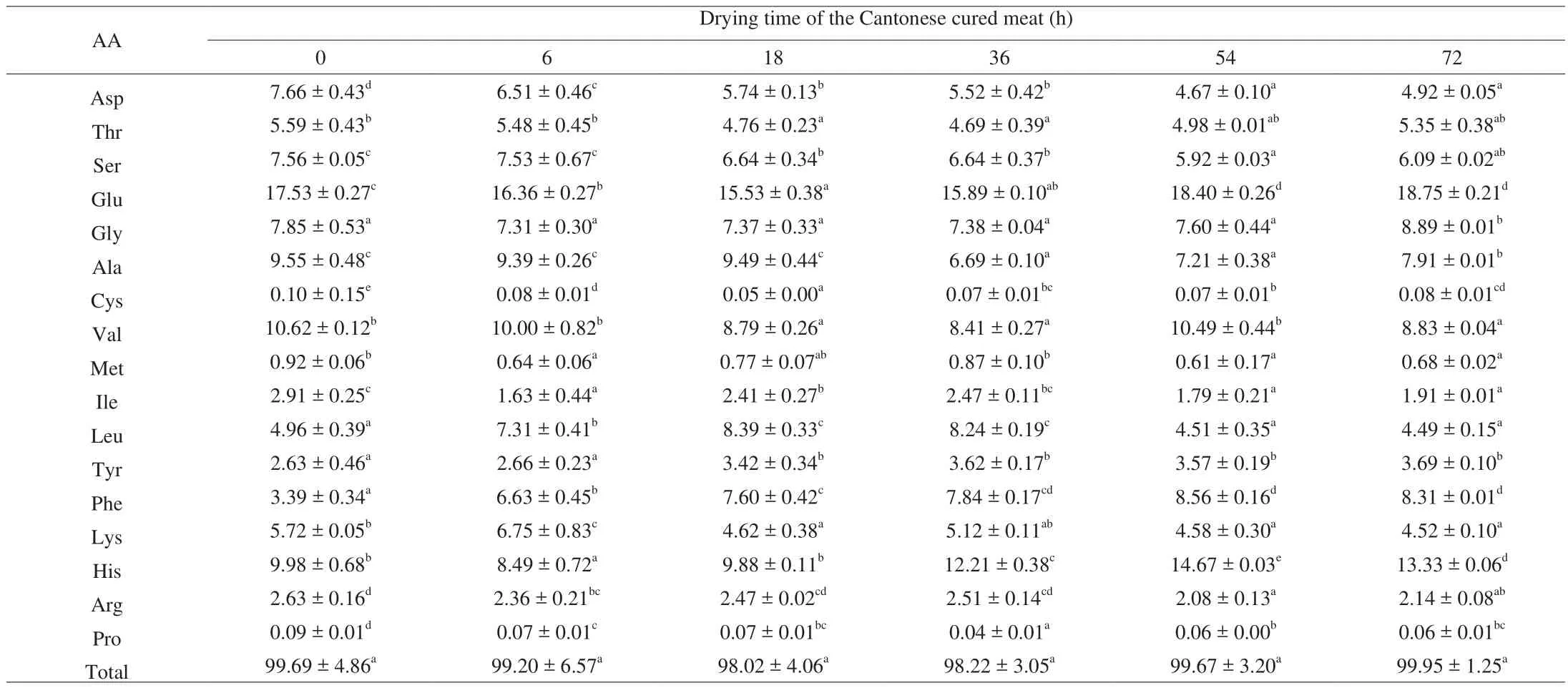
Table 3AA composition (g/100 g) of P1 of Cantonese cured meat during drying time.

Table 4AA composition (g/100 g) of P2 of Cantonese cured meat during drying time.
3.2 Results analysis of physical and chemical properties of proteins
3.2.1 Nitrogen content of sarcoplasm and myo fibrillar protein
The changes of the sarcoplasmic and myo fibrillar protein contents in Cantonese cured meat with the processing time were demonstrated in Fig. 4. The nitrogen contents of the sarcoplasmic proteins in the Cantonese cured meat were decreased from 246.67 mg/100 g DM up to 94.67 mg/100 g DM (P <0.05) during drying. The contents in 0-6 and 18-36 h were changed slightly (P >0.05). The myofibrillar proteins nitrogen contents were progressive decreased from 184.33 mg/100 g DM up to 17.00 mg/100 g DM (P <0.05). As we all know, the meat proteins are the main constituents making up the structure of meat product [46]. Protein characteristics, substantial composition and structure are changed during the process. Two reasons were mainly contributed to the decreasing of the content of sarcoplasmic proteins. One reason is that arcoplasmic protein denatured during heating changes its solubility and promotes the accumulation of alkali-soluble proteins. The other is that during the processing of dried preserved meat, the change of salt content and acid-base conditions leads to the loss of protein denaturation and solubility, and the sarcoplasmic protein is degraded into non-protein nitrogen, such as polypeptide, AA, creatine, ammonia and bilirubin, etc.
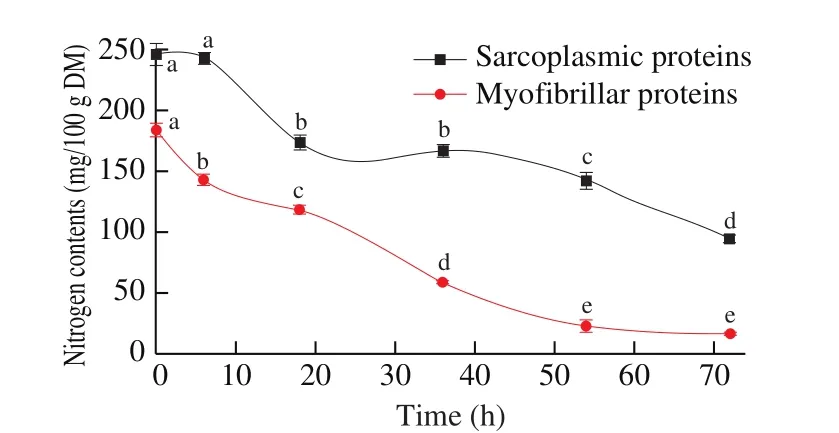
Fig. 4 Changes in the nitrogen contents of sarcoplasmic and myo fibrillar proteins in Cantonese cured meat during process. Values in a column followed by different letters are significantly different (P < 0.05).
3.2.2 SDS-PAGE electrophoretogram analysis of sarcoplasmic and myo fibrillar proteins
Electrophoretic analysis of sarcoplasmic and myofibrillar protein in Cantonese cured meat during drying was shown in Fig. 5. The approximate MWs of sarcoplasmic protein bands for ranged were from 31 kDa to 50 kDa. Visessanguan investigated that the bands could be classified as phosphorylase, creatine kinase, phosphoylycerate mutase or phosphoglycerate kinase, and triosephosphate isomerase, respectively [27]. They changed during the process due to proteolysis or salt-induced denaturation.

Fig. 5 SDS-PAGE electrophoretograms of sarcoplasmic proteins (A) and myo fibrillar proteins (B) in Cantonese cured meat during process.
The greatest change in the myo fibrillar protein pattern occurred between the beginning process (0–6 h), while only small differences were observed from 6 h to 72 h. The approximate MWs of the protein bands for ranged from 31 kDa to 66 kDa. Protein bands above 66 kDa disappeared during the first 6 h. Myo fibrillar proteins play the most critical role during meat processing as they are responsible for the cohesive structure and firm texture of meat products [47].
During the process of Cantonese cured meat, a great number of biochemical reactions occur associated with protein degradation.Cathepsin and muscle calpain are considered as the main enzymes,which can cause meat protein degradation [48]. For example, muscle calpain and cathepsin D lose their vitality in the cured process which might be playing the role in the beginning of the period [49].
3.2.3 Analysis of SH and S-S group levels of sarcoplasmic and myo fibrillar proteins
Protein oxidation is related with decreasing of sulphydryl groups,which are converted into disulphides [50]. The levels of SH and S-S group in sarcoplasmic and myofibrillar proteins from Cantonese cured meat during drying process was monitored. The results were exhibited in Fig. 6. The SH group levels of sarcoplasmic proteins in Cantonese cured meat at the beginning was the highest (100%),and then decreased during the whole drying process (P >0.05), but no signification difference (P >0.05) was observed during 18-54 h.The SH group levels of myofibrillar proteins at the beginning were 71.83 μmol/g, and then decreased during the whole drying process (P <0.05), but no significant difference (P >0.05) was observed during 0-6 and 36-54 h.
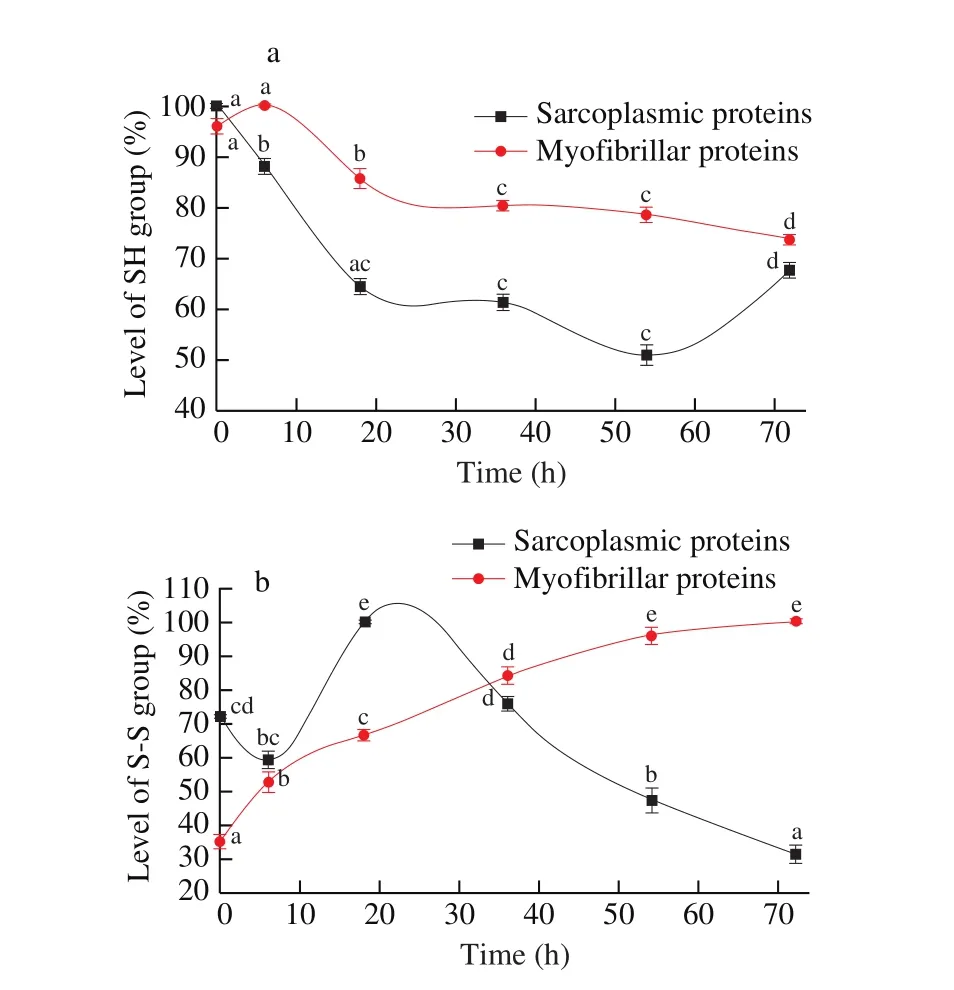
Fig. 6 Changes in level of SH (a) and S-S group (b) of sarcoplasmic and myo fibrillar proteins in Cantonese cured meat during process. Values in a column followed by different letters are significantly different (P < 0.05).
The level of SH group was decreased might contribute to the oxidation of accessible free thiol groups from cysteine residues at the protein surface [51]. Cysteine residues and the disulphide bonds play important roles in the aggregation of proteins [52]. During the meat processing, the tertiary structure of protein may change by heating,mechanical shear and exposure to air or oil-water interface. They can affect the release of sulfhydryl and cystine groups to the water and become chemically reactive to form the disulphide bonds [53].
The S-S group levels of myofibrillar proteins at 0 h was 23.96 μmol/g, increased in the whole process, and up to 67.53 μmol/g at 72 h. S-S group level lacks regularity, which may be due to the protein degradation and thermal effect. Protein degradation could result in the increase of new water-soluble fraction, and thermal effect could lead to water soluble fraction degeneration and loss of soluble components, and then change the proportion of sulfhydryl [54].
3.2.4 Carbonyl content of sarcoplasmic and myo fibrillar proteins
The carbonyl contents of sarcoplasmic and myo fibrillar proteins from Cantonese cured meat during drying were shown in Fig. 7. The amount of carbonyl groups significantly increased (P <0.05) during processing from 1.69 nmol/mg to 3.59 nmol/mg of sarcoplasmic proteins and from 1.58 nmol/mg to 4.45 nmol/mg of myofibrillar proteins, respectively. Du found that carbonyl content of myo fibrillar proteins of duck is 1.62 nmol/mg [55]. Morzel et al. [56]studied that the carbonyl content of myo fibrillar proteins of pork were presented as 1.7 and 1.4 nmol/mg, which is similar with our results. Another report suggested that during Frankfurt sausage storage, proteins were oxidized, and the carbonyl content was significantly increased from 3.7 nmol/mg to 5.4 nmol/mg [57].
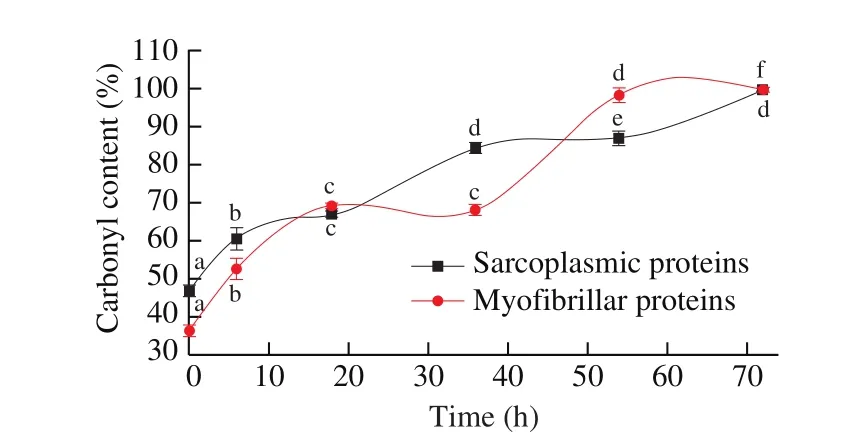
Fig. 7 Changes in the carbonyl content of sarcoplasmic and myo fibrillar proteins in Cantonese cured meat during processing. Values in a column followed by different letters are significantly different (P < 0.05).
AA with NH or NH2moiety on its side chain can react with free oxygenated radicals generated by lipid peroxidation [6]. Then the carbonyl groups were formed by these groups. The protein carbonyl derivatives can be generated through oxidative cleavage of proteins by eitherα-amidation pathway or oxidation of glutamyl side chains, leading to the formation of a peptide in which theN-terminal AA is blocked byα-ketoacyl derivative. In addition, the carbonyl groups might be introduced into proteins by reactions with aldehydes (4-hydroxy-2-nonenal,malondialdehyde) produced during lipid peroxidation [5,53]. The protein carbonyls are also formed by glycation and glycoxidation. As we know, the Cantonese cured meat had a high content of lipid, and contents in the muscle tissue are heme, metal ion and various oxidase,etc. During the meat processing, these substances are attacked the protein, which leaded to carbonyl value increased.
3.2.5 Analysis of sarcoplasmic and myofibrillar proteins surface hydrophobicity (H0)
The changes of chemical and physical states of protein were estimated by the surface hydrophobicity, which is a suitable parameter to protein changes [58]. Hydrophobic interaction is the main force of maintaining protein tertiary structure. It played an important role on stability of protein structure, conformation and functional properties.Due to the intermolecular interactions, surface hydrophobicity had greater impact on protein function than the overall hydrophobicity.TheH0of sarcoplasmic and myo fibrillar protein from Cantonese cured meat at drying process were shown in Fig. 8. During the processing of traditional Cantonese cured meat, theH0of sarcoplasmic proteins increased to a maximum at 54 h (P <0.05) and then decreased (P <0.05) gradually at the final phase of processing. Salt curing might lead to the increasing ofH0of myofibrillar proteins from 0 to 72 h. Melander and Horvath [59]have found that salt was positively associated with surface hydrophobicity of proteins.
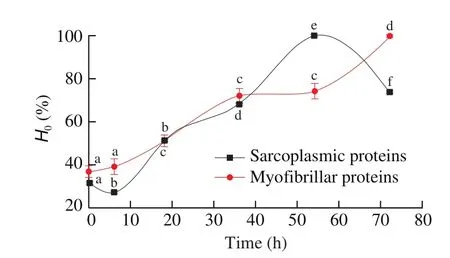
Fig. 8 Changes in H0 of sarcoplasmic and myo fibrillar proteins in Cantonese cured meat during processing. Values in a column followed by different letters are significantly different (P < 0.05).
Studies reported that protein hydrophobicity was related to oxidation. Through the exposition of hydrophobic AAs, oxidation enhances the hydrophobicity on the protein surface, which was buried in the inside of the protein structure under native conditions [60]. Chelh et al. [61]also observed a considerable increase of hydrophobicity, which was positively correlated to the heating time and temperature. In proteins, the heating treatment is generally associated with the breaking of hydrogen or electrostatic bonds [62].We speculate that the increase ofH0during Cantonese cured meat processing could ascribe to protein oxidation, thermal treatment and hydrolysis, etc.
3.2.6 Far-UV CD spectroscopy of sarcoplasmic proteins
The secondary conformation or structure of sarcoplasmic proteins from Cantonese cured meat during drying process were evaluated and made comparison by using of far-UV CD spectra (Fig. 9). The secondary structure compositions includingα-helix, antiparallel,parallel,β-turns and random coil of these samples were calculated by CONN CD spectra deconvolution software, as displayed in Fig. 10. Contents of α-helical and random coil were decreased as 19.94%-13.57% and 36.08%-32.68%, respectively. Antiparallel andβ-turns were increased from 17.78% to 28.51%, and from 16.22% to 15.47% respectively over time. The secondary structures of parallel remained stable during processing. Theα-helix structure is mainly stabilized by hydrogen bonds between the carbonyl oxygen (-CO) and amino hydrogen (-NH) of a polypeptide chain [63].
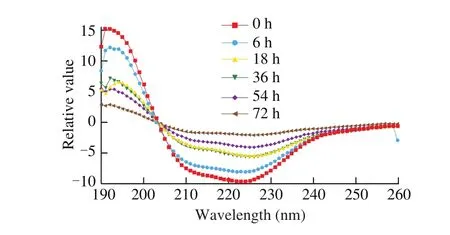
Fig. 9 Band scanning curve of the secondary structure of sarcoplasmic proteins by far-UV CD spectra.

Fig. 10 Secondary structural compositions of sarcoplasmic proteins in Cantonese cured meat during processing.
At the process, the decrease of residual nitrite level could be attributed to the following mechanisms: transformation into other compounds, reaction with other meat components and the presence of ascorbate acid and the salts. The textural characteristics were changed during the drying process of meat products, it is might due to the fact that there was the product shrinkage proportional to the water loss [64],increasing the dry matter content of the sample used in the texture analysis. Moreover, Randall and Bratzler [65]have proposed that drying of meat promoted a closer contact between proteins and new interactions were formed, further increasing the hardness.
4. Conclusions
Salting out, protein oxidation and heat treatment were closely related to surface hydrophobicity and the secondary structures of peptides were changed during the dry-cured meat processing. Reducing the levels of protein oxidation and improvement of the antioxidant properties should be of great interest to preserve the nutritional quality of meat products and prolong preservation period. The processing conditions and protein degradation play critical roles in protein reorganization. Further work will be carried out to separate, purify and identify the antioxidant peptides and protect protein against oxidation during Cantonese cured meat and to evaluate the relationship between protein functional characteristics and flavor release.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by Henan Province's key R&D and promotion projects (scientific and technological research) projects(222102310587), Key Scientific Research Project Plan of Henan Province (22A310011), Grants from the Henan University (Yellow River Scholar Fund for Shanqing Zheng), the National Natural Science Foundation of China (81872584), Key R&D and Natural Science Foundation of Shenzhen (JCYJ20210324093211030),Medical Scientific Research Foundation of Guangdong Province(A2020490), the Interdisciplinary Research for First-class Discipline Construction Project of Henan University (2019YLXKJC04).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
