The prevention role of Spirulina platensis (Arthrospira platensis)on intestinal health
2022-06-23EnderDenizAsmzNilySeyidoglu
Ender Deniz Asmz, Nily Seyidoglu*
a Department of Histology and Embryology, Veterinary Faculty, Bursa Uludag University, Nilufer 16059, Turkey
b Department of Physiology, Veterinary Faculty, Tekirdag Namik Kemal University, Suleymanpasa 59030, Turkey
Keywords:
Histochemistry
Duodenum
Spirulina platensis
Intestinal health
A B S T R A C T
Spirulina is a popular herbal food that has a preventive effect on health. In the study, it aimed to evaluate the effects of different doses of Spirulina platensis (Arthrospira platensis) on the morphological properties of the duodenum of rats, and to assign the effect of Spirulina on the expression of proliferating cell nuclear antigen (PCNA) in the rat duodenum, and thereby to observe the effects on intestinal health. 30 male Wistar albino rats were divided into 3 groups for 45 days. The first group was received the basal diet; the second group was given 500 mg/kg Spirulina daily by oral gavage; the third group was given 1 000 mg/kg Spirulina daily. The duodenum segments were taken at the end of the trial and processed for histological assay. Although the total mucosa, villus height and villus/cript ratio were found higher in high dose Spirulina, the lower cript depth was detected in same group compared to control and low dose group. A significant increase was observed at high dose compared to the control and low dose group in terms of PCNA expression intensity and proliferation index.Findings suggest that high dose of Spirulina may support the duodenal growth, and thereby intestinal health.
1. Introduction
The intestinal tissue plays an important role in homeostasis via protection against toxic and pathogenic substances present in intestinal system. Physiological stress, chemicals substances and pathogens may alter the normal intestinal micro flora and epithelium. Belongs to the importance of acting of intestinal epithelium as a natural barrier,nutritious can be crucial to improve intestinal microbiota. Insight of literatures, using dietary probiotics, prebiotics or some other food additives could successfully protect intestinal health. Recently,there has been a growing interest in natural herbal supplements and their extracts for intestinal health, immunity and growth. The action of herbals or extracts is explained by either preventing intestinal system from harmful microorganism or supporting the important microorganism for absorption and digestion [1]. An important alga namedSpirulina, formally calledArthrospira, has high nutrition properties as well as potential therapeutic effects on intestinal mucosa [2,3].Among a large number ofSpirulinaspecies, 3 species are the most intensively investigated, includingS. platensis(A. platensis),S. maxima(A. maxima)andS. fusiformis(A. fusiformis).S. platensis(A. platensis) contains many important bioactive components such as proteins, polysaccharides, amino acids, minerals,antioxidants (carotenoid) and phycocyanin which give a great attention for growth and immunity. It was also reported thatS. platensisstimulates the growth of probiotics and maintains the nutrient digestion due to its cell structure. It has cellulose on its cell wall which can be absorbed by intestinal mucosa easily, and thereby improve the mucosal digestion and function [4,5].
The morphological parameters such as villi height, crypt depth and total mucosa thickness, which are frequently used by researchers to provide the detailed information about intestinal absorption. Some researchers indicated that high villus height and high villus height crypt ratio could be the indicators of intestinal epithelial development [6-8].Pluske et al. [8]indicated that improvement of absorptive function is linked with activities of brush border enzymes via high crypt/villus ratio in weaned pigs. It was observed that higher villus height, crypt depth and their ratio are important for gut health due to effect of nutrients on absorption surface. Also, Pluske et al. [7]rewieved the decrease of villus height with a decrease in absorption and digestion. Nevertheless, the immunohistochemistry (IHC) detection of proliferating cellular nuclear antigen immunoreactivity (PCNA)is the most common method used to identify cells proliferating in tissue sections in studies on the digestive system. The new intestinal epithelial cells are produced by stem cells continuously in the crypts which subsequently migrate along the crypt-villi axis [9]. An increase in PCNA labelling, therefore, signals marked increases in the rate of cellular division. Expression of PCNA increases during the G1-phase and peaks at the S phase, and following declines during G2/M-phases of the cell cycle. These immunostaining aspects allow the identification of cells in the different phases of the cycle [10].Many researchers have obtained definitive results about the treatment method used by determining the increase or decrease in cell proliferation on the digestive system after the treatments given as supplementary nutrition using this method [11,12]. Garcia et al. [11]mentioned that cell proliferation in the gut, especially in crypts, which was expressed by PCNA, can also be considered as the ability of the gut to cope with pathogens and support gut health. In addition,proliferative activity of enterocytes is a sign of healthy tissue cycle and maintenance.
It’s known that natural additives have an important role in improving feed avaliability and thereby intestinal micro flora [13,14].Spirulinahas a great place among these additives. However, to our knowledge, there exist limited data regarding the effects ofS. platensison duodenum morphologically and histologically in healthy rats. Therefore, in this study, it was aimed to evaluate the utility of different doses ofS. platensison the morphometric properties of the duodenum in rats using morphological and histological parameters.
2. Materials and methods
2.1 Animals and feeding
The experiments were performed in accordance with the guidelines provided by the National Institute of Health for Animal Research and were approved by the ethical committee on animal research at University Animal Experiments Local Ethics Committee (Approval No.: 2017/04-4). In the study, 30 male Wistar albino rats were allocated to 3 experimental groups. The groups were control group(basal diet); group SP-1 (added 500 mg/kgS. platensisdaily); Group SP-2 (added 1 000 mg/kgS. platensisdaily). Feed and water were offeredad libitumfor all groups.S. platensiswere diluted and given by oral gavage daily throughout the 45 trial days.S. platensis(Egert,Izmir-Turkey) doses were provided and modified according to literatures [15].
2.2 Measurements
2.2.1 Neutrophile:Lymphocyte (NL) ratio
NL ratio has been accepted a physiological welfare parameter during diseases or stress as well as normal conditions[16,17]. In this study, NL ratio was recorded for evaluating the conditions of rats whether in stress or not. According to literatures, NL ratio was calculated from complete white blood cell counts by dividing absolute count of neutrophils to lymphocytes [18].
2.2.2Histology and morphometric analysis of the duodenum
At the end of the study, all rats from each group were weighted and sacrified. Rats were dissected according to CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guideline approved method (CPCSEA), and duodenum samples were taken out approximately 3-4 cm below the pylorus.Then, samples were fixed in 10% neutral buffered formalin. The routine histological methods were applied, and embedded the samples in paraffin. 5 mm thick sections were cut from paraffin blocks and dried overnight. After procedures of dewaxing and rehydration,sections were stained by the Crossman triple stain for morphometric and morphological assay [19]. After the process, the villus height and crypt depths were measured and micrograps were photograped by Nikon 80i microscope. Measurement of the villus height was from villus tip to villus-crypt junction and the crypt depth was from villus-crypt junction to lower limit of the cript [20]. The level for randomly 15 vili and 15 corresponding crypts per section were estimated. The total mucosa and villus:crypt ratio was also calculated for each segment.
2.2.3 Immunohistochemistry analysis
The standard streptavidin biotin peroxidase complex technique were applied for immunohistochemistry analysis. For antigen retrieval, the boiling step was performed in a microwave oven of 750 W with sodium citrate buffer (1 mol/L, pH 6.1) for 3 × 5 min.After the sections were washed with PBS, for endogenous peroxidase activity the sections were blocked for 10 min. The horse serum was applied to sections for 20 min to reduce nonspecific antibody binding. After that, sections were incubated overnight 4 °C with anti PCNA primary antibody (sc-7907) diluted 1:50 as recommended by manufacturer. Then, samples were washed 3 times with PBS, and were incubated with ImmPRESS reagent at room temperature for 30 min. After washing again with PBS for 3–5 min, the tissues were washed with distilled water, and they were incubated with 5 min DAB for imaging. Haematoxylin was used as counterstaining [21].Sections were cleared with xylol, and then they were covered with the entellan. Proliferative index (PI) rate was calculated as the ratio of the number of crypt cells positive for PCNA staining to the total number of crypt cells. The number of proliferating cells per crypt was defined as the mean of proliferating cells in 15 crypts [22]. In addition,intensity and localization of PCNA expression was assessed by two independent observers with scoring system: 0, no immunoreaction;1, weak immunoreaction; 2, moderate immunosuppression; 3, strong immunoreaction [23].
2.3 Statistical analysis
Statistical analyses were performed with SPSS (Version 17.0;Chicago, IL, USA). Data were examined for normality distribution and variance homogeneity assumptions (Shapiro-wilk test). If normally distributed, one-way ANOVA test was applied, and the differences between groups were analysed by thepost hocTukey test. The differences were considered significant atP< 0.05, and the means and standard errors were calculated.
In the study, nonparametric tests were used as the data did not provide normal assumptions. So, the differences between groups were analysed by Kruskal Wallis, and Mann Whitney U test was used between groups. Also, the differences were considered significant atP< 0.05, and the median values (minimum-maximum) were calculated.
3. Results
The NL ratio and morphometric analysis of the total mucosa,villus height, crypt depth and villus/crypt ratio of the control and experimental groups are presented in Table 1. The NL ratio was found lower in group SP-2 compared to control group (P= 0.025). The total mucosa and villus height of duodenum were found significant higher in group SP-2 than control group (P< 0.000 1). However cript depth was determined the lowest value in group SP-2 (P< 0.000 1). Also,villus/cript ratio was found in a high value in group SP-2 compared to control (P< 0.000 1). The morphological measurements of duodenum such as villus height, thickness of total mucosa and depth of crypt of all groups are figured in Figs. 1-3.

Table 1The NL ratio and morphometric analysis of the total mucosa, villus height,crypt depth and villus/crypt ratio of the control and experimental groups.
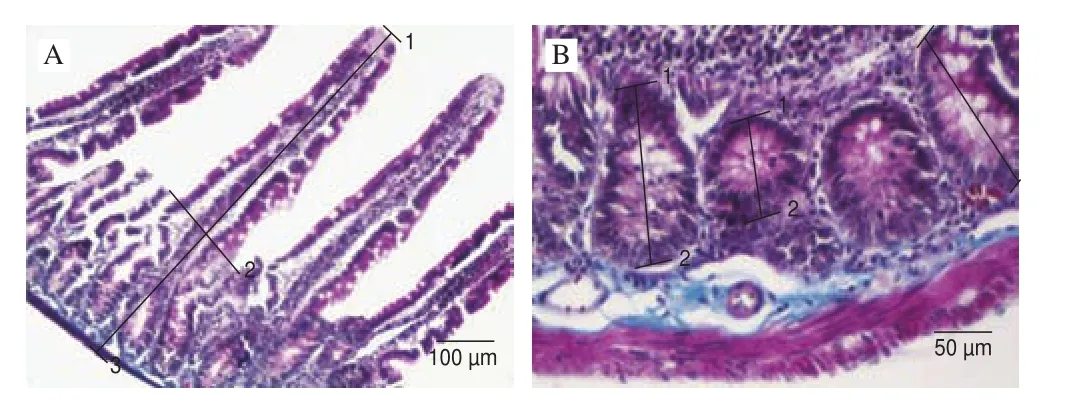
Fig. 1 Morphological measurements in the duodenum of the control group.(A) (1-2) villus height, (1-3) thickness of total mucosa; (B) (1-2) depth of crypt, Crossman’s modified triple stain.
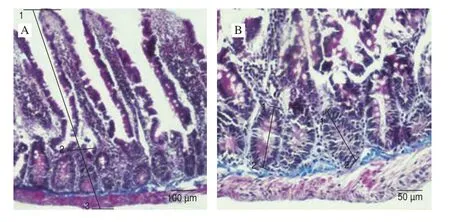
Fig. 2 Morphological measurements in the duodenum of group SP-1.(A) (1-2) villus height, (1-3) thickness of total mucosa (B) (1-2) depth of crypt, Crossman’s modified triple stain.

Fig. 3 Morphological measurements in the duodenum of group SP-2.(A) (1-2) villus height, (1-3) thickness of total mucosa; (B) (1-2) depth of crypt, Crossman’s modified triple stain.
The expression severity of PCNA in the duodenal tissue of the control group, groups SP-1 and SP-2 were evaluated. Statistical differences in terms of expression severity between the control group and experimental groups were determined in Lieberkühn crypts of the duodenum. On the other hand, there was no difference for PCNA expression in the intestinal villi and muscle layer among all groups.PCNA expression was determined from weak to moderate in the control group. However, a moderate expression was observed in group SP-1, whereas a moderate to strong immunoreaction was detected in group SP-2. There is no significant difference between the control and the SP-1 group in terms of expression severity. A significant increase was observed in group SP-2 compared with group SP-1 (P< 0.05) in terms of expression severity. On the other hand, there was an increase in group Sp-2 in comparison to control (P< 0.001; Figs. 4, 5A).

Fig. 4 PCNA expression in duodenum of control and experimental groups. (A, B) Control group; (C) negative control; (D, E) group SP-1; (F) low dose Spirulina negative control; (G, H) group SP-2; (I) high dose Spirulina negative control. Magnification: A, D, G, 40 ×; B, C, H, 10 ×; E, F, I, 4 ×.

Fig. 5 Effect of S. platensis for 45 days on duodenal PCNA immunoreactivity and PI in male rats of all groups. (A) PCNA expression severity of all groups.(B) Percentage of PCNA/cyclin positive cells (PI) in the duodenal Liberkühn crypts. All data are presented as the mean ± SE (n = 30). For PI, P < 0.001. For PCNA expression between ab and c P < 0.001, between b and c P < 0.05.
In addition, a comparative PI of the control group and experimental groups was determined for Lieberkühn crypt cells.The proliferation index was determined as the percentage of PCNA positive cells to the total crypt cells in 10 crypt cells randomly selected from different regions in each group. A statistical significance increase was determined in group SP-2 compared to control. There was also a significant increase in group SP-2 compared to SP-1(P< 0.001; Figs. 4, 5B).
4. Discussion
In this study, we assessed the association between feeding with a low and a high dose ofS. platensisand duodenal mucosa of rats using histological and morphological methods. Here we provide evidence that 45 days of feeding withSpirulinaobserved a protective effect on intestinal health.
Researchers have noticed that regulating intestinal mucosa and its ecosystem could protect organism from pathogens, malnutrition and inflammations [24]. For this homeostatic balance, especially natural supplements or additives have been used, for example herbal ones. The nutrients digestibility and utilization ofS. platensiswas reported by some researchers [25]. In the present study, the total mucosa, villus height, crypt depth and the ratio were increased in allSpirulinagroups (high and low dose) than control group. However,although no significant difference was found in low doseSpirulinagroup, increases in villus height, crypt depth and villus/cript ratio were found in high dose ofS. platensisgroup. As well as our results,Furbeyre et al. [25]suggested that 1%Spirulinacan improve the intestinal mucosal digestibility with increasing the villus height, and can balance the digestive system in weaning piglets. They indicated that high villus length may be associated with protein and amino acids contents ofSpirulina.
Enterocytes, placed in duodenal crypt cells, are the major cell type in the intestinal epithelium. They are simple columnar epithelial cells which have important roles in nutrient absorption (e.g., ions, water,sugar, peptides, and lipids) and also in secreting immunoglobulins.While the crypt cells rich in lysozyme enzyme play an active role in protecting the intestinal flora, it provides regular regeneration of the intestinal epithelium with the undifferentiated cells it contains.Therefore, the cell proliferation severity and proliferation index were evaluated in Liberkühn crypts, where the most mitotic activity occurred in the intestinal epithelium. Previous researches have indicated that the concentrations of PCNA change during the cell cycle [26]. Most G0/G1 cells did not express a significant amount of PCNA. PCNA synthesis in S phase was significantly higher than G0/G1 and G2/M, and also the amount of PCNA in G2/M phase was still elevated relative to G0/G1 cells. Each phase is under the general control of specific cyclin-dependent kinase (CDK)-cyclin complexes in the cell cycle. PCNA forms complexes with CDK-cyclin as well as critical checkpoint proteins in cell cycle regulation [27].As an example, PCNA plays an important role in G2/M phase by forming complexes with CDK1-cyclin-B the transducing negative signals. In the present study, percentage of PCNA/cyclin positive cells and proliferation index in the duodenal Liberkühn crypts were found higher in group SP-2 compared to control group, and in group SP-2 compared to group SP-1. It was thought that the increase in PCNA expression in crypt cells caused by the cell entering the S phase faster and more intensely than the control groups by triggering the cell replication region which may be due to an activation of polysaccharides and protein contents ofSpirulina.
There were some important reports about polysaccharides and proteins ofSpirulinathat studied for intestinal health. It was reported that polysaccharides ofSpirulinacould improve the cell nucleus enzyme activity and also repair the DNA’s synthesis damage [28,29].González et al. [29]reported that myeloperoxidase is an enzyme in neutrophils which directly linked with intestinal inflammation. They suggested that phycocyanin had an anti-inflammatory activity on colitis within increased intestinal myeloperoxidase enzyme levels in rats. Another study reported thatSpirulinaimproves intestinal health by improving the lactic acid bacteria in rats’ intestine by molecular mechanisms due toSpirulina’s crude protein content [30]. The researchers indicated thatSpirulinaincreased the S phase of the cell cycle and G1/S transition during the epidermal growth factor receptor and mitogen-activated protein pathways which were activated by phosphorylation. It was also determined thatSpirulinaproteins play role on migration and proliferation of IEC-6 cells and EGFR and MAPK pathways’ activities. Thereby, researchers suggested thatSpirulinamay be an important pharmacological agent for homeostasis of intestinal epithelium.
In the present study, NL ratio was found to be lower in high dose ofSpirulinacompared with control group. It can be suggested that either long trial or application high doses ofSpirulinacould not exist a stress on animals. It can be suggested that, duodenal mucosal features were developed normally and healthy in rats. As well as our results, Yoon et al. [13]reported that a greater diversity of intestinal microbiota is associated with a lower NL ratio in a healthy population.
This study provides the effects of high doses ofS. platensison intestinal morphology and health. The results could be correlated with the high and good quality protein content ofS. platensis.It was suggested that protein and polysaccharide content ofSpirulinamay support the growth of epithelial cells, and thereby intestinal mucosa.We hope this study will simplify our knowing of the therapeutic potential ofS. platensisamong the intestinal health.
Declaration of competing interest
There is no conflict of interest.
Acknowledgements
Special thanks to Prof. Dr. Berrin Zik for scientific advances and assistance during research. This study was supported by grant from the Research Foundations of University (NKUBAP.10.GA.16.074).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
