Orientin and vitexin attenuate lipopolysaccharide-induced inflammatory responses in RAW264.7 cells: a molecular docking study,biochemical characterization, and mechanism analysis
2022-06-23YueYuFushengPeiZhanmingLi
Yue Yu, Fusheng Pei, Zhanming Li*
School of Grain Science and Technology, Jiangsu University of Science and Technology, Zhenjiang 212004, China
Keywords:
Anti-inflammatory
Mitogen-activated protein kinase
Plant extract
NF-κB
Molecular docking
A B S T R A C T
Plant flavonoids have received considerable attention for their health benefits. However, structure-activity relationships between flavonoids such as orientin and vitexin with similar structures are rarely reported. In the present study, molecular docking study suggested that orientin and vitexin suppressed inflammatory responses through the modulation of the mitogen-activated protein kinase (MAPK) signaling pathway. Moreover,RAW264.7 cells with lipopolysaccharide-induced inflammation were used to evaluate the anti-inflammatory activities of orientin and vitexin, based on the inflammation-related cytokines production, quantitative realtime reverse transcription PCR, and western blotting analysis. As a result, orientin or vitexin attenuated inflammatory responses through modulation of the MAPK/NF-κB signaling pathway by suppressing NF-κB translocation.
1. Introduction
Recently, high-energy dietary patterns have increased the lipid accumulation associated with overweight or obesity [1,2]. In addition,systemic low-grade inflammation is associated with overweight and obesity [3]. Appropriate levels of inflammation play a vital role in maintaining health, whereas excessive inflammation can induce insulin resistance, obesity, and other complications [4]. Dietary intake of natural plant flavonoids can effectively alleviate inflammatory responses. Previous studies have investigated the functional properties of plant flavonoids with practical applications [5,6].
Lipopolysaccharide (LPS), as one of the cell wall components of Gram-negative intestinal bacteria, might be a trigger to induce chronic inflammation. A high-fat diet was observed to increase the delivery of LPS from the intestinal tract to the rest of the body, causing an increase in adipose tissue, inflammation, and insulin resistance [7].Furthermore, a highfat diet can increase pro-inflammatory cytokines,which supports evidence for the relationship between a high-fat diet and health status [8,9]. Indeed, the suppression of inflammatory responses is crucial to sustain the cellular steady state.
Many studies have revealed the anti-inflammatory effects of plant flavonoids [10,11]. The flavonoids profile fromCitrus reticulataprovided potentially superior anti-inflammatory benefits.Gnaphalium affineextracts also inhibited LPS-induced nitrous oxide (NO), inducible nitric oxide synthase (iNOS), and pro-inflammatory cytokines production [12].Besides, the flavonoid glycosides ofRubus chingiiHu displayed anti-inflammatory activity via suppression of extracellular signal-regulated kinase (ERK) and JUN N-terminal kinase (JNK), as well as decreasing pro-inflammatory cytokines production in LPS-stimulated macrophages [13]. It was also suggested that the bioactivities of plant flavonoids were related to their structures [14]. Therefore, it is interesting to explore the structure-activity relationships of plant flavonoids with similar structures from the same source.
Bamboo leaf flavonoid extracts have received increased attention because of their health benefits. Moreover, bamboo leaf extracts have been authorized as food materials by the Ministry of Health, China [15].The 4 most important flavonoids in bamboo leaf extracts are isoorientin, orientin, isovitexin, and vitexin [16,17]. The flavonoids were also reported in many other plants, includingPassi flora,Trolliuschinensisflowers and tulsi(Holy basil)leaves [18-20], however, little research has been performed to reveal the different anti-inflammatory activities of these bamboo leaf monomeric flavonoids. In addition, to the best of our knowledge, studies have never been performed to verify the anti-inflammatory performance of different bamboo leaf flavonoid compounds. Thus, it is crucial to determine the structure-function relationships of these plant functional compounds.
Plant flavonoids could suppress the LPS-induced inflammatory responses in both cell and animal models [20,21]. Toll like receptor 4 (TLR4) is an important receptor for LPS-induced inflammatory responses. Although the cellular signaling pathways of modulating inflammation are complex and involve crosstalk, as an effective signaling pathway, modulation of the mitogen-activated protein kinase (MAPK) signaling pathway is important to suppress the proinflammatory cytokines in LPS-induced inflammatory responses [22].Generally, the interactions between flavonoids and protein receptors are associated with their bioactivity. Molecular docking is an attractive technology to study the bioactivity of the compounds with promising applications in the pharmaceutical industry [23].
In this study, a molecular docking study was performed to investigate whether orientin or vitexin could block LPS-induced inflammation by binding to TLR4 competitively with LPS. In addition, the mechanism of the alleviation of inflammatory responses was also analyzed through the modulation of MAPK signaling pathways. Moreover, RAW264.7 macrophages with LPS-induced inflammation were employed to clarify the differences in antiinflammatory activities of orientin and vitexin with similar structures.The potential regulatory mechanism of orientin or vitexin intervention in anti-inflammatory responses was also discussed.
2. Materials and methods
2.1 Materials and reagents
Bamboo leaf flavonoids, orientin and vitexin with the purities of more than 97%, were prepared according to the method detailed in our previous study [19]. Enzymelinked immunosorbent assay (ELISA)kits were supplied by the Nanjing Jiancheng Bioengineering Institute(Nanjing, China). Human RAW264.7 macrophages were purchased from the Shanghai Institute for Biological Sciences (Shanghai, China).Primary and secondary antibodies (recognizing phosphorylated (p)-mitogen-activated protein kinase 14 (p38), p-JNK, and p-ERK) for western blotting analysis were purchased from Affinity Biosciences(Cincinnati, OH, USA). Fetal bovine serum and other reagents for cell treatment were purchased from Gibco/BRL Life (Grand Island, NY,USA). Reagents such as LPS, 2,7-dichloro fluorescin diacetate (DCF-DA),and other chemicals used for analysis were supplied by Sigma-Aldrich(St. Louis, MO, USA).
2.2 Docking analysis
A molecular docking study was performed based on the model of the TLR4 complex (PDB ID: 2Z62) and MAPK (PDB ID: 5MTY)to orientin or vitexin. Hydrogen atoms, force field (CHAEMm),and atomic charges were added. Docking of orientin or vitexin to the protein was conducted using Discovery Studio 2016 software(Accelrys, San Diego, CA, USA).
2.3 Cell culture
RAW264.7 macrophages were recovered and incubated at 37 °C with 5% CO2according to previous studies [20]. Cell solutions(5 × 104cells/mL) were incubated in 96-well microplates after several passages. Cells treated with phosphate buffered saline (PBS) were used as control. According to the previous research [24], LPS with the concentration of 1 μg/mL cells was employed to treat the cells as the positive group. LPS (1 μg/mL) plus orientin (80 μg/mL), and LPS (1 μg/mL) plus vitexin (80 µg/mL) were also used to treat the cells for 24 h. The cells were harvested and used to determine anti- or proinflammatory cytokines, antioxidant enzymes, and reactive oxygen species (ROS) production.
2.4 Cytokines production
RAW264.7 macrophages were seeded onto 12-well plates in triplicate (at 5 × 104cells/well) and incubated at 37 °C with 5% CO2.After 24 h, the medium was replaced and incubation continued for a further 24 h. Then, LPS, LPS plus orientin, and LPS plus vitexin were used to treat the cells for 24 h. Moreover, tumor necrosis factor alpha (TNF-α), interleukin (IL)-10, IL-6, IL-1b, transforming growth factor beta 1 (TGF-β1), and iNOS (also known as NOS2) levels were analyzed in the samples using ELISA kits according to the manufacturer’s instructions.
2.5 Intracellular ROS production
Harvested cells were washed twice using PBS after centrifugation (1 200 ×g, 5 min). The fluorescent probe (diluted DCF-DA solution) was added into wells containing cells in PBS and then incubated at 37 °C according to the manufacturer’s protocols.Cells incubated in medium (serum-free) were used as negative controls. Flow cytometry (Beckman, Pasadena, CA, USA) was used to analyze ROS production.
2.6 Superoxide dismutase (SOD) and glutathione peroxidase(GSH-Px) production
It is believed that LPS stimulation upregulates oxidative stress connected with the production of intracellular free radicals. Flavonoid treatment could reverse the effects of enhanced antioxidant enzymes production [25]. The effect of orientin and vitexin on antioxidant enzymes, including SOD and GSH-Px levels, were measured using ELISA kits according to the manufacturer’s instructions.
2.7 Measuring ATP levels
The treated cells were collected by centrifugation at 3 000 ×g.Hot purified water was then added and the cells were broken by homogenization. The cell suspension was heated for 10 min in a boiling water bath. The extracted cell suspension was centrifuged at 3 000 ×gfor 10 min, and the supernatant was collected for ATP detection. Cellular ATP levels under different treatments were measured using ATP ELISA kits according to the manufacturer’s instructions. An ATP calibration curve was used to determine the ATP concentration. The cellular protein content was determined according to a previously published method [26].
2.8 Quantitative real-time reverse transcription PCR(qRT-PCR) analysis
The TRIzol reagent was used to isolate total RNA and qRT-PCR was performed using a Roche LightCycler 480 II system. The PCR primers were designed using Primer Premier 6 software and are shown in Table S1. All samples were analyzed in triplicate and the relative expression ofCOX2(cyclooxygenase 2),CYCS(cytochrome C),andNFKBIA(NF-κB inhibitor alpha) were normalized to that ofGAPDH(glyceraldehyde-3-phosphate dehydrogenase) as an internal control.
2.9 Western blotting analysis
Western blotting was used to determine the levels of the phosphorylated (p) forms of three subunits of MAPK, including p-JNK, p-ERK, and p-p38, which are considered as biomarkers of the MAPK signaling pathway, to reveal the effects of LPS stimulation [27].Western blotting was performed according to a previously published method [28]. The levels of p-JNK, p-ERK, and p-p38 were normalized to that of GAPDH as an internal control.
2.10 Statistical analysis
The quantitative results for the responses to the four interventions were analyzed using one-way analysis of variance (ANOVA) in SPSS 17.0 (IBM Corp., Armonk, NY, USA) with statistical significance set atP< 0.05. All the tests were performed in triplicate and the mean value ± standard deviation was reported for each analysis.
3. Results and discussion
3.1 Molecular docking analysis
Molecular docking simulation is used to deduce the best conformation of the interaction between the receptor small molecule and the target protein through continuous optimization. Molecular docking study of orientin and vitexin and TLR4 is shown in Fig. 1.Both orientin and vitexin docked to the same binding region of TLR4,which was different to LPS binding region (Fig. 1). Three hydrogen bonds were formed between orientin or vitexin and the TLR4 complex. These results suggested that any alleviation of inflammatory responses by orientin or vitexin were not induced by competitive binding with LPS for TLR4.
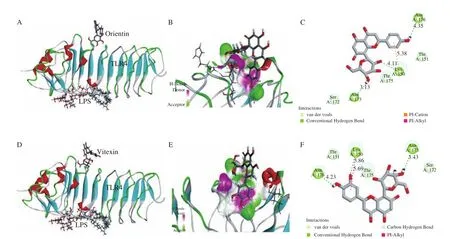
Fig. 1 Molecular docking study of orientin and vitexin and TLR4 (PDB ID: 2Z62). (A) Molecular docking of orientin and LPS to TLR4; (B) 3D image of hydrogen bonds of orientin and TLR4; (C) 2D interaction plot showing the interactions of orientin and amino acid residues; (D) molecular docking of vitexin and LPS to TLR4; (E) 3D image of hydrogen bonds of vitexin and TLR4; (F) 2D interaction plot showing the interactions of vitexin and amino acid residues. Green dotted lines in (C) and (F) indicate conventional hydrogen bonds.
The proposed binding position of orientin and MAPK indicated that orientin could fit well into the active site, with a predicted binding energy of -51.87 kcal/mol, while the predicted binding energy of TLR4 was -22.95 kcal/mol. The proposed binding position of vitexin had a predicted binding energy of -49.29 kcal/mol,which is also greater than the predicted binding energy of TLR4(-24.89 kcal/mol). The larger binding energy indicated better binding efficacy, supporting the view that orientin or vitexin might target the signaling molecules of MAPK to block inflammation, as reported in previous studies [29,30], without competitive binding with LPS, as shown in Figs. 2A and B.
Five hydrogen bonds were formed between orientin and the His107, Met109, Gly110, Ser154, and Asp168 residues of MAPK(Fig. 2C). Four hydrogen bonds were formed between vitexin and the Ser37, His107, Met109, and Asp168 residues of MAPK(Fig. 2D). Ser, His, Met, and Asp residues were associated with the protein activities and the interactions between these amino acids residues were proposed to be important features of the antiinflammatory activity. Additionally, important hydrophobic interactions were formed between vitexin and the Val30 (π–alkyl),Ala51 (π–alkyl), and Leu167 (π–alkyl) residues. The Val30 (π–alkyl),Ala51 (π–alkyl), Ala111 (Amide-π stacked), and Leu167 (π–alkyl)residues of MAPK also produced hydrophobic interactions with the hydroxyl groups of orientin.

Fig. 2 Superimposed binding modes (A) of OR (Grey) and VI (Green) (B) in the active site of MAPK (PDB ID: 5MTY); 2D interaction plot showing the interactions of orientin (C) or vitexin (D) and key amino acid residues. Proposed binding pose of flavonoids to the active pocket of MAPK showing the hydrophobicity of OR (E) and VI (F).Hydrogen bonds between OR (G) or VI (H) and protein. Green dotted lines in (C) and (D) indicate conventional hydrogen bonds.
Besides, the proposed binding pose of flavonoids to the active pocket of MAPK was employed to show the hydrophobicity of orientin (Fig. 2E) and vitexin (Fig. 2F) and hydrogen bonds of orientin(Fig. 2G) and vitexin (Fig. 2H). The model showed that orientin and vitexin could be well-fitted with the active pocket and the formed hydrophobic interactions between orientin or vitexin and MAPK molecules played important role in the anti-inflammatory activity.In all, the molecular docking analysis suggested that hydrophobic interactions and hydrogen bonds formed between orientin or vitexin and the amino acids residues of MAPK could produce antiinflammatory activity.
3.2 Biochemical characterization
3.2.1 Inflammation-related cytokines production
Biochemical characterization was conducted to verify the docking analysis results. According to the previous research, LPS (1 μg/mL)stimulation of RAW264.7 macrophages produced significant differences in the levels of anti-inflammatory and pro-inflammatory cytokines [24]. The antioxidant activity of bamboo leaf flavonoid extracts has been investigated at different concentrations in our previous research, which verified that the bamboo leaf extracts(0–480 μg/mL) exhibited different antioxidant activities [19]. In this study, the orientin or vitexin concentration of 0.18 μmol/mL (equal to 80 μg/mL) were selected for the treatment and it was suitable to evaluate their anti-inflammatory activities on LPS-treated RAW264.7 macrophages.
As shown in Fig. 3, LPS stimulation significantly increased the levels of pro-inflammatory cytokines, including TNF-α, IL-6,IL-1b, TGF-β1, and NOS2/iNOS (Figs. 3A-E). In addition, the level of the anti-inflammatory cytokine IL-10 level significantly decreased(P< 0.05) (Fig. 3F). After the treatment with the flavonoids, the proinflammatory cytokines levels decreased compared with those of the LPS treatment; meanwhile, the levels of anti-inflammatory cytokines significantly increased (P< 0.05). Besides, orientin and vitexin intervention effectively alleviated the inflammatory responses. As shown in Figs. 4A and 4B, the chemical structures of orientin (with an extra 3’C-OH on ring B) and vitexin are similar. It was intriguing that the small difference in structure may result in significant differences in the anti-inflammatory response [31,32]. As reported, luteolin(extra 3’C-OH on ring B) showed lower tyrosinase inhibitory effect and higher affinity in comparison with apigenin (Figs. 4C and 4D),indicating that the bioactivity of extra 3’C-OH on ring B of flavones is weakened [33]. Therefore, the small difference in structure may result in significant differences in the bioactivities according to the previous research [31].
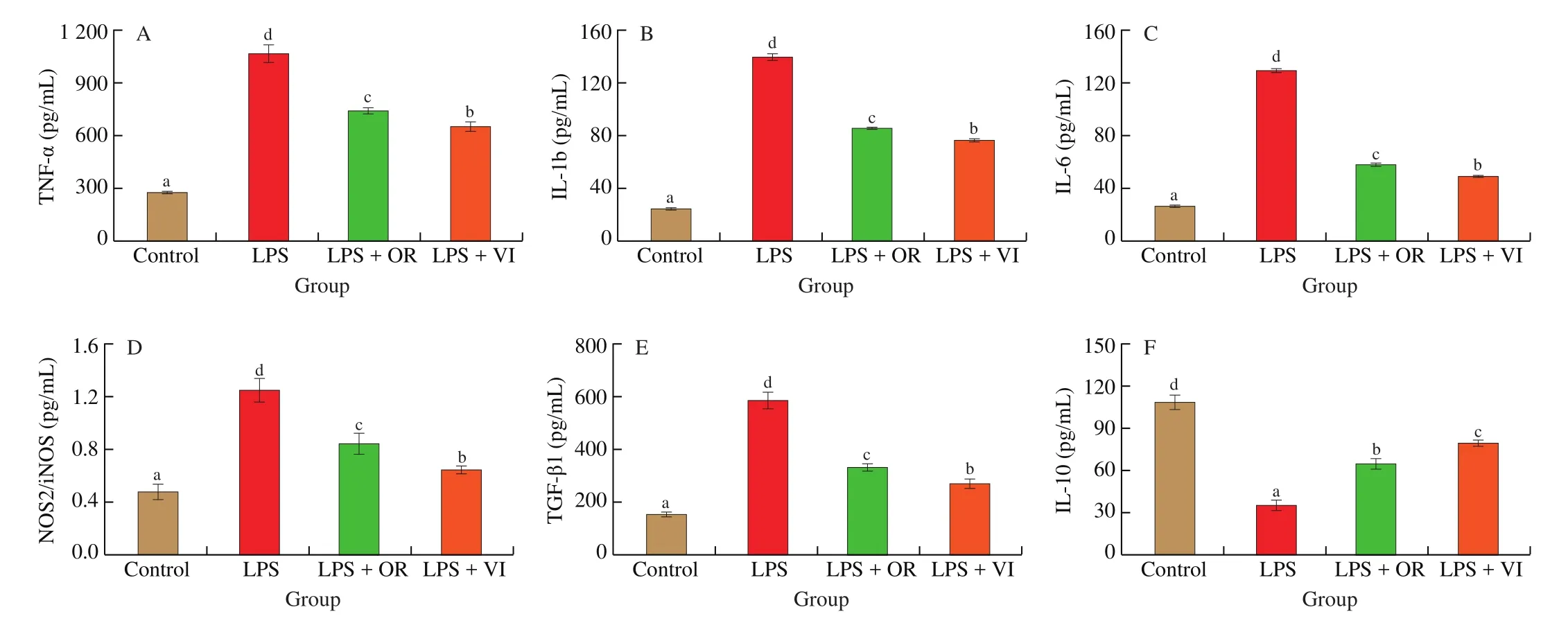
Fig. 3 Inflammation-related cytokines production in response to four treatments (Control, LPS, LPS + OR, LPS + VI). (A) TNF-α, (B) IL-1b, (C) IL-6,(D) NOS2/iNOS, (E) TGF-β1, and (F) IL-10. Letters a-d indicate significant differences (P < 0.05).
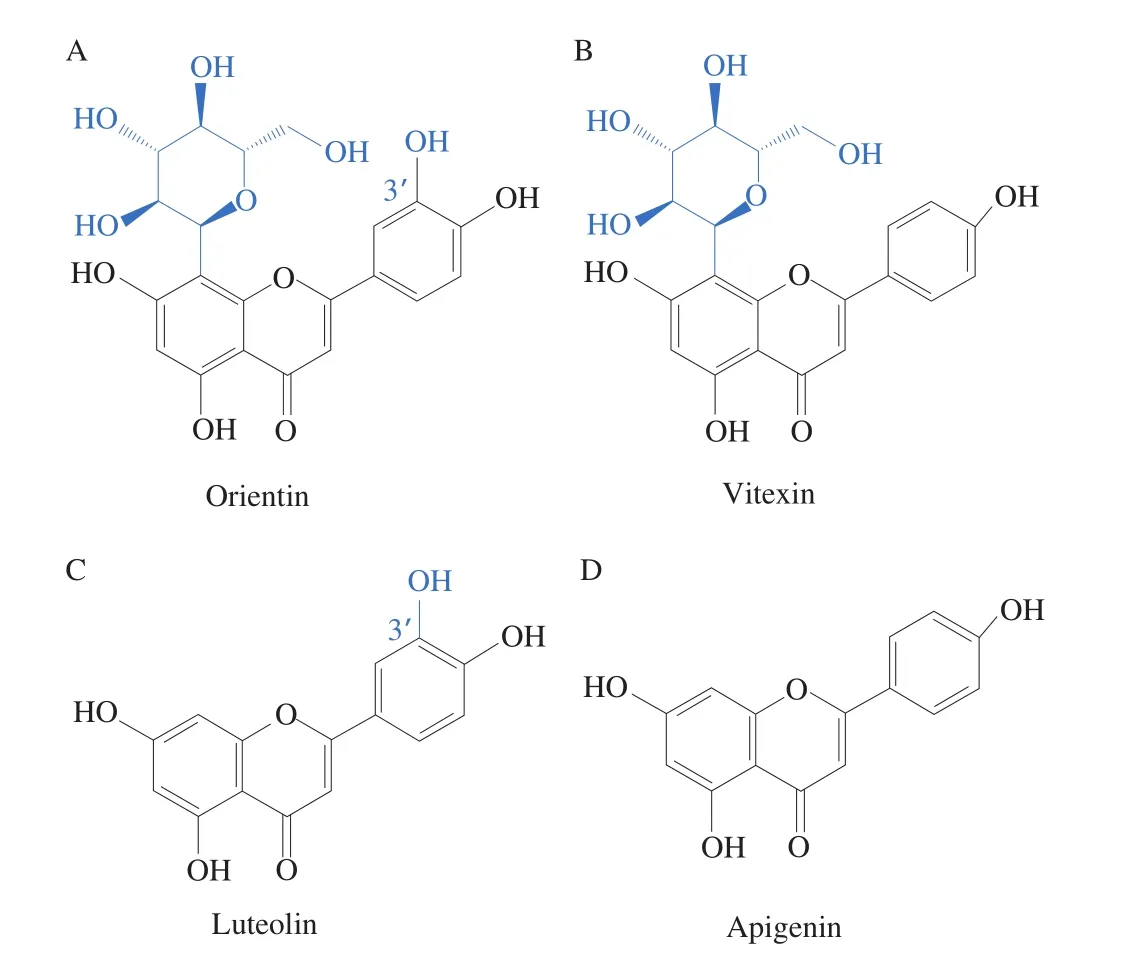
Fig. 4 Structures of flavonoids orientin (A), vitexin (B), luteolin (C) and apigenin (D).
3.2.2 ROS production
Moderate ROS production can be used to activate cytokines expression and other immunomodulatory factors. However, the excessive quantity of ROS damages DNA and proteins, even resulting in cell death. To sustain the normal cell steady state,ROS production should be maintained at an appropriate level [34].As shown in Fig. 5, inflammation induced by LPS stimulation caused an increase of 150% in the ROS production, which could be significantly reduced by orientin or vitexin treatment (P<0.05).However, the attenuation of ROS production was significantly different (P< 0.05) between orientin and vitexin treatments,which was similar to the results for cytokines production. Cells treated with vitexin showed moderately activated ROS production compared with the control, which was beneficial to induce related immune responses [35].
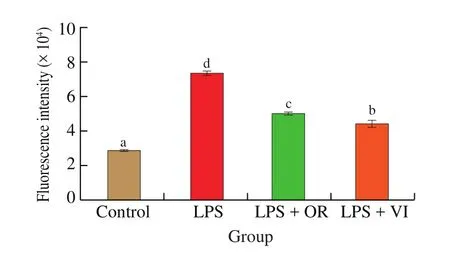
Fig. 5 ROS production in response to the four treatments. Letters a-d indicate significant differences (P < 0.05).
3.2.3 SOD and GSH-Px production
To better illustrate the positive effects of orientin or vitexin treatment, changes in antioxidant enzymes production (SOD and GSH-Px) were used to assess changes in cellular oxidative stress according to the previous research [36]. SOD production decreased significantly when the cells were treated with LPS (Fig. 6A).Meanwhile, SOD production in the orientin intervention group((1.23 ± 0.12) ng/mL) and vitexin intervention group ((1.58 ±0.09) ng/mL) significantly (P< 0.05) improved in comparison with that of the LPS group ((0.47 ± 0.04) ng/mL). As shown in Fig. 6B,GSH-Px production was decreased by 75% after LPS stimulation and the decrease was reversed by treatment with orientin ((81.73 ±9.13) U/g prot) and vitexin ((108.11 ± 7.87) U/g prot). The level of GSH-Px reduced after dealing with orientin and vitexin compared to that of the control treatment (P< 0.05). Meanwhile, the increase of the SOD and GSH-Px production indicated that the antioxidant defense responses were enhanced. These results showed that the increase in antioxidant enzymes might also contribute to alleviating excessive ROS level, which is consistent with previous studies [37,38].

Fig. 6 SOD (A), GSH-Px (B) and ATP (C) production in response to the four treatments (Control, LPS, LPS + OR, LPS + VI). Relative mRNA expressions of COX2 (D), CYCS (E), and NFKBIA (F) in response to the four treatments. Letters a-d indicate significant differences (P < 0.05).
3.2.4 ATP levels
Activation of inflammation activation is associated with changes in the mitochondrial membrane potential. Moreover, the intracellular ATP level is an important indicator of mitochondrial function [39].After treating RAW264.7 cells with LPS, the intracellular ATP level significantly decreased (P< 0.05) from (71.71 ± 3.89) μmol/g prot to (31.81 ± 3.97) μmol/g prot compared with the control treatment(Fig. 6C). Mitochondrial dysfunction might be caused by oxidative stress [19]. The LPS-induced decrease in the ATP production was significantly reversed (P< 0.05) by the addition of orientin ((47.25 ±2.98) μmol/g prot) and vitexin ((58.97 ± 3.39) μmol/g prot). It should be noted that upregulation of ATP levels by orientin and vitexin sustained the mitochondrial function [39].
3.2.5 COX2 and CYCS expression
Macrophages suppressCOX2expression to counteract inflammatory responses [20]. As shown in Fig. 6D,COX2expression was increased by LPS stimulation (P< 0.05) indicating activation of the inflammatory response. By contrast,COX2expression was decreased by orientin or vitexin treatment, indicating that inflammation was effectively alleviated (P< 0.05).
CYCSexpression was associated with excessive inflammation that may lead to the initiation of programmed cell death [40]. In Fig. 6E, treatment of LPS-stimulated cells with orientin or vitexin significantly decreasedCYCSexpression in comparison with that in the LPS stimulated cells. Our previous study revealed the positive effects of bamboo leaf flavonoids on mitochondria-associated apoptosis [19].Taken together, the present and previous studies suggested that the antiinflammatory responses and anti-oxidative stress activities of bamboo leaf flavonoids were mediated in part by modulation ofCYCSexpression.
3.2.6 NF-κB translocation
NF-κB signaling pathway plays a vital role in initiating immune responses. NF-κB can combine with its inhibitor IkBα (encoded by theNFKBIAgene) to activate the expression of related genes [20].When LPS, together with other stimulations, is used to induce inflammation, the NF-κB and IkBα dimer disassociates to NF-κB translocation into the nucleus to regulate gene expression [41]. As shown in Fig. 6F, the relative expression ofNFKBIAdecreased significantly after LPS stimulation (P< 0.05). Treatment of LPS-stimulated cells with orientin or vitexin increased the expression ofNFKBIAsignificantly (P< 0.05), although the expression level did not reach that of the control. This result highlighted the association of the NF-κB signaling pathway with the modulation of anti-inflammatory responses via NF-κB translocation suppression.Our findings were consistent with the report thatSyzyium cuminiseeds(70% methanol fractions) performed the potential to target LPS-induced inflammation by modulation of NF-κB translocation [20].
3.2.7 Western blotting analysis
The MAPK signaling pathway is associated with the modulation of the inflammatory responses and MAPK phosphorylation caused by LPS stimulation can be attenuated by plant bioactive components,such as olive oil polyphenols [42]and dihydromyricetin [43].Therefore, western blotting analysis was used to observe the levels of three phosphorylated subunits of MAPK.
The levels of p-ERK, p-JNK, and p-p38 increased dramatically in cells stimulated with LPS (P< 0.05) as shown in Fig. 7. For all three proteins, orientin or vitexin treatment suppressed phosphorylation in comparison with that induced by LPS treatment (P< 0.05). There was no significant difference in the levels of p-ERK (44 kDa) and p-JNK (54 kDa) between orientin and vitexin treatments (P> 0.05).However, vitexin treatment was more effective in modulating the levels of p-ERK (42 kDa), p-JNK (46 kDa), and p-p38 compared with that of orientin group (P< 0.05).
3.3 Mechanism of action
The results of qRT-PCR and western blotting analysis revealed that the anti-inflammatory activity of orientin (or vitexin) modulated by the MAPK/NF-κB signaling pathway or inhibited by NF-κB translocation. In addition, significant differences in anti-inflammatory activity were observed between orientin intervention and vitexin intervention (P< 0.05), although they shared similar structures. The lack of systematic studies on the structure-activity relationships can be attributed to the difficulty in purifying monomeric bamboo leaf flavonoid compounds. The results of the present study supported the hypothesis that flavonoids with similar structures would have different antiinflammatory activities.
As reported previously, the flavonoids 8-prenyl quercetin and quercetin directly target subunits of MAPK, ERK, and JNK, to induce the anti-inflammatory activity [27]. Orientin and vitexin, as similar flavonoids, might also exert their anti-inflammatory activities by modulating the phosphorylation of MAPK subunits. Assessing crosstalk between signaling pathways is also important when evaluating their mechanism of action [44]. It is possible that many different plant functional compounds might regulate biochemical processes based on the common crosstalk protein or receptors.
It is also suggested that the MAPK/NF-κB signaling pathways are involved in inflammation and oxidative stress regulation [45,46].According to our research, the possible signaling pathways for the modulation of the anti-inflammatory responses by orientin or vitexin are presented in Fig. 8. The phosphorylation of three subunits of MARK could be alleviated by orientin or vitexin treatment, possibly resulting in suppression of NF-κB translocation.
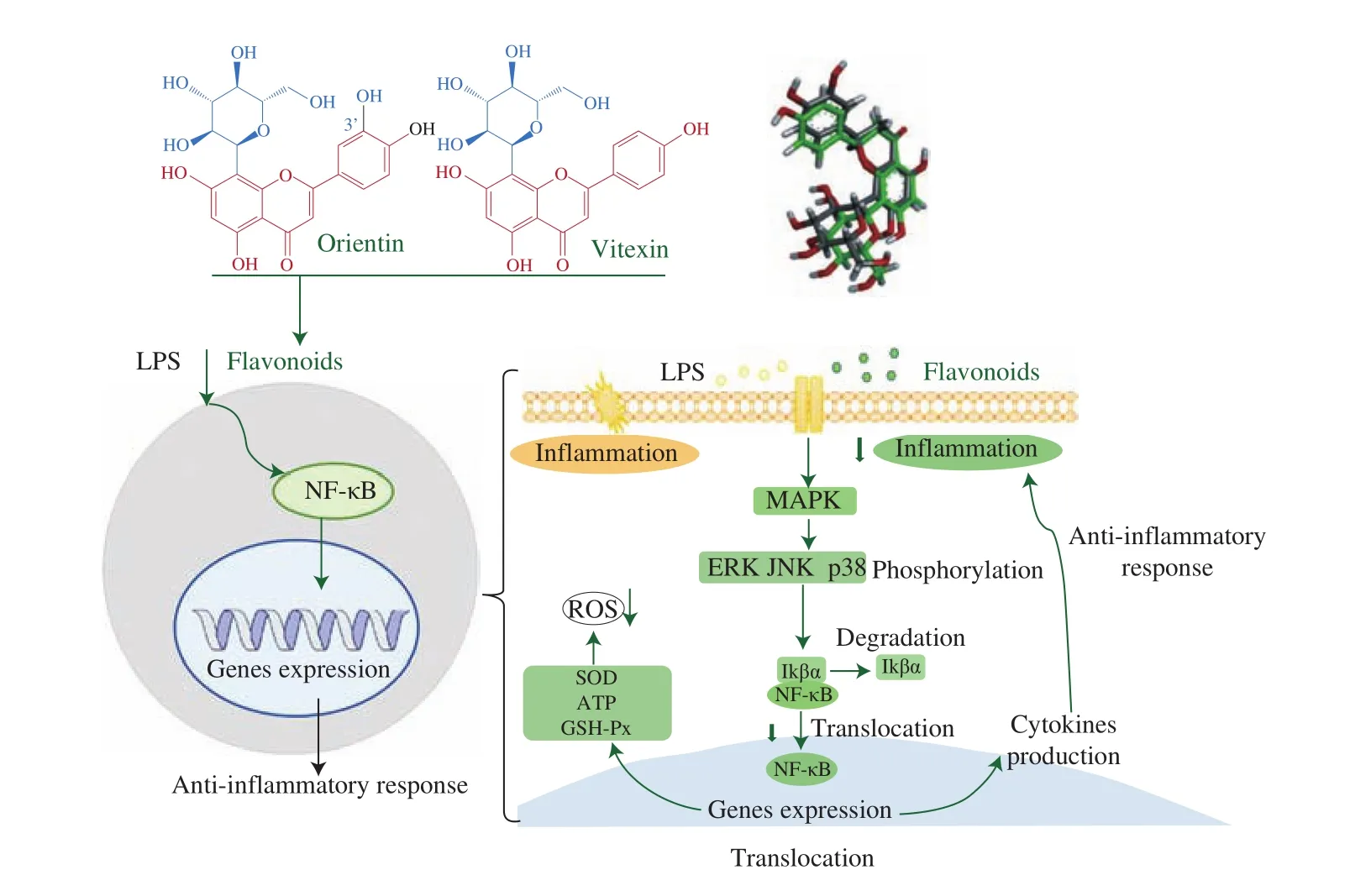
Fig. 8 Possible signaling pathways for the modulation of the anti-inflammatory responses by orientin or vitexin intervention. Anti-inflammatory responses were activated by orientin or vitexin intervention via modulation the MAPK/NF-κB signaling pathway by suppressing the NF-κB translocation.
4. Conclusion
The current study analyzed the anti-inflammatory activity and regulatory mechanisms of orientin and vitexin using RAW264.7 macrophages with LPS-induced inflammation. The molecular docking study revealed that the downregulation of MAPK signaling pathway activity by orientin or vitexin was involved in the suppression of LPS-induced inflammatory responses, however, it did not involve competitive binding with TLR4. The results of qRT-PCR and western blotting analysis indicated that orientin intervention and vitexin intervention could effectively alleviate inflammatory responses by modulating the MAPK/NF-κB signaling pathway, possibly by inhibiting NF-κB translocation. These findings promote the research to clarify the mechanism of the anti-inflammatory activity of plant flavonoids with similar structures.
conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant No. 31902204), the Public Welfare Project of Huzhou, China (Grant No. 2018GZ28) and the Start-up fund of Jiangsu University of Science and Technology (No.1182932009). We appreciate Professor Hongshun Yang in National University of Singapore for the useful comments and suggestions.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.04.024.
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
