Cow milk αs1-casein induces allergic responses in a mouse model of atopy
2022-06-23GungyuWngXiofengYuYnjunCongLinfengLi
Gungyu Wng, Xiofeng Yu, Ynjun Cong,*, Linfeng Li
a Beijing Higher Institution Engineering Research Center of Food Additives and Ingredients, College of Food and Health,Beijing Technology and Business University, Beijing 100048, China
b Department of Dermatology, Beijing Friendship Hospital, Beijing 100050, China
Keywords:
Cow milk
αs1-Casein
Allergen
BALB/c mouse model
Allergic mechanism
A B S T R A C T
αs1-Casein is a potential allergen to induce hypersensitivity in cow milk. We had identified αs1-casein and its epitopes in previous studies. The present study aimed to evaluate the allergic mechanism of αs1-casein in a BALB/c mouse model. The levels of specific IgE, mast cell proteinase, histamine and cytokines in sensitized mice were determined, and the clinical and pathological observation were evaluated. Results showed that the levels of specific IgE, mast cell proteinase, histamine, IL-4, IL-5, IL-10 increased significantly with a dose-dependent trend. The local alveolar septum collapsed or thickened, and lymphatic foci were produced in the spleen and thymus, and the inflammatory cells infiltrated in small intestinal mucosa mesenchyme.In conclusion, the levels of specific IgE, mast cell proteinase, histamine, IL-4, IL-5, IL-10 and some inflammatory factors could possibly serve as allergic biomarkers of αs1-casein, however, additional studies on signal transduction and gene expression are necessary in future.
1. Introduction
Cow milk is one of the most common food allergens for children younger than three years of age with IgE or IgG1 mediated allergic responses, and consists of more than 25 different proteins [1,2].About 65% of patients suffering from cow’s milk allergy are mainly sensitive toαs1-casein [3]. Our previous work showed thatαs1-casein was a potential allergen by ELISA and Western blotin vitro[4]. The other study demonstrated that the detection and evaluation of cow’s milkαs1-casein were mainly usingin vitromethods [5], whereas,thein vivomethods were few reported, it is very important to find a reliable biomarker to qualitatively analyze the allergenicity potential ofαs1-casein.
A number of advantages were shown for mice compared with other animal models in allergy research, such as, their small size,short breeding cycle and well-characterized immunology [6,7].The phenotypes of mouse models, BALB/c strain, C3H/HeJ strain,C57Bl/6 strain, etc., are greatly influenced by genetic background,gender, route of allergen exposure, the nature and concentration of food allergens, as well as the use of adjuvants [8]. BALB/c mice are generally believed to be a Th2-baised strain, however reported being resistant to the induction of hypersensitivity to cow’s milk or peanut,in contrast to the C3H/HeJ strain [9]. Although some mouse models have been successfully established using whole milk,very limited published models were established using a single milk casein protein.Nevertheless, the natural complexity of the allergic reactions makes it difficult to find a single reliable endpoint parameter for quantitative analyze the sensitization potential of an allergen [10]. Therefore, there is urgent need to use the reported models to evaluate food allergen and to screen the treatment methods.
The present work aimed to further evaluate allergic mechanism ofαs1-casein after gastrointestinal digestion in a BALB/c mouse model.Then, biomarkers were characterized by measuring the levels of allergen-specific IgE antibody, splenic cytokine, plasma histamine,mast cell proteinase, and histopathological observation was also carried out.
2. Materials and methods
2.1 Isolation of αs1-casein from cow’s milk
Isolation ofαs1-casein from raw cow’s milk (Beijing Sanyuan Foods Co., China) was achieved by ion exchange chromatography using previously published methods [11]with some modifications.Cow’s milk was heated to 45 °C and centrifuged at 1 650 ×gfor 30 min and then the fat was removed. Skim milk was reheated to 45 °C, the pH was adjusted to 4.2 with 2 mol/LHCl, and the milk was stirred for 30 min and then centrifuged at 1 650 ×gfor 15 min to recover the casein precipitate. Casein was washed (4 times) by resuspension in purified water (10 times the original milk volume)and stirring for 1 h before recentrifugation at 1 650 ×gfor 15 min.The casein was then freeze-dried, packed in vacuum, and stored at 4 °C until required. The casein was dissolved at 11.5 g/L in 25 mmol/L sodium formate buffers containing 7.5 mol/Lurea and 0.8 mmol/L dl-dithiothreitol (DTT), pH 4.0, stirred for 2 h at room temperature(21 °C), and then filtered through a 1.0 μm filter. 10 mL casein solutions were applied to a chromatography column (50 × 1.6 cm)equilibrated with 75 mmol/L sodium formate, 7.5 mol/Lurea, and 64 μmol/LDTT, pH 4.0. Elution was carried out at room temperature(21 °C) at a flow rate of 1.0 mL/min by a stepwise gradient of 0.11, 0.16, and 1.0 mol/LNaCl in 75 mmol/Lsodium formate,7.5 mol/Lurea, and 64 μmol/LDTT, pH 4.0. The protein peaks were collected in separate fractions and ultra-filtered using a 10 kDa membranes to remove urea and sodium chloride before freeze-drying. The fractions were assessed by SDS-PAGE.
2.2 Characterization of column fractions by SDS-PAGE and N-terminal amino acid sequencing
SDS-PAGE was carried out according to Laemmli [12]using an AE6400 Dual Mini Slab electrophoresis apparatus (ATTO Corporation, Japan) with acrylamide gels (90 mm × 73 mm × 1 mm)consisting of a 12.5% separating gel and 4.5% stacking gel.Pre-stained molecular weight markers (TIANGEN, Beijing, China)with molecular masses of 14.4, 20, 28.5, 35, 45, 66.2 and 94 kDa were invoked as a reference. The divide fractions were mixed in a one to one ratio with 50 mmol/L Tris buffers (pH 6.8) containing 1% SDS, 1%β-mercaptoethanol, and 20% (V/V) glycerin. The separate fractions were boiled for 5 min before loaded onto the gel (10 mg per well). Gels were stained with Coomassie brilliant blue R-250. After detaining, gels were scanned and analyzed with a gel image analysis system (GELPRO, America). The protein fractions were appointed by comparing with standard molecular weight marker.
After SDS-PAGE electrophoresis, lane 3 protein was transferred to a polyvinylidenti fluoride (PVDF) (Immobilon-P, Millipore Corp.,Bedford,MA) membrane using an electrotransfer buffer (10 mmol/L(3-cyclohexylamino)-1-propane sulphonic acid (CAPS), pH 11.0,10% (V/V) methanol) for 75 min at 0.8 mA/cm2. Prior to use, the membranes were rinsed in methanol (5 s) and eletrotransfer buffer(15 min). The proteins were visualized with 0.1% (m/V) Coomassie Blue R-250 in methanol/20% acetic acid (50/50,V/V) staining. After staining, PVDF membranes were detained with 50% methanol,then washed with double-distilled water (5 min) and air-dried [13].Microsequencing of the N-terminal amino acids forαs1-casein was performed by Edman degration with PROCISE 491 protein sequencer(Applied Biosystems Procise, Weiterstadt, Germany).
2.3 Purification of αs1-casein by HPLC
Protein fractions containingαs1-casein were purified by high performance liquid chromatography (HPLC). The proteins were purified on a Nucleosil C18preparation column (Macherey and Nagel, Düren, Germany; 21.5 × 250 mm, 5 mm, 30 nm), at a flow rate of 12.0 mL/min and a gradient of 1% (V/V) acetonitrile per min.Acetonitrile/water with 0.1% (V/V) trifluoroacetic acid was used as the eluting solvent [4].
2.4 Animals
50 female BALB/c mice with 5 weeks old were purchased from Beijing Weitong Lihua company (Beijing, China; permit number“SCXK (Jing) 2006-0009”). The mice were housed in an animal room with controlled temperature of 23 °C, relative humidity of 50%, and with the altering of 12/12 h light-dark cycle. The mice were housed in polypropylene cages (32 cm × 20 cm × 14 cm) and provided unlimited access to a commercially available rodent diet and water.Water was made available continuously through automatic port, and the diet did not contain any milk allergens which could influence the outputs of the experiments. Moreover, bedding (wood shaving) of mice was replaced every two days. Animals were acclimatized to the laboratory condition for 1 week before commencement of the animal experiment. All mice used in this study were cared for in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication 85-23, 1996), and all experimental studies were conducted under the program approved by the animal ethics committee of China Agricultural University (License No.: syxk (Jing) 2015-0045).
2.5 Oral sensitization and challenge of mice
As illustrated in Fig. 1, refer to the references [14-16], mice were randomly divided into five groups (n= 10/group). All the mice were sensitized by the intragastrically (i.g.) on days 0, 7, 14, 21, 28, 35,and orally challenged on day 42th. More specifically, the first group is the positive control (PC) group which was sensitized with ovalbumin(OVA, 2 mg) and cholera toxin (CT; Sigma-Aldrich, St. Louis, MO,USA) adjuvant (15 μg) in 200 mL phosphate buffer solution (PBS),and challenged with ovalbumin (10 mg) in 200 mL PBS. The second group was the high dose (HD) group which was sensitized withαs1-casein (3 mg) and CT (15 μg) in 200 mL PBS, and challenged withαs1-casein (15 mg) in 200 mL PBS. The third group was the medium dose (MD) group which was sensitized withαs1-casein (2 mg) and CT(15 μg) in 200 mL PBS, and challenged withαs1-casein (10 mg) in 200 mL PBS. The fourth group was the low dose (LD) group which was sensitized withαs1-casein (1 mg) and CT (15 μg) in 200 mL PBS,and challenged withαs1-casein (5 mg) in 200 mL PBS. The fifth group was the negative control (NC) group which was sensitized with the same volume of normal saline and 15 μg CT, and challenged with 5 times volume of normal saline. Then, 30 min after the final challenge, the allergic symptoms were monitored using an anaphylactic validated scoring. The symptoms of hypersensitivity were scored on a scale from 0 (no symptoms) to 5 (dead) as indicated in Table 1. Thereafter, blood samples, mouse lungs, spleens, thymus and the middle of small intestines (about 2 cm) were collected to measure the resulting different biomarkers. The body temperature of mice was evaluated before and after stimulation.

Fig. 1 Experimental scheme for oral immunization of BALB/c mice with αs1-casein. BALB/c mice were sensitized weekly by intragastric gavage with αs1-casein or OVA using CT as adjuvant. Positive control group received OVA. Negative control group received normal saline and CT. After challenge, blood samples,mouse lungs, spleens, thymus and small intestines were collected to measure the following different biomarkers.
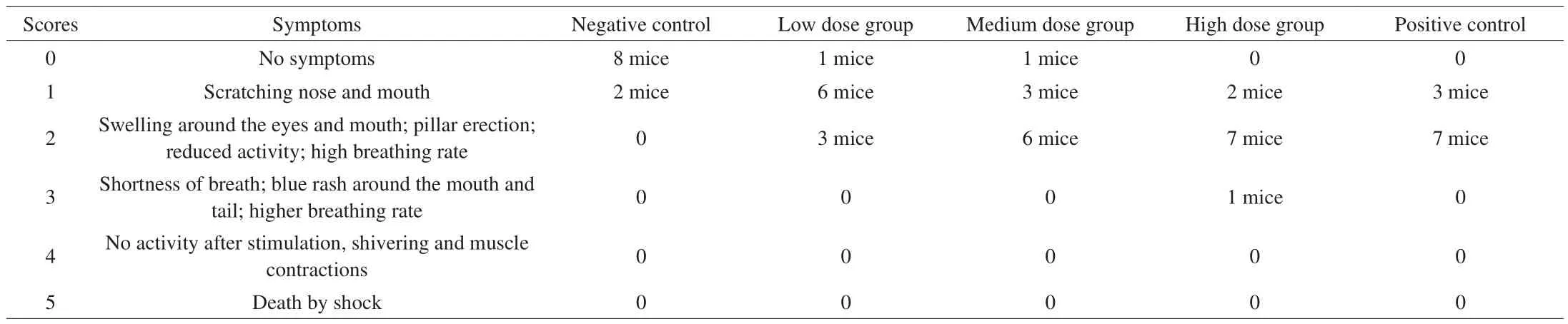
Table 1Anaphylactic symptom and the number of different symptoms appeared in mice.
2.6 Measurement of αs1-casein-specific IgE
To identify the animal model, serum allergen-specific IgE levels were determined by an indirect ELISA (n= 10/group) [17].Briefly, 100 µL of 5 µg/mLαs1-casein in 50 mmol/L sodium carbonate buffer (pH 9.6) was added to a 96-well microtiter plate per well, and the plate was allowed to incubate overnight at 4 °C.Each diluted serum was added (1:20 for specific IgE), followed by 100 µL of horseradish peroxidase (HRP)-conjugated goat antimouse IgE antibodies (1:6 000, Beijing Friendship Zhonglian Biotechnology Co., Ltd., Beijing, China) per well. Enzyme-catalyzed color development was accomplished by the addition of a solution of 3,3’-5,5’-tetramethylbenzidine. The optical density was measured at 450 nm by a microplate reader (Model 1860, Bio-Rad, USA).
2.7 Quantification of mouse mast cell protease-1 (mMCP-1)and histamine in blood
Serum concentrations of mMCP-1 were quantified following the manufacturer’s instructions using a commercially available ELISA kit (eBioscience, San Diego, USA) [18]. To determine the level of histamine in the plasma, 500 mL of a mouse blood sample(n= 10/group) was collected into anticoagulant tubes, followed by centrifugation for 10 min at 4 000 ×g. The supernatant was collected and stored at -80 °C until further analysis. Plasma histamine levels were measured using a histamine ELISA kit (eBioscience, San Diego.USA) in accordance with the manufacturer’s protocol [18].
2.8 Splenic cell cytokines evaluation
Individual spleen was aseptically collected and minced (n= 10/group).After erythrocyte lysis, spleen cells were suspended in complete medium (RPMI-1640 containing 10% fetal bovine serum, 2 mmol/LL-glutamine, 25 mmol/L HEPES buffer, 100 IU/mL penicillin, and 100 mg/mL streptomycin). Cells (2 × 106/well) were seeded in a 48-well plate. These cells were then stimulated with plain medium or in the presence ofαs1-casein or OVA (50 mg/mL) for 72 h (37 °C and 5% CO2). Subsequently, supernatants were collected and stored at-80 °C until further analysis. The levels of interleukin (IL)-4,IL-5, IL-10, and interferon (IFN)-γ were determined by a commercial ELISA kit (eBioscience, San Diego, USA) [19].
2.9 Histopathological observation
To visualize the pathologic changes in the tissue due toαs1- casein exposure, analysis of tissues was performed. In the present study, histopathological evaluation was performed by following a previously established procedure [20,21]. The lungs, spleen, thymus and intestines were washed in cold normal saline solution, fixed in 10% formalin, and embedded in paraffin. Sections of 5 mm thickness were cut and stained with hematoxylin and eosin for microscopic examination. The images were captured by the microscope.
2.10 Statistical analysis
Data are presented as means ± standard error of mean (SEM).Significant differences among different groups were analyzed by Fisher’s protected multiple comparisons using SPSS 19.0.
3. Results
3.1 Isolation and purification of αs1-casein
Isolation ofαs1-casein from skim cow’s milk by the ion-exchange column with increasing concentration of salt produced 3 protein peaks (Fig. 2A). Then, identification of the protein was confirmed by molecular weight size on SDS-PAGE (Fig. 2B). The first peak(1) elected in 0.11mol/L NaCl may containκ-casein. The second peak (2) elected with 0.16 mol/L NaCl may containαs1-casein. The third peak (3) elected with 1.0 mol/LNaCl may mainly containβ-casein. To further identifyαs1-casein, N-terminal sequence ofαs1-casein was analyzed as MKLLILTCLV(amino acids residues 1-10) by microsequence. In addition,αs1-casein was purified by HPLC(Fig. 2C), and the purity ofαs1-casein was 92.5%.
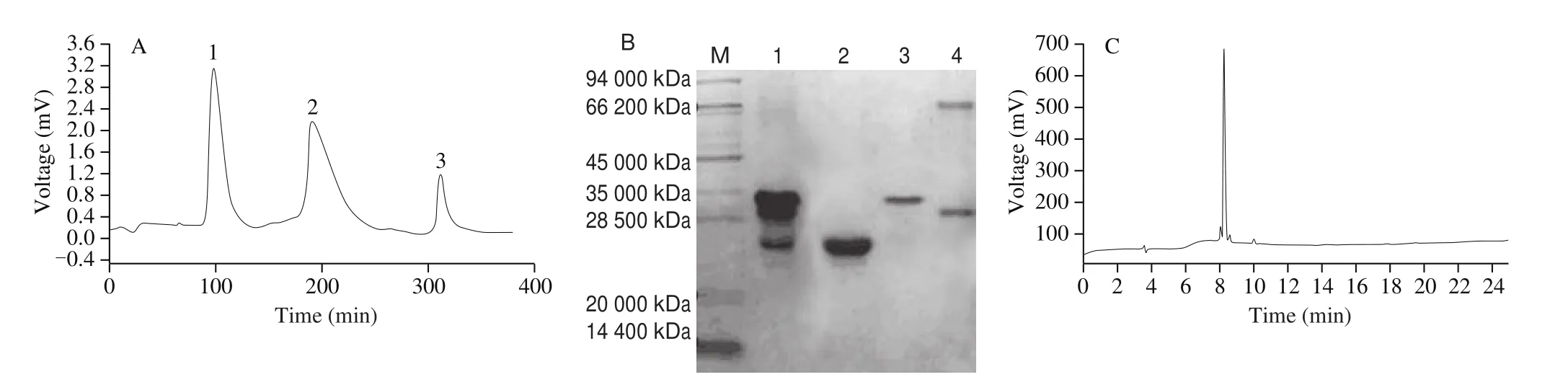
Fig. 2 Purification of αs1-casein from cow milk (A) and SDS-PAGE profile (B) and HPLC chromatogram (C). (1) Peak corresponds κ-casein; (2) peak corresponds αs1-casein; (3) peak corresponds β-casein. Lane M corresponds low molecular weight proteins marker at 94, 66.2, 45, 35, 28.5, 20 and 14.4 kDa;Lane 1 corresponds α-casein marker; Lane 2 corresponds κ-casein; Lane 3 corresponds αs1-casein; Lane 4 corresponds β-casein
3.2 Specific IgE antibody
As showed in Fig. 3A, the OD values of specific IgE in serum of each sensitized mice were significantly higher than the negative control group, in addition, the ratio of OD value of positive serum to that of negative serum is more than 2.1, which indicatedαs1-casein could induce BALB/c mice producing specific IgE antibody as an allergen. The level of IgE antibody in mice challenged byαs1-casein was increased with the increasing of the dose of gavage. The low dose group and the medium dose group were all significantly lower than the positive control, and there was no significant difference between the high dose group and the positive control.
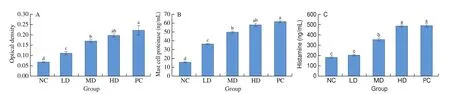
Fig. 3 The OD value of specific IgE (A), the content of mast cell proteinase (B) and the content of histamine (C) in blood of each mice group. Data are presented as mean ± SEM and are representative of at least 3 independent experiments (n = 10 mice per group). Significant differences among the 5 groups were analyzed by Fisher’s protected multiple comparisons. Values showing a different superscript letter among the five groups are significantly different (P < 0.05).
3.3 Mast cell proteinase
The content of mast cell proteinase in serum of each sensitized group was significantly higher than the negative control group,illustrated in Fig. 3B. In the challenged mice byαs1-casein, the amount of mast cell proteinase increased with the increase of the dose of gavage.The low dose group and the medium group showed significantly lower compared with the positive control, and there were not significantly different between the high dose group and the positive control group.
3.4 Histamine release test
As showed in Fig. 3C, the medium dose group, the high dose group was significantly higher than the negative control. The histamine content in the mice induced byαs1-casein increased with the increasing of the dose of gavage, the low dose group and the medium group were significantly lower than the positive control, and there were no significantly difference between the high dose group and the positive control group.
3.5 Cytokine production by spleen cells
Illustrated in Fig. 4, culture supernatants from cells isolated from the individual mouse spleens were analyzed for IL-4, IL-10,IL-5 and IFN-γ. Restipulations with allergenin vitroinduced almost no cytokine response in splenocytes from negative control mice.In contrast, when splenocytes from orally challenged mice were stimulated withαs1-casein, levels of IL-4, IL-10, IL-5 and IFN-γ all increased significantly compared with that of control mice. For IL-4,IL-10 and IL-5, levels rose in a dose-dependent manner, mice orally challenged with higher doses ofαs1-casein produced higher levels of cytokine in their splenocytes, whereas, the same dose response was very different for IFN-γ. The level of IFN-γ for the low dose group was significantly greater than other groups. Levels of IL-4, IL-10,IL-5 in mice challenged byαs1-casein significantly differ with the negative control group.
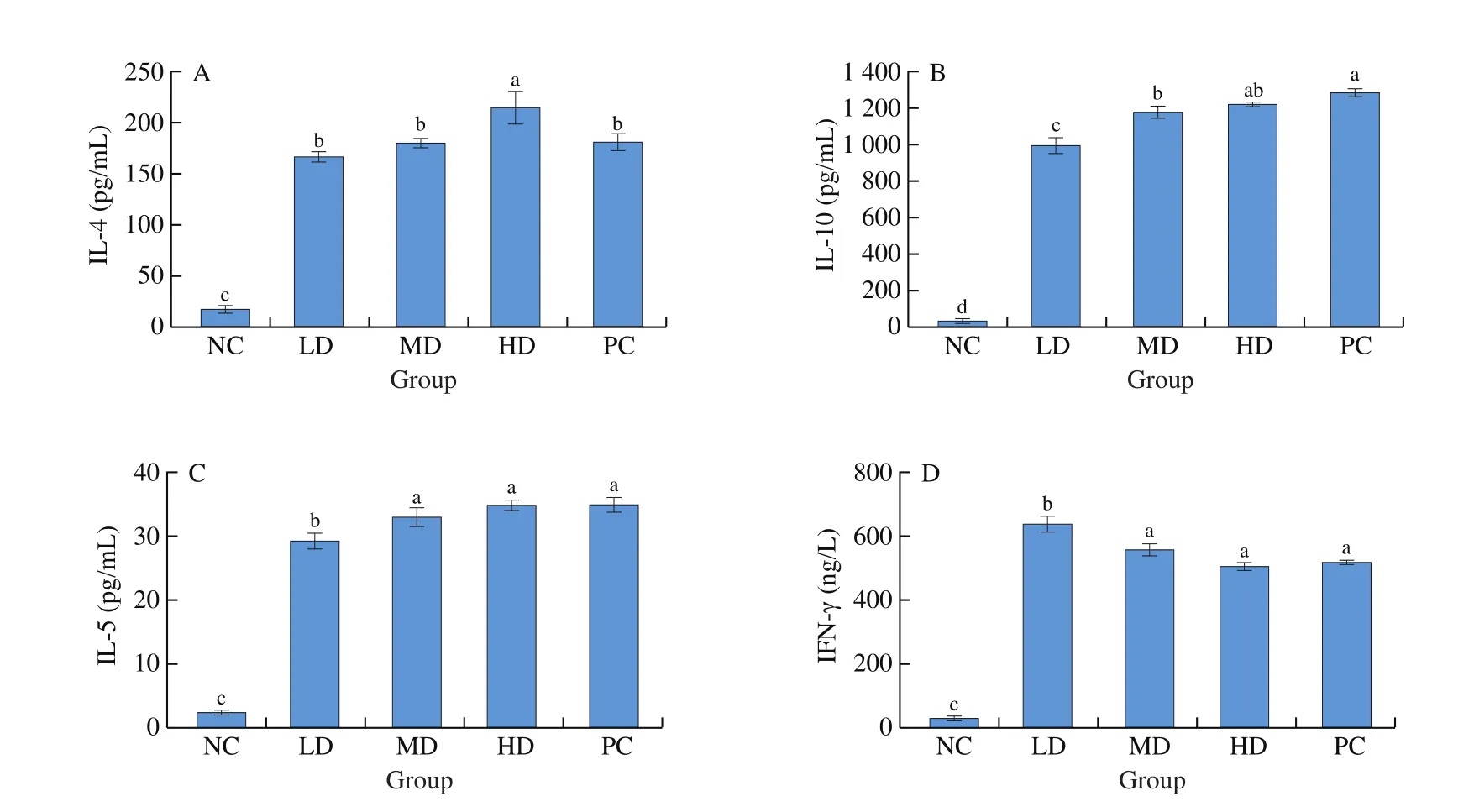
Fig. 4 Secretion of cytokine IL-4 (A), IL-10 (B), IL-5 (C) and IFN-γ (D) in splenic lymphocyte of each mice group. Data are presented as mean ± SEM and are representative of at least 3 independent experiments (n = 10 mice per group). Significant differences among the five groups were analyzed by Fisher’s protected multiple comparisons. Values showing a different superscript letter among the 5 groups are significantly different (P < 0.05).
3.6 Allergic symptoms
Thirty min after the last challenge, the allergic symptoms emerged in each sensitized mouse group were monitored using an anaphylactic validated scoring (Table 1). As showed in Table 1, the negative mice group almost no symptom and only 2 mice appeared scratching nose and mouth. For the low dose group, 1 mouse had no symptom,6 mice showed scratching nose and mouth, and 3 mice appeared swelling around the eyes and mouth, pillar erection, reducing activity and elevated breathing rate. For the medium dose group, 1 mouse showed no symptom, 3 mice showed scratching nose and mouth,and 6 mice appeared swelling around the eyes and mouth, pillar erection, reducing activity and high breathing rate. For the high dose group, 2 mice showed scratching nose and mouth, 7 mice appeared swelling around the eyes and mouth, pillar erection, reducing activity and elevated breathing rate, 1 mouse in shortness of breath, blue rash around the mouth and tail and higher breathing rate. For the positive control group, 3 mice showed scratching nose and mouth, 7 mice appeared swelling around the eyes and mouth, pillar erection,reducing activity and high breathing rate.
3.7 Histopathological observation
The organs of mice in negative control group present normal form by histopathological observation, whereas, for other mice orally challenged byαs1-casein, the lung, spleen, thymus and small intestines had different degrees of inflammation (Fig. 5).

Fig. 5 Pathological sections of the lung, spleen, thymus and small intestine of the sensitized mice.
For mice orally challenged by low doseαs1-casein, we observed the local alveolar hyperinflation, dilatation, and alveolar septum widening. The red pulp of the spleen was hyperemia, with a small number of lymph nodes in the marginal sinus. No abnormalities were found in the thymus form. Small amount of inflammatory cell was in filtrated in the interstitial tissue of the small intestines.
Mice were orally challenged by the medium dose ofαs1-casein showed inflammatory pathological features for visceral organs.Resident alveolar septum was collapsed and a small amount of alveolar was compensatory dilatation. The red pulp of the spleen was hyperemia, and there were more lymph nodes in the marginal sinus.The lymph nodes were prepared in the thymus. There were numerous inflammatory cells in the mesosoma of the small intestines.
Mice were orally challenged by the high dose ofαs1-casein showed more obvious inflammatory pathological features for visceral organs. Local alveolar septum was thickening, a small amount of alveolar was compensatory dilatation, and a small amount of bleeding in the local alveolar. The red pulp of the spleen was hyperemia, and the narrow marginal sinus had lymph node like a stripe. There was a larger lymph node in the medulla of the thymus. There were many inflammatory cells in filtrated in the mesosoma of the small intestines.
There were likewise more noticeable inflammatory pathological features in the positive control group. Resident alveolar septum was thickening, the alveolar were compensatory dilatation and hyperinflation. The red pulp of the spleen was hyperemia, with more foci and focal necrosis. There was a larger lymph node in the medulla of the thymus. There were many inflammatory cells in filtrated in the mesosoma of the small intestines.
4. Discussion
A potentially controversial issue is the selection of sensitization pathways in food allergy animal experiments, and the conventional methods are intraperitoneal injection and oral gavage [22]. The injection may be more likely to cause an allergic reaction in the animal than oral gavage without adjuvants, but its mechanism was different from that of oral gavage. CT is just an effective mucosal immune adjuvant which was studied more deeply so far as adjuvants [23,24].While exposure to CT is an unlikely mechanism of allergy induction in humans, data gleaned using adjuvants can give clues to the broader mechanisms by which innate stimuli initiate IgE responses to food [25].In the present study, CT-B subunit was used to immune BALB/c mice as the previous studies [26]. Also, Sun et al. [27]showed that there was no significant difference in serum specific IgE and IgG1 between CT group and normal saline group (P> 0.05), while OVA group and OVA+CT group were significantly higher than those in control group (P< 0.05), suggesting that in the presence of allergens,cholera toxin promotes Th2 cell differentiation and enhances the body’s immune response. In addition, Złotkowska et al. [16]using CT in challenge phase had studied differences in regulatory mechanisms induced byβ-lactoglobulin andκ-casein in cow’s milk allergy mouse model, and concluded that the applied BALB/c mice model exposure toβ-lactoglobulin andκ-casein showed significant differences in immunoreactivity mechanism, and may be useful for the estimation of allergenic potential of various food proteins. Further study needs to be done for the mechanism of CT in animal allergic model.
T lymphocyte (CD4+), as allergic effector cells, can differentiate between mature effector T-helper (TH) cells and secrete cytokines when they meet with antigens such as cow’s milk proteins [28].According to the different cytokines secret, T-helper cell can be divided into Thl and Th2 cells. Thl cells mainly secrete IFN-γ, IL-2 and TNF-α [29]. These cytokines were in charge of cell mediated immune responses. Th2 cells mainly produce IL-4, IL-5, IL-10 and IL-13, these cytokines can effectively activate B cells to produce IgE antibody, moreover, the combination of lgE and mast cells leads to release mast cell degranulation, histamine, mast cell proteinase, and other mediators, starting the allergic reaction to specific antigen [30].Mast cells are critical mediators of allergy and anaphylaxis [31]. The mast cell proteinase (mMCP-1) is released by mast cell activation within the gastrointestinal tract, which exerts profound effects on the intestinal epithelial barrier [32]. Increased concentration of mMCP-1 in sensitized mice indicates increased epithelial permeability, which promotes Th2-type inflammatory responses that might result in an intermediate or late-phase response in food allergy [33]. Histamine has been widely studied because of its role in increasing vascular hyper permeability, inducing edema, contracting the smooth muscle,causing mucus secretion, and inducing leukocyte chemotaxis.
In this study, the level of specific IgE antibody produced by theαs1-casein sensitized mice were significantly higher than that of the negative control group, this further indicatedαs1-casein was an allergenin vivo, and also verified the result by ELISA and western blottingin vitroin our previous studies [4]. The study conducted by Fotschki et al. [34]showed the serum specific IgE antibody content increased significantly when BALB/c mice were sensitized withβ-lactoglobulin andα-casein (aluminum as adjuvant). Compared with the negative control, the content of mast cell protease and histamine in mice oral challenged byαs1-casein increased significantly respectively, in addition, to elucidate the allergenic effects ofαs1-casein, the cytokines collected from individual BALB/c mice were quantified. Cytokine secretion patterns were achieved in spleen cell cultures. The level of IL-4, IL-5, and IL-10 secreted by Th2 cells increased significantly with the increase of the sensitization dose.The study carried out by Meng et al. [17]showed that the level of Th2 cytokines increased dramatically in BALB/c mice immunized withα-lactalbumin (CT as an adjuvant). The conclusion of Hodgkinson et al. [11]showed thatαs1-casein isolated from goat milk were used to sensitized BALB/c mice, serum IgG1 and IgE contents in mice were significantly increased, mast cell protease content increased 10 times compared with control group, cytokines IL-4 and IL-10 levels increased significantly. The data of this study found that IFN-γ secreted by Th1 cells also showed an increasing trend, and the IFN-γ level in the sensitized groups were higher than that of the negative control group, moreover, the low dose group was higher than other dose groups. Th1 cells mainly secrete IFN-γcytokines, which could inhibit Th2 allergic reaction. Th1 cells and Th2 cells restrict each other, and the balance between the two subgroups is the basic way of immune regulation [35].
Orally sensitized mice withαs1-casein allergens showed visible signs of anaphylaxis, shortness of breath, blue rash around the mouth and tail and higher breathing rate, indicating thatαs1-casein almost showed stronger sensitization than OVA. The pathological observation results further showed that the resident alveolar septa collapsed or thickened in the medium and high dose mice groups, and there were lymphatic foci in the spleen and thymus, and inflammatory cells infiltration in the intestinal mucosa. Specific and nonspecific mucosal barrier system in the human gastrointestinal tract can restrict the protein allergen fragments intake and absorption, but for some individual with poor gastrointestinal barrier, food allergen fragments easily went through the intestinal mucosa, causing inflammation [36].
Ovalbumin is one kind of proteins in egg white, which often causes strong allergic reactions, therefore, it is widely used in various disease models as the standard allergen [37]. For mice immunized by ovalbumin, the level of histamine, mast cell protease significantly increased, the lung, spleen, chest gland and small intestine showed different degrees of inflammation. At the same time, the negative control mice group was performed with normal saline and CT,during the whole experiment, the specific IgE level of mice, mast cell protease and histamine content were stable, the observation of lung,spleen, thymus and small intestine showed normal morphology.
Various methods were utilized to study on food allergens, but animal models were considered as the most direct and accurate method. BALB/c mice were initially used to evaluate the allergenicity ofαs1-casein in the present study. Main advantage is that intrauterine sensitization in BALB/c mice is similar to that of human. Another benefit is that a predominantly IgE-mediated mouse model for orally induced cow milk protein allergy, indicating that this model can be used extensively to evaluate the allergenicity of cow milk [38].The results showed that BALB/c mice were prone to produce Th2 cytokines and showed obvious inflammatory symptoms.
5. Conclusion
The allergenicity ofαs1-casein was verified byin vivoexperiment.The increasing significantly of specific IgE antibody, mast cell protease, histamine, IL-4, IL-5, IL-10 characterize the sensitization ofαs1-casein. Accordingly, obvious inflammatory reactions in challenged mice were observed. BALB/c mice were successfully used in a cow milk allergen-induced murine anaphylactic model. Bruton’s tyrosine kinase (BTK) is an enzyme thought to be essential for high affinity IgE receptor (FcεRI) signaling in human mast cells and basophils. The structure and gene expression of BTK in sensitized and challenged mice will be the focus of future research.
conflict of interest
The authors declare no conflict of interests.
Acknowledgements
This work was supported by National Science and Technology Project in Rural Areas (China, 2018YFC1604205), and the National Natural Science Foundation of China (Beijing, China; 31872886).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
