Effect of plant-based functional foods for the protection against salt induced endothelial dysfunction
2022-06-23SheungYinSanJenniferWanJimmyChunYuLouie
Sheung Yin San, Jennifer. M. F. Wan, Jimmy Chun Yu Louie*
Discipline of Food and Nutritional Sciences, School of Biological Sciences, Faculty of Science, The University of Hong Kong, Pokfulam 999067, Hong Kong, China
Keywords:
Endothelial dysfunction
Salt intake
Functional foods
Nitrate
Flavonoids
Flow-mediated dilation
A B S T R A C T
This study aimed to compare the efficacy of four formulations of plant-based functional foods on the protection against salt-induced endothelial dysfunction. A randomized crossover design was employed.Ten healthy subjects were recruited, and on five separate occasions they received, in random sequence one of the following 5 treatments: 250 mL of plain water (control) alone, and with beetroot powder,celery powder, green tea extract or beetroot powder with green tea extract prior to consuming 150 mL of high-salt chicken broth. Flow-mediated dilation (FMD), blood pressure (BP), heart rate (HR) and pulse-wave velocity (PWV) were measured at fasting and at 30, 60, 90 and 120 min postprandial.Comparing with control, beetroot supplementation led to a significantly increased HR at 30, 60 and 90 min postprandially (P = 0.025, 0.004, < 0.001, respectively). No significant difference was observed for FMD, BP and PWV between control and any of the treatments. Salt reduction may still be the most effective strategy to improve vascular health.
1. Introduction
Consisting of only a monolayer of endothelial cells, the vascular endothelium is not only a physical barrier between the blood and other tissues, but also an important paracrine, endocrine,and autocrine organ for the regulation of vascular tone and the maintenance of vascular homeostasis [1,2]. Normal endothelial cells are capable of metabolizing, synthesizing and releasing a variety of substances that perform the above functions [2]. Among all, nitric oxide (NO) is an essential vasodilator that contributes to normal endothelial function [1-3]. Reduced bioavailability of NO results in the impairment of endothelium-dependent vasodilation, a condition known as “endothelial dysfunction” [1-3]. Endothelial dysfunction is an important initial step in the development of atherosclerosis [4],thus serving as an early target for the identification of cardiovascular disease risks.
Diet is one factor leading to endothelial dysfunction, and studies have provided compelling evidence for the adverse effect of high-salt intake on endothelial function [5-8]. Plasma sodium concentration is increased after a high-salt meal, which is shown to stiffen human endothelial cells, thus reducing nitric oxide production [5]. It is a global trend that our average daily salt intakes exceeds the levels recommended by the World Health Organization (WHO) [9].However, dietary habits are not easy to change. Therefore, alternatives have to be found for the protection against salt-induced endothelial dysfunction, and plant-based functional foods are one possibility.Plants contain a variety of different compounds, including nitrates,which has been shown to have beneficial effects on vascular health. Dietary inorganic nitrate, once ingested, will undergo a series of biochemical reactions in the body, producing NO, the key vasodilator [1-3].
This study aimed to compare the efficacy of four different formulations of plant-based functional foods for the protection against salt-induced endothelial dysfunction. The four formulations include beetroot powder, celery powder, green tea capsule, and a combination of beetroot and green tea. Studies have demonstrated the beneficial effect of fruits and vegetables on cardiovascular health [10,11]. Beetroot and celery are chosen for this study because they are high in nitrate, with more than 250 mg of nitrate present per 100 g of the fresh food [12]. In addition to nitrate, beetroot contains the pigment betalain, which is also claimed to have many benefits on cardiovascular health [13]. An investigation of both beetroot and celery supplement would allow us to study the effect of nitrate from different dietary sources, as well as to study the possible involvement of other compounds like betalain in the improvement of endothelial function. Green tea, though not a good source of nitrate,is included in the study because it is high in catechin, a flavonoid with many proclaimed health benefits, including the improvement of endothelial functions [14,15]. A combination of beetroot and green tea supplementation is included to investigate if a combination of all the targeted compounds would produce a maximal protective effect. Therefore, we hypothesized that dietary supplementation of nitrate and flavonoids may improve endothelial function, and that a combination of beetroot and green tea, when consumed simultaneously, would provide a synergism in their beneficial effects and thus is the most effective for the protection against salt-induced endothelial dysfunction.
2. Material and methods
2.1 Subjects
Fig. 1 summarizes the study flow for subject recruitment. A total of twenty volunteers, recruited by personal contact and recruitment posters at the University of Hong Kong (HKU), were screened for eligibility between 9 May to 21 June 2018. After screening,10 healthy volunteers aged between 18 and 23 years met all the inclusion criteria and agreed to participate in the study. Inclusion criteria included 18-40 years of age, body mass index (BMI) between 18.5 and 23.0 kg/m2(i.e. healthy weight as defined in the WHO Asian BMI cut-off [16]), systolic BP (SBP) < 140 mmHg, diastolic BP (DBP)< 90 mmHg, no current or history of hypertension, cardiovascular diseases including hyperlipidemia, blood-related diseases, type 1 or type 2 diabetes or impaired glucose tolerance, liver diseases or gastrointestinal diseases. Participants were excluded if they were intolerant or allergic to beetroot, green tea or celery, on regular medication except oral contraceptives, intentionally losing weight,women who were pregnant or planning to be pregnant during the study, with special dietary requirements including vegetarianism,current or former smokers, users of mouth wash, regular alcohol consumers, or endurance sport participants. This study follows the guidelines laid down in the Declaration of Helsinki. All participants gave written informed consent before the commencement of the study. The study was approved by the HKU Human Research Ethics Committee (EA1703023).

Fig. 1 Consolidated standards of reporting trials study flow for subject recruitment.
2.2 Study design and protocol
The study employed a randomized crossover design. Each participant completed 5 sessions on 5 separate mornings. Each session was separated by a washout period of at least 4 days. A randomized test sequence was assigned to each participant using random numbers generated by Excel (Microsoft, Redmond, WA). No participants received the same test sequence. In each session, participants consumed 150 mL of high salt chicken broth (Swanson) with 3.8 g added salt (5.56 g total salt, including the salt present in the soup),together with one of the following 5 treatments (Table 1): 250 mL of water only (control), 24.5 g beetroot powder (Bioglan) dissolved in 250 mL water, 10.6 g freeze-dried celery powder (Eclectic) dissolved in 250 mL water, one capsule of green tea extract (GNC) with 250 mL water, or 24.5 g beetroot powder in 250 mL water plus one capsule of green tea extract. Though different amount of beetroot powder and celery powder was used, they had similar amounts of inorganic nitrate (approximately 350 mg). The betalain content of the beetroot powder was not determined in this study, but a previous study reported it ranges 7.2-17.24 mg/g dry mass (i.e. 176.4-422.38 mg per 24.5 g serving of beetroot powder) [17]. The amount of nitrate in each of the treatments was determined by high-performance liquid chromatography (HPLC) prior to the commencement of the study.The green tea capsules do not contain nitrate but had around 125 mg of epigallocatechin gallate per the manufacturer’s specification.

Table 1Content of bioactive compounds (mg/treatment serving) in the study treatments.
Prior to the test sessions, participants were asked to refrain from consuming caffeinated drinks and alcohol for three days, and refrain from vigorous physical activity on the day before. On the night before the test session, participants were required to consume a standard dinner, and no foods or drinks except water were allowed after that.At least 8 h of fasting was required. For females, they were not allowed to perform the test during their menstruation.
The test sessions were conducted in a temperature-controlled room at the School of Biological Sciences, HKU. On arrival,participants were asked to empty their bladder. Then, body weight was measured to the nearest 0.1 kg using an electronic digital scale(Seca, 769). After 5 min of rest, baseline flow-mediated dilatation(FMD) was measured. Baseline blood pressure (BP), heart rate (HR)and pulse-wave velocity (PWV) were also measured in triplicates.After the baseline measurements, subjects were asked to consume the test drinks (high salt broth and one of the treatment drinks) within 5 min. The same set of measurements (FMD, BP, HR and PWV) was measured every 30 min until 120 min. Each session lasted 2.5 h. To facilitate the measurement of PWV and avoid unnecessary movements of the participants, the same semi-supine position with an angle of 30 degrees was maintained throughout the whole session.
2.3 FMD
FMD was measured as previously described [18,19]. The measurements were done on the non-dominant side of the participants(all left in this study), using an ultrasound machine (Mindray DP50,Shenzhen Mindray Bio-Medical Electronics, Shenzhen, China) with a 7.5 MHz linear probe (Model 75L38EA, Mindray, Shenzhen,China). The longitudinal view of the left brachial artery was captured by ultrasound, following a 5-min forearm occlusion at 250 mmHg by a sphygmomanometer. Images were obtained at baseline (before occlusion), 30 s before cuff release, and then every 30 s after cuff release for 2 min. Shear rate was not determined. All images were stored for offline analysis and were measured by the same, single observer. Arterial diameter was measured by ultrasonic calipers at end diastole using the in-built software of the ultrasound machine.FMD was then expressed as the percentage change from the baseline diameter, calculated by the following formula:

2.4 Blood pressure, PWV and heart rate
BP was measured with a specialist device (Vicorder, SMT Medical, Wuerzburg, Germany) on the dominant arm of the participant (which was all right arm in this study). The participants maintained the semi-supine position with the arm resting at heart level throughout the measurements. The BP cuff was placed at the upper right arm. At each time point, at least three consecutive BP measurements were taken, until SBP readings fell within 10 mmHg and DBP readings within 5 mmHg. The mean values were then taken for analysis. Carotid-femoral PWV was measured using the same Vicorder device, following the instructions from the Vicorder User Manual [20]. Same as BP, PWV was measured on the dominant side of the participant (which was all right sides in this study). An inflatable neck pad was placed over the subject’s neck with the pressure sensor securely placed on the right carotid area. A BP cuff was placed around the upper right thigh in a position as high as possible. The distance (in centimeters) between the suprasternal notch and the midpoint of the thigh cuff was determined. The carotid sensor and thigh cuff were then simultaneously inflated and PWV value was recorded when several steady waveforms were obtained.Three consecutive PWV measurements were made at each time point.HR was also determined at the same time as the PWV measurements using the same Vicorder device and the same program.
2.5 Statistical analysis
The results were expressed as mean ± SD unless otherwise specified. All the outcome variables (FMD, SBP, DBP, HR and PWV)were plotted as postprandial curves, and the areas under the curve(AUC) of the FMD graphs were determined. The AUC was expressed as % AUC relative to the control. Differences in the % AUC between the control and each of the treatments were compared using pairedt-test. These were done using Excel 2016 (Microsoft, Redmond, WA).Also, differences between the control and each of the treatments in the outcome variables at each time point were tested using Linear Mixed Models with the baseline measurement as the covariate [21]using SPSS 25 for Windows (SPSS Inc, Chicago, IL). Significance was set at two-sidedP< 0.05.
3. Results
All 10 volunteers (7 men and 3 women) completed the study,and their key characteristics at screening are summarized in Table 2.There was no significant change in their BMI during the whole study period. All of their BP values were within the normal range at screening. All of the test drinks were well tolerated and consumed within 5 min, and no adverse events were reported during the study.

Table 2Baseline characteristics of participants.
The mean ± SD baseline diameter, peak diameter, absolute change in diameter and shear rate of the subjects at various timepoints under different treatment conditions were presented in Table 3. There was no significant difference between treatments in all of these parameters at any timepoints.

Table 3Mean ± SD baseline diameter, peak diameter and changes in diameter from 0 to 120 min by treatment conditions.
Postprandial FMD decreased in all treatments (Fig. 2a). For the control, the change in FMD was (9.07 ± 3.54)%, (8.94 ± 3.61)%,(9.25 ± 3.24)% and (9.69 ± 2.87)% at 30, 60, 90 and 120 min postprandial, respectively. None of the treatments had postprandial FMD significantly different from the control at any of the time points.Treatment with celery (92.89 ± 24.57)% had % AUC lower than the control (100%), while treatments with beetroot (105.74 ± 36.38)%,green tea (101.49 ± 36.35)%, and the combination of beetroot and green tea (109.39 ± 38.84)% had % AUC higher than the control(Fig. 2b). Beetroot + green tea produced the highest % AUC.However, none of the differences was statistically significant.

Fig. 2 (a) FMD and (b) AUC at fasting and in response to consumption of the treatments, represented by % AUC using control as reference (100%).Values presented as mean ± SEM. n = 10 (7 men and 3 women). For FMD,linear mixed models were used to test for difference between control and each treatment at each time point, adjusted for baseline values. Paired t-test was used to test for differences in % AUC between control and each treatment. No significant difference was found.
Postprandial blood pressure (both SBP and DBP) increased in general. For SBP, the maximum increase occurred at 120 min postprandial for all treatments, but the same could not be observed for DBP (Figs. 3a,b). No significant difference was observed between the control and any of the treatments at any of the time points. Except for green tea, all other treatments had postprandial HRs higher than the control (Fig. 3c). Treatment with only beetroot showed HRs of(56 ± 8), (55 ± 7) and (58 ± 11) beats/min at 30, 60 and 90 min postprandial respectively. These were all significantly different comparing with the control ((53 ± 7), (52 ± 6), (52 ± 5) beats/min;P= 0.025, 0.004, < 0.001 respectively). The treatment with only green tea resulted in higher PWV than the control (Fig. 3d). Yet, no significant difference was observed between the control and any of the treatments at any of the time points.
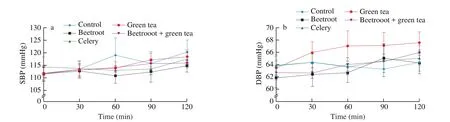
Fig. 3 (a) SBP; (b) DBP; (c) HR; and (d) PWV at fasting and in response to the treatments. n = 10 (7 men and 3 women). Linear mixed models were used to test for difference between control and each treatment at each time point, adjusted for baseline values. For heart rate, significant difference was found between beetroot and control at 30, 60 and 90 min (P = 0.025, 0.004, and < 0.001, respectively). No significant difference in SBP, DBP and PWV was found at any time point for any of the treatments.
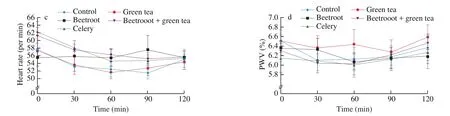
Fig. 3 (Continued)
4. Discussion
This study showed no significant improvement in endothelial function after consuming a high-salt meal with different plantbased functional foods. Although slight improvements of FMD could be observed in three of the treatments, these were statistically insignificant. Our study also showed no significant difference in terms of PWV, a reflection of arterial stiffness and a marker of endothelial dysfunction, after the supplementation of the test drinks. This matches the result of a meta-analysis [22]which concluded that PWV was not able to show the significant effects of nitrate supplementation, while FMD could. Therefore, in future studies, FMD should be the choice of measurement of endothelial function, whereas the use of PWV is limited. Our study also showed no significant difference between controls and treatments in terms of BP.
Our results were consistent with some other studies. Bahra et al. [23]found that nitrate supplementation of 500 mg had no effect on acute FMD in 14 healthy subjects. Gilchrist et al. [24]studied the effect of 2-week beetroot juice supplementation (containing 465 mg nitrate)on 27 patients with type 2 diabetes mellitus and found no significant improvements in FMD. Bondonno et al. [25]were able to show significant improvement in acute FMD with 182 mg of nitrate from spinach in 30 healthy subjects but reported the difference in FMD being very small. Therefore, in our study with only 10 subjects, and a mean participant age of only (20.8 ± 1.2) years, the difference in FMD may even be harder to detect.
There were different pathways involved regarding how nitrate and flavonoids may affect endothelial function. NO, the key vasodilator and key mediator of FMD, can be produced by different mechanisms in our body. Flavonoids enhance endothelial function and NO production primarily via an endogenous pathway [25,26].Endothelial NO synthase (eNOS), one key enzyme of NO production,is activated by flavonoids [26]. Besides, flavonoids also work on inhibiting NO breakdown [25], thus improving endothelial function.On the other hand, nitrate is involved in the exogenous nitrate-nitritenitric oxide pathway [25]. Ingested nitrates have two routes in the body. Most of the nitrates are absorbed into plasma for circulation,while some remained in the mouth. The dorsal surface of the tongue is concentrated with bacteria that are capable of converting nitrate to nitrite virtually instantly by the action of nitrate reductase [27]. The nitrite is then swallowed and the acidic environment in the stomach favors its non-enzymatic reduction into NO. Around 25% of absorbed nitrate enters the enterosalivary circulation pathway [12,28]. In the salivary glands, there is an active uptake of nitrate from blood,resulting in a 10 to 20-fold higher concentration of nitrate in the saliva than in blood [28]. The exact mechanism of this salivary uptake is still unknown [12], but it contributes to a major source of nitrite and NO in the body, due to the action of oral bacterial nitrate reductase.The release of active nitrite into the blood happens within 15-30 min of consumption [10,29]which should have occurred within the timeframe examined in the current study under normal physiological conditions, but since our study involved the high salt condition,the above mechanisms might not apply. Further study is needed to identify the interaction between salt, nitrate and flavonoids.
We also hypothesized there may be synergism between the effects of nitrate and flavonoids on endothelial function. However,this could not be shown in our study. Bondonno et al. [25]also tried to study the synergism between nitrate and flavonoids, using spinach and apple as the respective sources, and their results were consistent with ours. In fact, inhibition instead of synergism was shown in their study. They found decreased plasma concentration of nitrite after the simultaneous ingestion of nitrate and flavonoids and hypothesized that NO production from nitrite in the stomach was augmented, thus less nitrite was available for the absorption into the circulation [25].The same mechanism might also be true in our study. Therefore, the synergism between nitrate and flavonoids might not be as powerful as originally assumed.
Webb et al. [10]showed that plasma nitrite concentration peaked at around 3 h after dietary nitrate supplementation. Nitrite plays an important role in the synthesis of NO through the nitrate-nitrite-nitric oxide pathway. The fact that its plasma concentration peaked at 3 h after supplementation implied that its beneficial effects on endothelial function may also be the most prominent at or after the 3-h interval.Our study, lasting for only 2 h, may not be long enough to detect such beneficial change. Though in a study conducted by Bahra et al. [23]in which they looked at endothelial functions only at the 3-h time point, still no significant change could be detected in FMD, although improved vascular compliance was shown. The same reason may apply to the insignificant results of BP and PWV. In different studies looking at the effect of beetroot or nitrate supplementation on BP, the maximum blood pressure-lowering effect occurred at 2.5 h to 3 h after the supplementation [10,12,30]. Once again, this suggested that our study could be improved by extending each session to at least 3 h or longer.
Our results suggested that supplementation of inorganic nitrate,flavonoids, or even a combination of the two, did not improve endothelial function as impaired by a high salt meal in healthy young adults in a 2 h period. This implies that the best way to protect against salt-induced endothelial dysfunction may still be to reduce the amount of salt in our diets. Dickinson et al. [31]found that a reduction of salt intake from 9 g/day to 6 g/day (which is still higher than the WHO recommendation of 5 g/day) is already able to reverse the impaired FMD in overweight and obese subjects. This suggested that salt reduction should be the top priority when dealing with endothelial dysfunction.
A limitation of our study was that biomarkers such as plasma sodium, nitrate and nitrite were not measured. Without information on these biomarkers, we could not understand the underlying mechanisms of our observations, and the effectiveness of our treatments in raising nitrite levels could not be determined. Furthermore, the high-salt diet consumed in this study was only a bowl of chicken soup, which is not a good representation of the usual diet of an individual. Highsalt diets with comparable carbohydrate and fat contents as our usual diets would be more useful in understanding the efficacy of the tested functional foods, as this provides conditions closer to the way salt is consumed in real life. We also did not assess shear rate, but it has been suggested that normalizing FMD using shear rate is not statistically sound and meaningful [32].
The lack of a time control where only the treatment drinks but not the high salt broth was consumed also means that we are unable to confirm the consistency of the vascular measures. We also only controlled for the effect of menstruation on vascular functions by disallowing female subjects to attend the test sessions during their menstruation. It has been suggested that vascular function can vary during various periods of the full menstrual cycle [33,34], and most studies perform treatment/intervention on women only during their early follicular phase to minimize such effect. Nonetheless, only 3 female subjects were included in our study, and excluding their data did not materially change the results and conclusions (data not shown). Furthermore,a priorisample size calculation was not conducted. In fact, it was suggested that in order to show significant change in FMD, a typical sample size of 20 to 30 was needed for a crossover design study [18]. Therefore, the lack of observed differences between treatments may be a result of inadequate statistical power.Last, our study failed to consider the other nutrients present in the test drinks. We only focused on having similar amounts of nitrates in the treatments but ignored the amount of other nutrients in the test drinks.For instance, the beetroot powder used contained around 70% sugar.However, the celery and green tea used did not have comparable amounts of sugars. Presence of sugar in one treatment but not others may have biased the result because high-sugar diets have also been shown to impair endothelial function [35]. Increases in plasma glucose level have been shown to induce postprandial endothelial dysfunction by increasing oxidative stress and decreasing the bioavailability of nitric oxide [35].Therefore, the sugar present in the treatments might have masked the beneficial effects of the treatments.
5. Conclusion
We found no significant improvement of FMD, BP and PWV by any of the tested treatments after a high-salt meal in a group of healthy young men and women. Supplementation of nitrates and flavonoids alone or in combination seemed ineffective in reversing the adverse effects on endothelial function caused by a high salt meal in this population. Salt reduction may still be the most effective method for the protection against endothelial dysfunction. Further study is needed to investigate how the body responds to nitrate and flavonoid supplementations, with a longer duration for each session.
conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgement
This study was funded by the Seed Funding for Translational and Applied Research, University Grants Council, The University of Hong Kong (Ref: 201611160038).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
