Fabrication and evaluation of a portable and reproducible quartz crystal microbalance immunochip for label-free detection of β-lactoglobulin allergen in milk products
2022-06-23MingfeiPanLipingHongJingyingYangXiaoqianXieKaixinLiuShuoWang
Mingfei Pan, Liping Hong, Jingying Yang, Xiaoqian Xie, Kaixin Liu, Shuo Wang,*
a Key Laboratory of Food Nutrition and Safety, Ministry of Education of China, Tianjin University of Science and Technology, Tianjin 300457, China
b State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science & Technology, Tianjin 300457, China
Keywords:
β-Lactoglobulin allergen
Quartz crystal microbalance
Immunochip
Milk products
A B S T R A C T
In this study, a label-free, portable and reproducible immunochip based on quartz crystal microbalance(QCM) was developed for the qualitative detection of β-lactoglobulin (β-LG), an allergen, in milk products.Experimental parameters in the fabrication and regeneration procedure such as pH of the coupling microenvironment, amount of anti-β-LG antibody and regeneration reagent were optimized in detail. Under optimal conditions, the proposed QCM immunochip exhibited good recognition of β-LG, with a calibration curve of ΔF = 12.877Cβ-LG0.480 9 (R2 = 0.998 2) and limit of detection of 0.04 μg/mL. Additionally, this portable QCM immunochip had good stability, high specificity, and no obvious cross-reaction to three other milk proteins (α-casein, α-lactalbumin, and lactoferrin). It could compete a qualitative measurement within 5 min, and could be reused at least ten times. In the β-LG analysis of actual milk samples, the developed QCM immunochip yielded reliable and accurate results, which correlated strongly with those from the standard HPLC method (R2 = 0.996 9). Thus, the portable, stable, and reproducible QCM immunochip developed in this study allowed the rapid, cost-effectively and sensitively measure the β-LG in milk products.
1. Introduction
Food allergy refers to an abnormal, reproducible and adverse health reaction triggered by the immune system after exposure to a specific food [1,2]. The level of an allergen in food is considered important for food safety, as well as food quality evaluation and quality supervision. Therefore, allergenic ingredients or technical adjuvants must be indicated on the corresponding food labels [3-5].Milk and dairy products are one of the food rich in high-quality proteins, providing nutritional benefit for humans worldwide.Nevertheless, more than 30 types of proteins present in milk are potential allergens, the most common being casein and whey protein, which can cause severe allergic reactions [6,7]. Among them,β-lactoglobulin (β-LG), which accounting for 50% of the whey protein and its average concentration reaching 2-3 g/L in milk, belongs to the Lipocalin family and is one of the most strongly allergenic milk proteins allergen [8-10]. According to the latest results of a recent epidemiological survey, approximately 2%-7% of infants and young children suffer from vomiting, diarrhea, rashes, and other phenomenon related to food allergies due to ingestion of milk,among which approximately 82% of patients with milk allergy are allergic toβ-LG [11]. In food processing,β-LG is also widely added to various foods to increase its nutritional value, but it simultaneously increases the risk of exposure to milk allergens [12,13]. Consequently,monitoring the amount ofβ-LG will contribute to standardizing the labeling of food packaging, which is of great significance to the health security of people with milk allergies.
To date, various methods have been reported to measure the amount ofβ-LG in milk and dairy products, most of which are based on chromatographic separation and immunoassays [14,15].High-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) are standard strategies for the quantitative analysis ofβ-LG in various food matrices [16,17].Because of the precision requirements of the instruments, these methods usually require strict sample pretreatment processes,a larger sample volume, and a longer analysis time, resulting in a higher detection cost. Immunoassays based on specific antigen-antibody recognition can rapidly, reliably, and sensitively detect the target analytes in complex samples. Development of immunoassays has pushed the analysis of trace hazardous substances to new heights [18,19]. Traditional methods, including enzyme-linked immunosorbent assay (ELISA) and immunoassay strips, are simple to operate and can reach a lower detection limit forβ-LG [20-22]. These methods are low-cost and have an irreplaceable advantage in the rapid screening ofβ-LG in a large number of food samples. However,after heating, enzymatic hydrolysis, and other food processing, the conformational epitope ofβ-LG is destroyed which reduces the immunoreactivity, resulting in inaccurate test results. Additionally,under the requirement of accurate quantification, errors caused by inter-batch variation and cross-reaction can easily yield false-positive results. Moreover, most of these detection devices, such as ELISA kits and strips, are for one-time use, which leads to higher testing costs.Emerging technologies, such as electrochemical methods, biomimetic molecular imprinting, and high flux protein microarrays, have been developed in the recent years, which overcome the deficiencies of the above methods and also provide advanced research strategies for the analysis and detection ofβ-LG [23-25]. However, sample preparation for these methods is cumbersome, and the detection process is easily affected by the external environment. Therefore, developing a portable, accurate, sensitive, and low-cost method for monitoringβ-LG in milk and dairy products is necessary.
As an important branch of immunoassays, immunosensors have the merits of high sensitivity, simplicity of equipment, and convenient operation, which have been widely used in clinical diagnosis,environmental monitoring, and food analysis [26-29]. Among them,label-free immunosensing devices do not involve labeling with enzymes, isotopes, or fluorescent reagents required in the traditional enzyme-linked immunoassay and luminescence or fluorescence immunoassay, and can use the signal response of other instruments to achieve quantitative analysis, which has aroused great interest in researchers [30-32]. Quartz crystal microbalance (QCM) is a device that can monitor the resonant frequency shift caused by mass change on the chip surface through the Sauerbrey equation [33,34]. As a quantitativein situindicative device, QCM detects the universal and sensitive resonance frequency shifts. Thus, it has been widely used in the study of biomolecular interactions at different sensing interfaces [35,36]. The immunoassay strategies based on QCM can directly monitor the frequency signal generated by the specific antigen-antibody reaction, and can complete online data collection and analysis. Because these methods combine the convenience of QCM and the high specificity and sensitivity of immunoassays, they have the characteristics of simple operation, easy on-site detection,stable detection results, and good reproducibility, which have great prospects for practical applications. In this study, we directly immobilized the polyclonal antibody ofβ-LG on a QCM Au chip surface to develop a portable QCM immunochip for label-free,accurate, and sensitivequantitative analysis ofβ-LG in milk products.
2. Materials and methods
2.1 Instruments
A QCM instrument (No. 922) from Princeton Applied Research(USA) was used to measure the oscillation frequency. Gold-polished ando-AT-cut quartz crystal chips with a fundamental resonant frequency of 9.0 MHz were purchased from Seiko EG&G Company(Japan). An HPLC, equipped with a UV detector from Agilent Company (USA) was used to verify the results ofβ-LG measurement in milk products. A C4separation column (4.6 × 250 mm, 3.5 μm)from Waters Corporation (USA) was employed for the separation of milk extracts using a mixture of H2O (with 0.1% tetrahydrofuran,THF) (A) and acetonitrile (with 0.1% THF) (B) as the mobile phase at a flow rate of 1.5 mL/min at 60 °C. Millipore ultrafine filters from Merck Company (Germany) and a centrifuge machine from Eppendorf(Germany) were used for the pre-treatment of milk products.
2.2 Chemicals and reagents
The target analyteβ-LG (CAS: 9045-23-2, purity ≥ 90%)was purchased from Sigma-Aldrich (USA). The polyclonal antibody ofβ-LG (anti-β-LG Ab) was purchased from Beijing Bossbio Bio-Technology Co., Ltd. (China). The 1-ethyl-3-(3-(dimethylaminopropyl)carbodiimide (EDC) andN-hydroxysuccinimide (NHS) for Ab immobilization were obtained from General Electric Company (USA). 11-Mercaptoundecanoic acid (> 95%) for QCM chip activation, and ethanolamine (> 99.5%)for blocking were purchased from J&K Scientific Ltd. (China). The other chemicals including glycine (Gly), HCl, NaOH, sodium dodecyl sulfate (SDS), Na2HPO4, and H3PO4were obtained from Tianjin Chemical Reagent Factory (China), and were at least of analytical grade. Doubly deionized water (DDW) used in the experiment was prepared from a Direct-Q ultrapure water system from Millipore(USA). Milk products, including raw milk, pure milk, and infant formula, were purchased from a local supermarket (China).
2.3 Immobilization of anti-β-LG Ab on the QCM chip surface
The quartz crystal chips with an initial frequency of approximately 9.0 MHz were sonicated in ethanol, cleaned using freshly prepared “Piranha” solution (30% H2O2: 98% H2SO4= 1:3,V/V),and rinsed using DDW and ethanol and dried under a steady N2stream. A 10 mmol/L solution of 11-mercaptoundecanoic acid in ethanol solution (100 μL) was used to treat the surface of QCM chip overnight to introduce the —COOH groups by self-assembly (Au-S bond). Furthermore, the carboxylated chip surface was activated using 200 μL of the mixture of EDC (0.4 mol/L) and NHS (0.1 mol/L) (1:1,V/V) to import the ester groups, and fully rinsed using phosphate-buffered saline (PBS). Thereafter, 200 μL of anti-β-LG Ab, at the concentration of 0.2 μg/μL in PBS (pH 7.5), was added onto the QCM chip surface and incubated at 37 °C for 2 h. The chip surface was fully rinsed with PBS and dried using a stream of N2. As a result, the anti-β-LG Ab for specific identification was effectively immobilized on the QCM chip surface. Finally, 100 μL of ethanolamine solution (1.0 mol/L) was applied to block the untreated sites on the chip surface. After rinsing the surface using PBS containing 0.1% Tween, and drying using N2, the fabricated QCM chip was stored at 4 °C under buffered humidity prior to use.
2.4 Measurement of β-LG using the developed QCM chip
Working solutions ofβ-LG at different concentrations(0.5-1.0 mg/mL) were prepared using PBS (pH 7.4). The developed QCM immunochip was fixed into a flowing cell and 100 μL of the tested working solution was added for 5 min to allow the anti-β-LG Ab to react with the targetβ-LG, causing a shift in the QCM frequency (ΔF, Hz). The standard working curve was obtained according to the resulting ΔFvalues at the different tested concentrations ofβ-LG, the standard working curve was obtained.Fig. 1 shows a schematic representation of the principle of directly measuringβ-LG using the developed QCM chip.

Fig. 1 Scheme for the fabrication of QCM immunochip for β-LG detection.
2.5 Chip regeneration procedure
Different regeneration solutions including NaOH (0.05 mol/L),SDS (0.5%,m/V), Gly-HCl (pH 1.5, 0.01 mol/L) and HCl (0.1 mol/L)were prepared and applied to disassociate theβ-LG from the chip surface without destroying the immobilized anti-β-LG Ab layer.After each measurement forβ-LG, 100 μL of regeneration solution was applied to the QCM chip surface for 5 min, followed by rinsing with PBS to regenerate the chip. The regeneration procedure for each regeneration reagent was repeated three times.
2.6 Cross-reactivity evaluation
The specificity of the developed QCM immunochip was evaluated usingα-casein,α-lactalbumin, and lactoferrin, which can easily interfere withβ-LG detection. The resulting ΔFvalues of the different analytes at a concentration of 100 μg/mL were compared.
2.7 Sample preparation and validation of method
β-LG is a typical allergen in milk and dairy products. In this study, raw milk, pure milk, and infant formula were selected as the actual samples to investigate the applicability of the developed QCM immunochip. Accurately weighted milk products, viz. raw milk (5.0 g),pure milk sample (5.0 g) or infant formula (2.0 g), were taken in a 25 mL volumetric flask, respectively. After reaching a constant volume and being fully mixed, the pH of mixture was adjusted to 5.3 using acetic acid. After allowing to stand for 1 h, and centrifugation for 15 min (4 000 r/min), the supernatant was collected, and filtered through a 0.22-μm filter, and stored at 4 °C before use. The extract of milk products was further diluted 100 times using DDW, and a certain volume (100 μL) of the diluent was added into the cell for QCM analysis. The resulting frequency shift (ΔF, Hz) was recorded to calculate the amount ofβ-LG. An HPLC system equipped with a UV detector was used to validate the analysis results from the QCM immunochip. The LC separation was achieved on an Xbridge Protein BEH C4column (4.6 × 250 mm, 3.5 μm) using the mixture of H2O(0.1% THF) (A) and acetonitrile (0.1% THF) (B) as the mobile phase.The gradient conditions at a flow rate of 1.5 mL/min were as follows:A, from 95% to 62% (0-6.5 min); A, 62% (6.5-10 min); A, from 62% to 40% (10-22 min); A, 40% (22-28 min); A, 95% (28-35 min). The injection volume and detection wavelength were 20 μL and 210 nm,respectively. The results ofβ-LG analysis from the QCM immunochip and HPLC were compared, and the correlation coefficient (R2) was calculated. All the tests using QCM immunochip and HPLC were performed in triplicate.
3. Results and discussions
3.1 Fabrication of the QCM immunosensing surface
In this study, anti-β-LG polyclonal Ab was used for the specific identification ofβ-LG in milk products. Therefore, it was necessary to maintain the activity of Abs and effectively immobilize them on the surface of the QCM chip. The QCM Au chip was first carboxylated by the self-assembly of 11-mercaptodecanoic acid through the Au-S bond, which then formed a covalent bond with the -NH2group of the anti-β-LG polyclonal Ab. To ensure that enough Abs are immobilized on the surface of the QCM chip so that the developed immunochip possesses high accuracy and sensitivity forβ-LG detection, controlling the ionic strength (pH) of the microenvironment is necessary. PBS with different pH values (6.0, 6.5, 7.0, 7.5 and 8.0) was used to prepare the anti-β-LG Ab solution at 0.2 μg/μL for the immobilization on the QCM chip surface. The resulting QCM frequency shifts caused by anti-β-LG Ab modification are listed and compared in Fig. 2.

Fig. 2 Results of anti-β-LG Ab immobilization onto the QCM chip surface under different pH values.
It was observed that the resulting frequency shift increased from 174.6 Hz to 394.3 Hz with the increase in buffer pH from 6.0 to 7.0,signifying that more anti-β-LG Abs are immobilized on the QCM chip surface. This is because when the pH of the coupling buffer is lower than the isoelectric point of the anti-β-LG Ab (approximately 7.4),the anti-β-LG Ab is positively charged and the carboxylated QCM Au chip surface is negatively charged. The resulting electrostatic attraction is conducive to the immobilization of the anti-β-LG Ab on the surface of the chip. When the pH of the microenvironment is higher than 7.5, the Ab protein and the chip surface are both negatively charged, forming an electrostatic repulsion, and thus,fewer anti-β-LG Abs are immobilized. According to the values of the obtained frequency shift, the frequency at pH = 7.5 (576.2 Hz) was markedly greater than that at pH 7.0 (394.3 Hz) and 8.0 (343.2 Hz).A microenvironment close to the isoelectric point can maintain the stability of the protein structure, which is conducive to the specific recognition of the target. Therefore, a microenvironment of pH = 7.5 was used for the adhesion of the anti-β-LG Ab on the chip surface.
3.2 Optimization of anti-β-LG Ab concentration
The concentration or amount of Ab used in the analysis is a crucial parameter for the QCM immunosensing platform. A high Ab concentration can increase the steric hindrance on the chip surface,which will not only suppress the specific binding betweenβ-LG and anti-β-LG Ab, but also greatly increase the cost of detection.Comparison of the frequency shifts resulting from anti-β-LG Ab modification at different concentrations on the surface of the QCM chip (loading volume: 200 μL) are shown in Fig. 3. It was evident that with the increase in anti-β-LG Ab concentration, the frequency shift of the QCM chip changed significantly. In the lower concentration range(0.05-0.20 µg/μL), the gradual increase in frequency shift indicates that more anti-β-LG Ab proteins are bound on the chip surface. When the anti-β-LG Ab concentration reached 0.2 µg/μL, the frequency shift achieved a value of 392.4 Hz. When the concentration of anti-β-LG Ab was increased further, the frequency shift do not increase significantly, indicating that the Ab protein immobilized on the chip surface was nearly saturated. Thus, considering the cost of the Ab and the sensitivity of the developed immunochip, 200 μL of anti-β-LG Ab solution at a concentration of 0.20 µg/μL was used for the construction of the QCM immunochip.
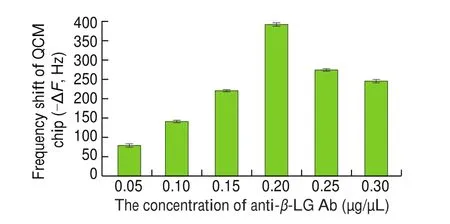
Fig. 3 Frequency shifts observed when using different concentrations of antiβ-LG Ab immobilization.
3.3 Regeneration procedure for the developed QCM immunochip
This study aimed to develop a QCM immunochip that can detectβ-LG allergen in milk products rapidly, accurately, and at a low cost.The regeneration capability of a chip directly affects the detection accuracy, sensitivity, and cost. An ideal regeneration process should effectively destroy the specific binding between theβ-LG and anti-β-LG Ab, thereby removing the boundβ-LG from the surface of the chip, without effecting the recognition ability of the anti-β-LG Ab on the chip surface in the next cycle. Several solutions with different properties, including NaOH (0.05 mol/L), SDS (0.5%,m/V), Gly-HCl(pH 1.5, 0.01 mol/L) and HCl (0.1 mol/L) were prepared and applied for studyingstudy of the chip surface.
According to the obtained results (Fig. 4), using NaOH (0.05 mol/L)and SDS (0.5%) solutions as the regeneration solvent reduced the base frequency of the QCM immunochips significantly, and the difference in frequency for three consecutive regeneration cycles reached 451 and 271 Hz, respectively. This is because the two alkaline regeneration reagents destroyed the binding ofβ-LG and anti-β-LG Ab, and simultaneously eluted the anti-β-LG Ab pre-modified on the chip surface. As a result, the frequency response obtained in the next measurement process was significantly reduced,attenuating to 65.3 and 45.6 Hz after three regeneration cycle,respectively. When acidic solutions (Gly-HCl and HCl) were used as regenerating agents, the base frequency of the QCM immunochip reduced by only 255 and 118 Hz, respectively, after 3 repeated regeneration processes, and the attenuation of the frequency response from three consecutive measurements was only 31.4 and 7.5 Hz.Based on the above results, HCl (0.1 mol/L) was selected as the reagent for chip regeneration.
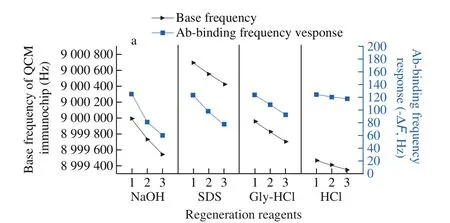
Fig. 4 Results of base and Ab-binding frequency response using different regeneration reagents.
3.4 Measurement of β-LG using the developed QCM immunochip
The response of the constructed QCM immunochip toβ-LG was investigated by measuring the frequency shift using different concentrations ofβ-LG standard solutions dripped onto the surface of the QCM immunochip. After eliminating the base frequency of the QCM chip, the obtained frequency shift response at each concentration (0.5-1.0 mg/mL) was used to plot the calibration curve forβ-LG detection (Fig. 5). The obtained calibration curve had a good nonlinear correlation, and as theβ-LG concentration increased, the QCM frequency shift tended to saturate. The equation of the calibration curve obtained was ΔF= 12.877Cβ-LG0.4809with anR2of 0.998 2. The limit of detection (LOD) of the developed QCM immunochip forβ-LG was determined from the relative standard deviation of three blank injections, which was calculated to be as low as 0.04 μg/mL. The limit of quantification (LOQ) of the developed immunochip forβ-LG analysis was calculated as the standard deviation (SD) of ten blank injections, and the calculated result was 0.132 μg/mL.

Fig. 5 Calibration curve for β-LG detection using the developed QCM immunochip.
The standard deviation of the frequency shift response of seven parallelly-prepared QCM immunochips against theβ-LG standard solution at the same concentration (100 μg/mL) was only 0.72 Hz.Fig. S1 showed that the frequency shift from ten consecutive analysis cycles using one QCM immunochip. The frequency shift at the tenth analysis can still reach 114.8 Hz with less response attenuation(< 7.9%), indicating the excellent stability and reusability of QCM immunochip. After storage at 4 °C for one week, the frequency response to the same concentration ofβ-LG solution hardly changed,with a deviation was 2.7% (n= 7), less than 5%. These results indicate that the constructed QCM immunochip has low inter-assay variation, good stability in preparation and storage procedure and can provide a portable, accurate, sensitive, and low-cost analysis method forβ-LG.
3.5 Specificity research
In this study, theα-casein,α-lactalbumin and lactoferrin, proteins that easily interfere withβ-LG detection were selected to evaluate the specificity of the constructed QCM immunochip. At the selected concentration (100.0 μg/mL), the average frequency shift responses to the interfering proteinsα-casein and lactoferrin were only 8.9 and 11.8 Hz, with SDs (n= 3) of 2.8 and 2.8, respectively, which are much lower than that caused byβ-LG (124.2 Hz) at this concentration.This result signified that theβ-LG analysis using the developed QCM chip was unaffected by these two interfering proteins. The frequency response ofα-lactalbumin at the concentration of 100.0 μg/mL achieved to 48.9 Hz (SD: 2.0,n= 3), nearly 40% ofβ-LG frequency response, meaning the obvious cross-reaction toβ-LG. This might be because that the major antigenic site ofα-lactalbumin is located on peptide corresponding to amino acids (AA) 5-18, which corresponds to the homologous sequence AA 124-134 on theβ-LG molecule,leading to its sequential epitopes structure can also be bound by the anti-β-LG Ab used in this study.
3.6 Sample analysis and validation of method
To evaluate the potential of application of the proposed QCM immunochip for analyzingβ-LG in milk products, typical milk products, such as raw milk, pure milk, and infant formula,were selected and spiked withβ-LG at three levels (0.5, 1.0, and 2.0 mg/mL). After simple sample treatment, the resulting extracts were used to measure the amount ofβ-LG using the developed QCM immunochip, and the standard HPLC method was employed to verify the accuracy of the QCM analysis. The data obtained forβ-LG in both QCM and HPLC measurements are illustrated in Table 1. Three replicates of each experiment were performed for all tested samples.
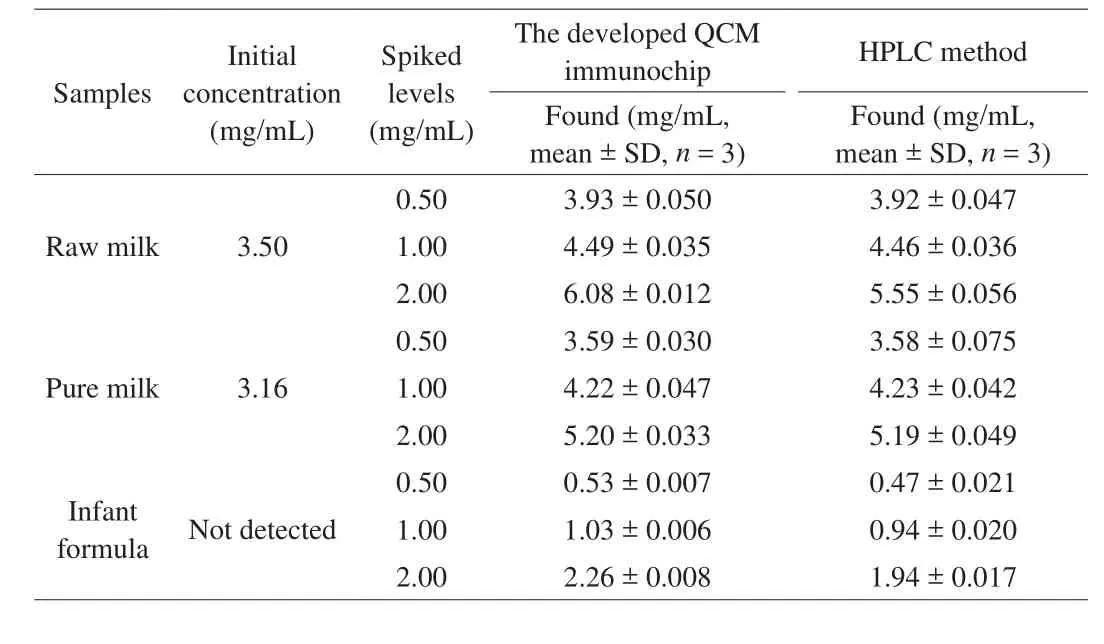
Table 1Results of β-LG detection in milk products using the developed QCM immunochip and HPLC method.
It was clear that at all the tested concentrations, the QCM immunochip yieldedβ-LG concentrations similar to those obtained using the HPLC method, with a correlation coefficient achieving of 0.996 9 (Fig. S2). This means that the QCM immunochip developed in this study can be used for reliable and accurate analysis strategy ofβ-LG. Additionally, the developed QCM immunochip is portable, can be easily stored, and can be reused many times. The entire detection process required a shorter analysis time (less than 5 min). These characteristics indicate that the constructed QCM immunochip can meet the requirements for large-scale number of sample screenings and rapid on-site detection, which is an ideal strategy forβ-LG analysis. Comparison of the results of the reported strategies used for β-LG analysis in various matrices has been provided in Table 2,whereinthe merits of the developed QCM immunochip have been mentioned.
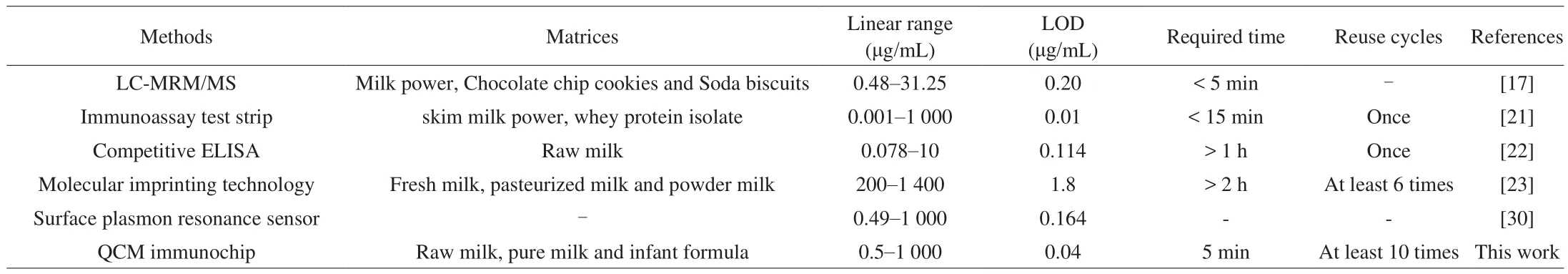
Table 2Comparison of the merits of the reported assays for β-LG detection in sample matrices.
4. Conclusion
In this study, we successfully fabricated a QCM immunochip for the detection ofβ-LG in milk products, which showed good specificity (low cross-reactivity) and sensitivity. Compared to the conventional methods currently in use, the proposed QCM immunochip offers a real-time, rapid and low-cost strategy forβ-LG detection, which can be applied for large-scale milk sample screening and rapid on-site detection. The QCM-based immunochip detection technology developed in this study can also be extended for the detection of other food allergens. It is a very promising technology that has broad prospects in the screening of raw material for processed of food and in monitoring the various aspects of food processing.
conflict of interest
The authors confirm that the contents of this article have no conflict of interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (31972147), Project of Tianjin Science and Technology Plan (19PTSYJC00050), the Open Project Program of State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science and Technology (SKLFNS-KF-202011), and Project program of Key Laboratory of Food Nutrition and Safety,Ministry of Education, Tianjin Key Laboratory of Food Nutrition and Safety, China (JYB202002).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.04.015.
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
