Alteration of fecal microbiome and metabolome by mung bean coat improves diet-induced non-alcoholic fatty liver disease in mice
2022-06-23DinzhiHouJinTngMeiliHunFngLiuSumeiZhouQunShen
Dinzhi Hou, Jin Tng, Meili Hun, Fng Liu, Sumei Zhou,*, Qun Shen*
a Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Engineering and Technology Research Center of Food Additives,School of Food and Health, Beijing Technology and Business University, Beijing 100048, China
b College of Food Science and Nutritional Engineering, Key Laboratory of Plant Protein and Grain processing, China Agricultural University, Beijing 100083, China
c COFCO Nutrition and Health Research Institute, Beijing 102209, China
d College of Tobacco Science, Henan Agricultural University, Zhengzhou 450002, China
Keywords:
MBC
Hepatic steatosis
Gut microbiota
Short-chain fatty acids
Metabolites
A B S T R A C T
Dysbiosis of gut microbiota and its derived metabolites has been linked to the occurrence and development of nonalcoholic fatty liver disease. Our previous study has demonstrated that mung bean coat (MBC) might be mainly responsible for the beneficial effects of whole mung bean on high fat diet (HFD)-induced metabolic disorders. To investigate whether MBC, which is rich in dietary fiber and phytochemicals, can protect against HFD-induced hepatic steatosis in mice via targeting gut microbiota and its metabolites, we conducted this study. Results showed that MBC could effectively alleviative the obese phenotype, reduce the lipid accumulation and insulin resistance, and improve the hepatic oxidative stress and inflammatory response.Furthermore, MBC significantly prevented the HFD-induced changes in the structure and composition of gut microbiota, characterized by promoting the bloom of Akkermansia, Lachnospiraceae_NK4A136_group, and norank_f_Muribaculaceae, and along with the elevated short-chain fatty acids concentrations. Non-targeted metabolomic analysis indicated a metabolism disorder that was obviously improved by MBC via regulating sphingolipid metabolism and α-linolenic acid metabolism. These findings suggested that MBC could improve hepatic steatosis through manipulating the crosstalk between gut microbiota and its metabolites.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a symptom of liver metabolic disorder, with the main feature being the increased hepatic fat deposition in the absence of persistent excessive alcohol consumption [1]. At present, NAFLD has emerged as a major threat to public health worldwide with the majority of countries demonstrated> 25% increase between 2009 and 2019 [2]. Although the exact pathogenesis of NAFLD is still not fully understood, the multipleparallel-hits hypothesis highlights that genetic and environmental modifiable factors can lead to gut microbiota imbalance, insulin resistance, and obesity, thus consequently promoting the occurrence and development of NAFLD [3]. However, no specific NAFLD-targeted drugs are currently available due to its complex and multifactorial pathological mechanism [4]. There is thus an unmet need for exploring new therapeutic strategies for reducing NAFLD risk.
The gut microbiota, which consists of trillions of metabolically active microorganisms, can serve as an important regulator between diet and host healthy [5]. There is a growing body of evidence supporting the fact that the gut microbiome-liver axis plays a critical role in the pathogenesis of NAFLD [6,7]. Interestingly, the mice are resistant to diet-induced obesity and hepatic steatosis in the absence of gut microbiota [8], and germ-free (GF) mice colonized with the fecal microbiota from donor mice with metabolic syndrome exhibited increased NAFLD susceptibility [9]. These data highlight a causal link between the gut microbiota and NAFLD, which suggests that targeting the gut microbiota might help identify novel preventative strategies and precise therapeutic approaches to intervene the disease.Furthermore, it is believed that the effects of the gut microbiota on the host physiological functions are partially mediated by the metabolites derived from commensal bacteria, which are greatly affected by the intake of dietary nutrients [10,11]. Emerging evidence has demonstrated that many endogenous metabolites, such as shortchain fatty acids (SCFAs) and amino acid-derived metabolites, are involved in the progression of NAFLD [12]. Nevertheless, a quantity of metabolites can be produced by gut microbes utilizing dietary nutrients as precursors, implying that diet has a pivotal impact on the metabolites of gut microbiota. Thus, gut microbial metabolites can act as important mediators in the maintenance of gut-liver axis homeostasis, highlighting the participation of host-diet-microbiotametabolite interactions in NAFLD.
Seed coats of pulses are the main by-products during pulsebased food processing, which have been considered as an important source of dietary fibre and phytochemicals and used as functional food ingredients [13]. Intriguingly, in our previous study, we found that the seed coat of mung bean played a crucial role in the regulation efficacy of whole mung bean on HFD-induced hepatic steatosis and gut microbiota disorder [14,15]. Furthermore, it was reported that the water exacts of MBC could effectively alleviate LPS-induced acute liver injury mice [16]. Besides, both thein vitroandin vivostudies have demonstrated that MBC or its exacts exerted various health effects [17,18], such as antioxidant, anti-inflammatory, and antihyperlipidemia, which are conducive to the improvement of NAFLD.Therefore, we speculated that the MBC might alleviate the dietinduced NAFLD. Notably, the rich dietary fiber and phytochemicals in MBC may also provide a substrate for gut microbiota metabolism.
However, whether MBC can target the modulation of gut microbiota and its metabolites for attenuating NAFLD and the potential underlying mechanism have yet to be elucidated. Herein,in the present study, we demonstrated that MBC was effective in protecting against the diet-induced NAFLD. Integrative analysis of the fecal microbiome and untargeted metabolomics revealed that MBC significantly prevented HFD-induced changes in the gut microbiota composition and its metabolites. More importantly, the production of SCFAs were significantly promoted after dietary supplementation with MBC. Our results provided novel insights into underlying mechanisms of MBC in preventing NAFLD, thus enlarging the application of MBC as a potential functional food.
2. Materials and methods
2.1 Materials and diets
MBC power used in this study were prepared in our lab without any extraction process. In detail, mung bean seeds (cultivated in Datong, China) were soaked in the distilled water for 8 h at room temperature, then were rubbed manually to obtain seed coats. The collected MBCs were dehydrated using freeze dryer, smashed into powders using a grinder, sieved at 80 of mesh size, and stored at-80 °C until subsequent usage. The major nutritional components of MBC powder were provided in Table S1. The animal diets, including normal control diet (NCD, 10% energy derived from fat, 70% energy derived from carbohydrate, 20% energy derived from protein,3.85 total kcal/g, D12450J) and high-fat diet (HFD, 60% energy derived from fat, 20% energy derived from carbohydrate, 20% energy derived from protein, 5.24 total kcal/g, D12492), were purchased from Research Diets Inc. (New Brunswick, NJ, USA). More importantly,based on the formulation of HFD, the MBC power was incorporated into the HFD as described previously [19,20]. The HFD and HFD supplemented with MBC (HFD-MBC) diets were contributed equally to the nutrients and caloric density.
2.2 Animals and experimental design
All animal experimental protocols in this study were performed in accordance with the ethical guidelines of the National Research Council Guidelines and were approved by the Institutional Animal Care and Use Committee of China Agricultural University. Fourweek-old male C57BL/6J mice (specific-pathogen-free (SPF) grade,weighted 16-18 g) were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice were raised four per cage in SPF grade holding house with food and waterad libitumunder controlled environment ((24 ± 2) °C at (60 ± 5)% relative humidity, 12-h light/dark cycle). The schematic overview of the animal experiment procedure is depicted in Fig. 1A.
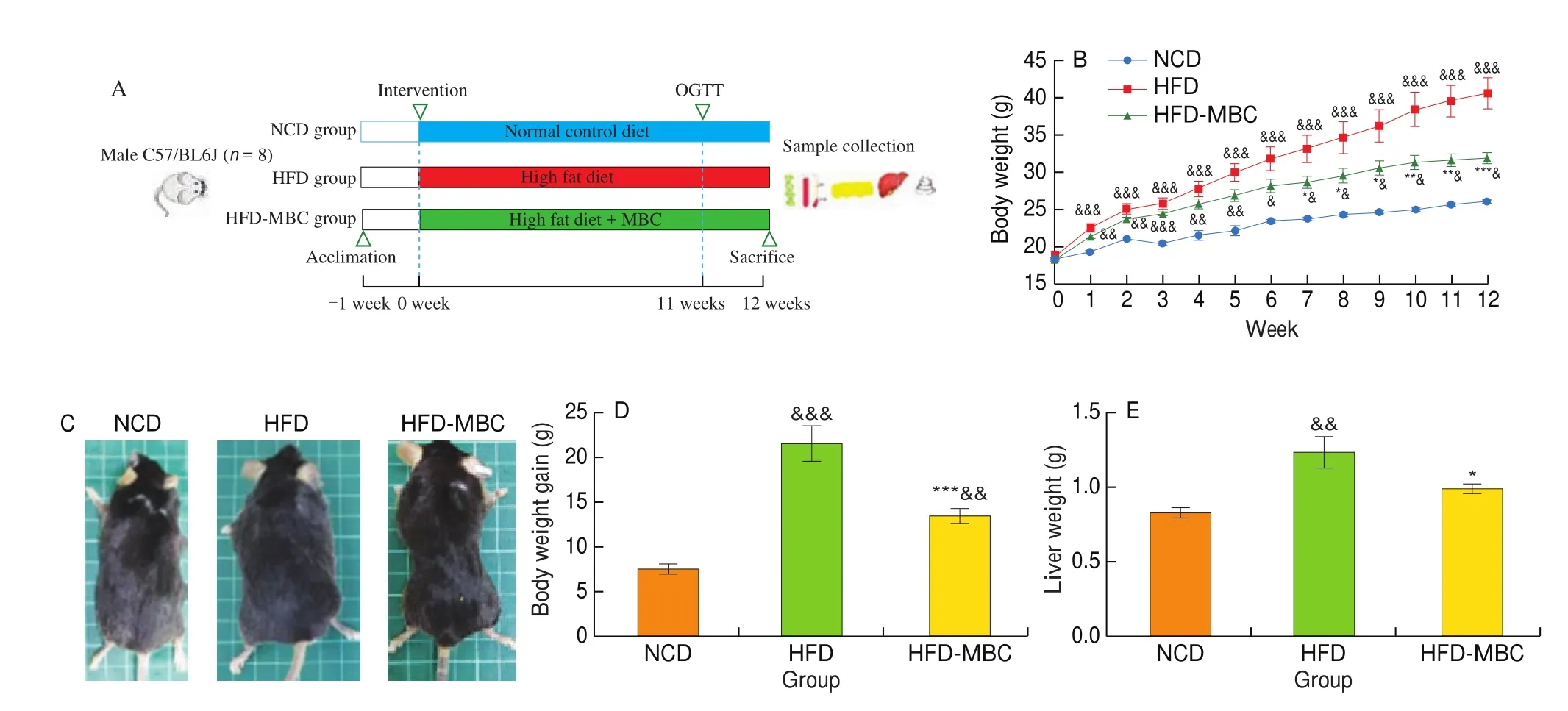
Fig. 1 Effect of MBC on body weight and liver steatosis in HFD-fed mice. (A) Animal experiment design. (B) Respective appearance of mice in different groups.(C) Body weight of mice was recorded every week. (D) Body weight gain. (E) Liver weight. (F) Representative morphology of the livers (top) and liver Oil Red O staining (bottom, magnification 200 ×). (G) Hepatic TC and TG contents. (H) Serum ALT and AST levels. &P < 0.05, &&P < 0.01, &&&P < 0.001 vs NCD. *P < 0.05,**P < 0.01, ***P < 0.001 vs HFD.
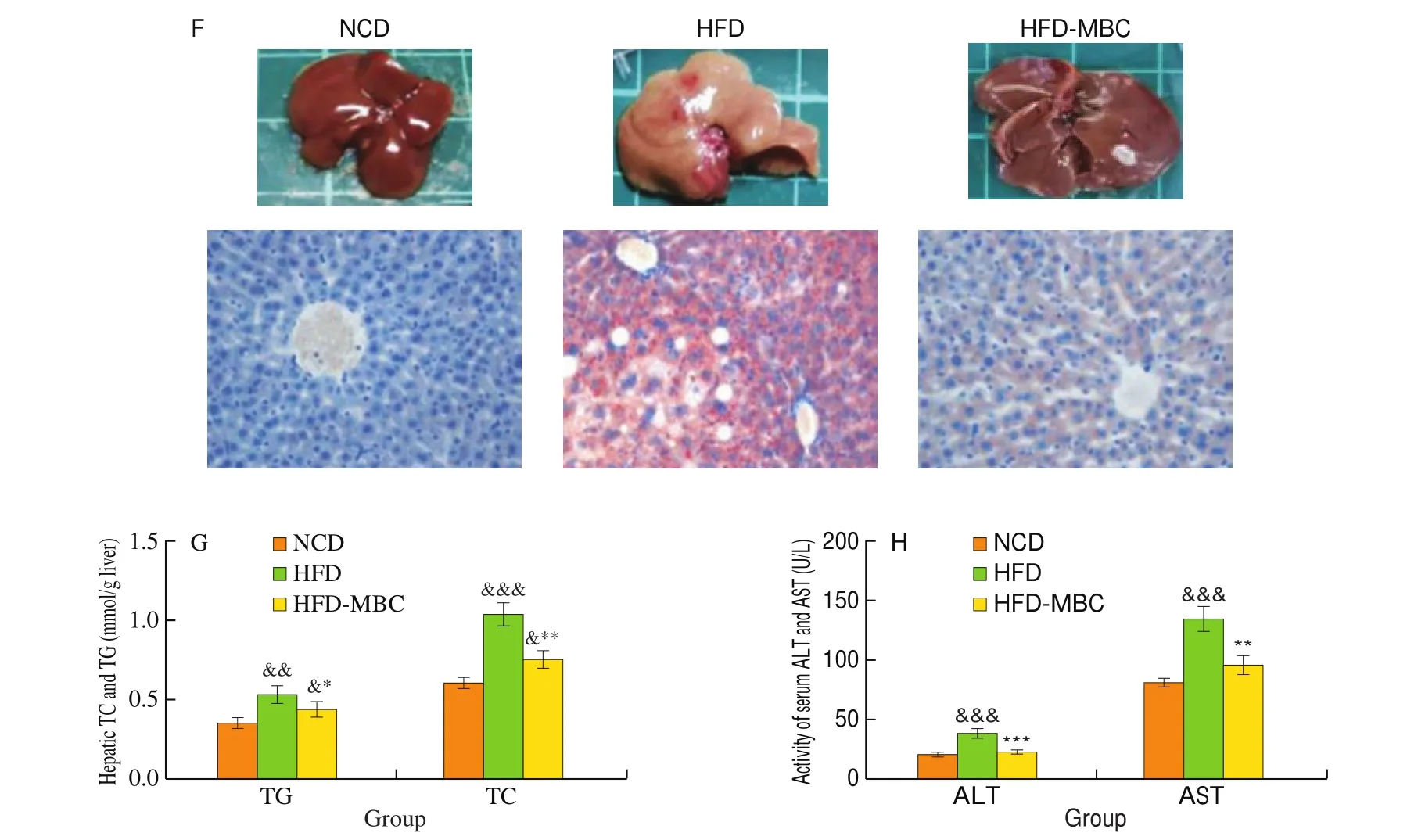
Fig. 1 (Continued)
The mice were fed with a NCD for one week to adapt to the environment and later randomly divided into 3 groups (8 mice per group): the normal control group (NCD group) was fed a NCD,whereas the other 2 groups were fed HFD (HFD group) or HFD supplemented with 6% MBC (HFD-MBC group), respectively.According to the Dietary Guidelines for Chinese Residents and our previous study [21], 30% whole mung bean powder could exert health benefits on HFD-induced metabolic disorders. Due to the MBC accounting for about 10%-20% of mung bean seed, the 6% (m/m)MBC supplementation levels were used in this study. The food intake and body weight were measured weekly during 12 weeks of intervention experiment. When the experiment was completed, all mice were sacrificed by cervical dislocation combined with mild pentobarbital anesthesia after a 12-h overnight fast. Subsequently, the liver tissues were collected and weighted. Part of the liver was fixed in 4% paraformaldehyde, and the others were snap frozen with liquid nitrogen and stored at -80 °C.
2.3 Oral glucose tolerance test (OGTT)
OGTT was carried out in mice following 6 h fasting at 12-week.In detail, each mouse was given glucose solution (2 g/kg body weight)by oral gavage based on their body weight. The glucose levels of blood samples taken from the tail vein at 0, 30, 60, 90, 120 min were determined by using a glucometer (Bayer Contour, Tarrytown, NY,USA). The area under the curve (AUC) corresponding to the OGTT data was calculated for each mouse.
2.4 Histological analysis
The hepatic steatosis was evaluated by Oil Red O staining. The Oil Red O staining of liver was performed according to the method described before with slight modification [22]. In detail, the optimal cutting temperature (OCT)-embedded liver tissues were serially sectioned at 5 μm, mounted on slides and allowed to dry. The dried slides were stained in 0.5% Oil Red O solution for 30 min after placing in propylene glycol for 2 min. Subsequently, the stained slides were rinsed with PBS for 2 changes, and processed with hematoxylineosin. The sections were imaged by using an inversion microscope(Axio Observer A1; Carl Zeiss, Oberkochen, Germany).
2.5 Biochemical assays of the liver and serum samples
Hepatic lipids content, including the total cholesterol (TC) and triglyceride (TG) in liver, was determined as described previously [23].Briefly, the total lipids from liver tissues were extracted with icecold normal saline (0.86%), the obtained supernatant was used to measure hepatic TC and TG. The detailed methods referred to the manufacturers’ instructions of commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanning, China). In addition, a 3100-automatic biochemistry analyzer (Hitachi Ltd., Tokyo, Japan)was used to determine the serum levels of total cholesterol (TC), total glyceride (TG), high-density lipoprotein cholesterol (HDL-C), lowdensity lipoprotein cholesterol (LDL-C), alanine aminotransferase(ALT), and aspartate aminotransferase (AST), according to the manufacturer’s instructions of corresponding commercial kits(Maccura Biotechnology Co., Ltd., Chengdu, China).
2.6 Oxidative stress and inflammatory response evaluation
Hepatic malondialdehyde (MDA) level and the activities of superoxide dismutase (SOD) and catalase (CAT) were measured by using commercially available biochemical assay kits following the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China). The levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the livers were determined with the enzymelinked immunosorbent assay (ELISA) kits (Shanghai Enzymelinked Biotechnology Co., Ltd., China) according the manufacturer’s instructions.
2.7 Gut microbiota analysis
Fresh fecal samples from each mouse were collected in individual sterile EP tubes at week 12, then immediately frozen in liquid nitrogen and stored at -80 °C. The bacterial genomic DNA was extracted from the fecal samples using an E.Z.N.A.®soil DNA Kit as per the manufacturer’s instruction. The purity and concentration of the extracted DNA were monitored by 1% agarose gels and a NanoDrop 2000 UV-vis spectrophotometer (Thermo Fisher Scientific, Wilmington, USA), respectively. The extracted genomic DNA was amplified with barcoded conventional primer pairs 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R(5’- GGACTACHVGGGTWTCTAAT-3’) based on the bacterial 16S rRNA gene V3–V4 hypervariable region. The resulted PCR products were extracted from a 2% agarose gel, purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City,CA, USA), and further quantified using the Quantus™ Fluorimeter(Promega, USA). The purified amplicons were pooled in equal amounts and paired-end sequenced (2 × 300 bp) on an Illumina MiSeq platform following the standard protocols at Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
After the accomplishment of sequencing, the obtained original data were quality filtered and merged to get the effective reads.The unique sequences were clustered into the same operational taxonomic units (OTUs) at a 97% similarity threshold using Uparse(Version 7.0, http://www.drive5.com/uparse/). Based on the results of OTUs clustering, the corresponding microbial species information and its abundance distribution were obtained through annotating the representative sequences of each OUT.α- andβ-diversity were determined using Mothur (V 1.30.2) and QIIME (V 1.7.0),respectively, and they were displayed with R software (V 3.3.1).Principal Coordinates Analysis (PCoA) was performed to evaluateβ-diversity by using the non-phylogenetic (Bray-Curtis) distances matrices. Significant differences inβ-diversity were confirmed by ANOSIM statistic test. Hierarchical clustering of samples calculated by using Unweighted Pair-group Method with Arithmetic Mean(UPGMA). Furthermore, the linear discriminant analysis (LDA) effect size (LEfSe) with a threshold of 3.0 was conducted to distinguish the significantly important microbial taxa among different groups.
2.8 Non-targeted metabolomics analysis
Ultra-high performance liquid chromatography tandem massspectrometry (UPLC-MS/MS)-based metabolomics was performed by Majorbio Bio-Pharm Technology (Shanghai, China). Feces metabolites extraction were performed as previously described method [24]. Briefly, accurately weighed 50 mg of fecal samples were extracted with 400 μL of pre-chilled methanol and acetonitrile mixture solution (1:1,V/V). The L-2-chlorophenylalanine solution(0.2 mg/mL) as an internal standard was added in the mixtures.After homogenization and sonication, the mixtures were incubated at -20 °C for 30 min to precipitate proteins. The supernatant was transferred and evaporated under a gentle nitrogen stream, and then the dried residue was re-dissolved in 100 μL of pre-chilled ethanol and acetonitrile mixture solution. After centrifugation, the collected supernatants were carefully filtered with a 0.22 μm filtering membrane and transferred into auto sample vials for UPLC-MS/MS analysis.The detailed methods of UHPLC-MS/MS analysis are provided in the Supplementary Information.
The acquired raw UHPLC-MS/MS data files were imported to into the progenesis QI 2.3 (Nonlinear Dynamics, Waters, USA) for peak detection and alignment analysis. After baseline correction and screening processing, peak intensities of metabolites were normalized to the total spectral intensity. Multivariate statistical analysis including the principle component analysis (PCA) and orthogonal partial leastsquares discriminant analysis (OPLS-DA) were subsequently used to assess the overall difference between groups. The metabolites were identified based on adduct ions, molecular ion peaks and fragments by matching with HMDB (http://www.hmdb.ca/) and ChemSpider (http://www.chemspider.com/) database. For identification of differential metabolites, those with VIP > 1 andP-value < 0.05 (two-tailed student’st-test) and fold change (FC) ≥ 1.5 or FC ≤ 0.5 among the three groups were included. FC was presented as a binary logarithm of the average normalized peak area ratio. The main biochemical metabolic pathways and signal transduction pathways enrichment of differential metabolites were studied based on the KEGG database(Kyoto Encyclopedia of Genes and Genomes, http://www.genome.jp/kegg).
2.9 SCFAs analysis
The fecal SCFAs (acetic acid, propionic acid, butyric acid,isobutyric acid, valeric acid, isovaleric acid, and hexanoic acid) in the feces were determined by gas chromatography tandem massspectrometry (GC-MS) according to the previously described method [25]with slightly modifications. In brief, 50 mg of lyophilized stool samples were diluted with 0.8 mL of distilled water by vortexing.Subsequently, 500 μL of ether and 0.4 mL of H2SO4(50%,V/V)were added to extract the SCFAs. After shaking for 3 min at room temperature and centrifuging at 12 000 ×gfor 10 min at 4 °C,anhydrous CaCl2was added to remove residual water. The collected supernatant was filtered through the 0.22 μm membrane and then was analyzed by GC-MS system.
The analysis of SCFAs were conducted by using a Thermo TRACE 1310-ISQ LT equipped with an Agilent HP-INNOWAX capillary column (30 m × 0.25 mm × 0.25 μm). The injection volume and the split ratio were 2 μL and 10:1, respectively. The initial oven temperature was maintained at 90 °C for 1 min, increased to 120 °C at a rate of 10 °C/min, then raised to 150 °C at a rate of 5 °C/min, finally increased to 250 °C at a rate of 25 °C/min, and held on this temperature for 2 min. The temperature of inlet was 220 °C, while the ionization source, transmission line, and quadrupole temperature were 230, 250, and 150 °C, respectively. The constant flow rate of carrier gas (He) was set at 1.0 mL/min. The concentration of each SCFA was calculated based on the corresponding standard curve.
2.10 Statistical analysis
Statistical difference was performed using GraphPad Prism 8.0(GraphPad Software, CA) based on an unpaired student’st-test (for comparison between two groups) or one-way analysis of variance(ANOVA) with Tukey’s multiple comparisons test (for comparison among three groups). The experimental data are presented as means ±standard error of the mean (SEM). The level of statistical differences was considered as significant atP< 0.05.
3. Results
3.1 MBC attenuated HFD-induced obese phenotypes and hepatic steatosis
To evaluate the effects of MBC on the obese phenotypes and hepatic steatosis, mice were subjected to HFD with or without MBC supplementation for 12 weeks (Fig. 1A). As anticipated, significant increases in the body weight of HFD-fed mice were observed from 1 to 12 weeks, as compared with those fed with a NCD (Fig. 1B).It was worth noting that from the week 7, MBC supplementation significantly prevented the HFD-fed mice from gaining more weight.At the end of the animal experiments, visual observation found that mice in the HFD-MBC group were thinner than these in the HFD group (Fig. 1C), as evidenced by the significantly decreased body weight gain in the HFD-MBC group (Fig. 1D). Furthermore, MBC supplementation did not alter the food and energy intake of mice as compared to the HFD group (Table S2).
As shown in Fig. 1E, mice in the HFD group displayed a higher liver weight than those in the NCD group, while MBC supplementation remarkably reduced the liver weight in HFD-fed mice (P< 0.05). The characteristic manifestations of NAFLD was the abnormal hepatic lipid accumulation, which was mainly attributed to the imbalance of lipid metabolism in the hepatocytes. The liver of mice in the NCD group were dark red. In contrast, the liver of HFD-fed mice lost blood color and looked light yellow and pale in color,which was a typical and intuitive manifestation of hepatic steatosis.However, dietary supplementation with MBC markedly recovered the dark brown-red color of the liver (Fig. 1F). The results of Oil Red O staining demonstrated that MBC supplementation remarkably mitigated the deposition of lipid droplets in liver tissues of HFD-fed mice (Fig. 1F). The hepatic TC and TG contents were significantly reduced by MBC supplementation (Fig. 1G), which was alignment with pathological results. The results of serum ALT and AST levels also reflected that the liver injure was significantly alleviated by MBC supplementation (Fig. 1H).
In addition, the serum lipid levels, including TC, TG, and LDL-C,were significantly elevated in HFD-fed mice as compared to the NCD-fed mice, however, they were significantly decreased by MBC supplementation (Fig. S1A). Furthermore, the results of OGTT and AUC (Fig. S1B) further confirmed that MBC could effectively attenuate insulin resistance of HFD-fed mice, which was closed associated with the improvement of the NAFLD. Meanwhile, mice treated with MBC presented lower levels in the fasting blood glucose and fasting serum insulin than these in the HFD group (Table S2).
3.2 MBC improved oxidative stress and inflammation in the liver
HFD resulted in a significant increase in the hepatic MDA level,which was significantly inhibited by MBC (Fig. 2A,P< 0.05).Moreover, the intracellular antioxidant enzymes, including SOD and CAT, in HFD-fed mice were significantly decrease as compared with that of the NCD group. In contrast, MBC could markedly increase the hepatic SOD and CAT activities in mice fed with a HFD(Figs. 2B and C,P< 0.05). Inflammatory response was another important clinical manifestation of NAFLD. Our results showed that the levels of hepatic TNF-α and IL-6 in the HFD group were significantly higher than those in the NCD group. However, the inflammatory levels were notably reduced by MBC supplementation(Figs. 2D and E,P< 0.05). Taken together, these findings suggested that MBC could effectively improve the hepatic oxidative stress and the inflammatory in HFD-fed mice.
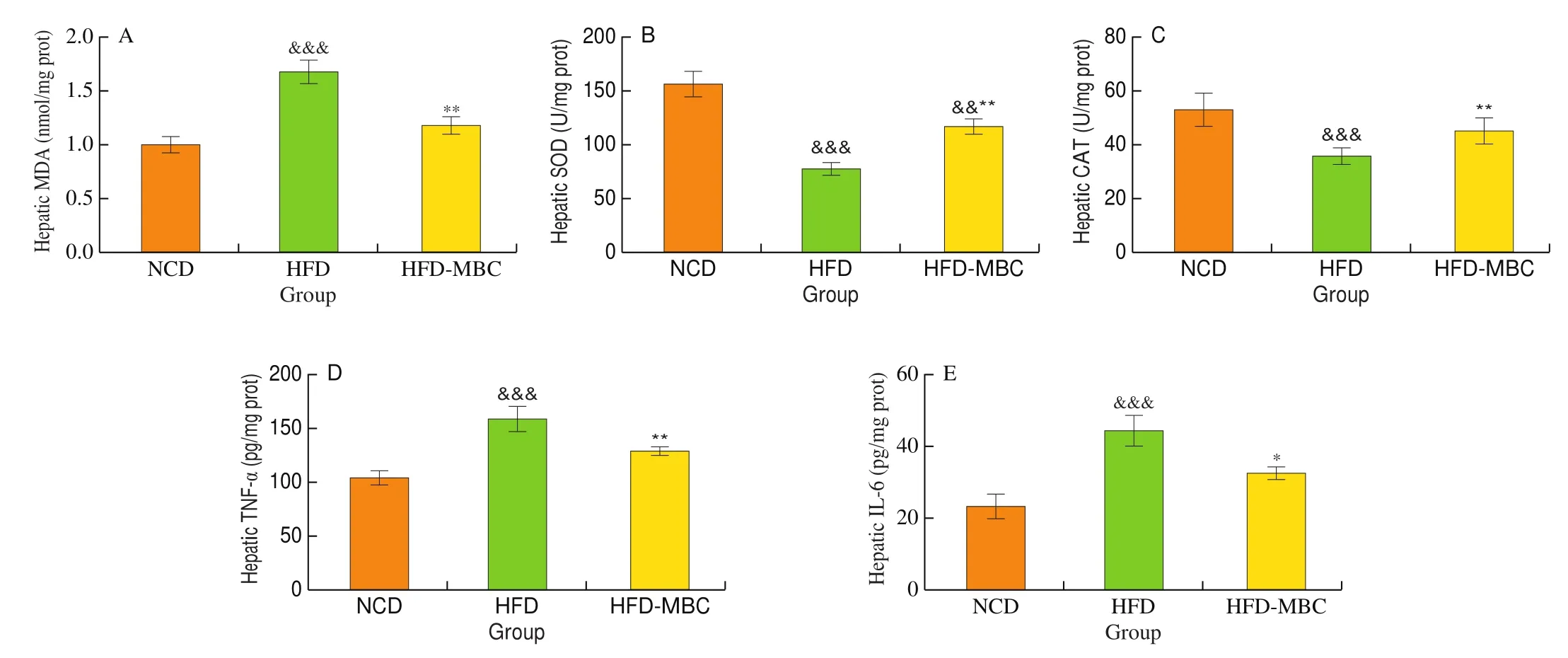
Fig. 2 Effects of MBC on the oxidative stress and inflammation of liver. (A) Hepatic MDA level. (B) Hepatic SOD activity. (C) Hepatic CAT activity. (D) and(E) Hepatic inflammation levels. &P < 0.05, &&P < 0.01, &&&P < 0.001 vs NCD. *P < 0.05, **P < 0.01, ***P < 0.001 vs HFD.
3.3 MBC modulated the overall structure and composition of gut microbiota in HFD-fed mice
We performed a pyrosequencing-based analysis of bacterial 16S rRNA V3-V4 region to evaluate the effects of MBC supplementation on the structure and composition of gut microbiota. After a series of processing (quality- filtered, merged and chimera-checked), a total of 940 201 high-quality reads were obtained from 24 fecal samples. The Rarefaction curve and Shannon index curve (Fig. S2A, B) revealed that the sequencing data covered vast majority of the diversity and rare new phylotypes.
Furthermore,α-diversity analysis showed that MBC supplementation effectively prevented the HFD-induced reduces in the microbial community richness, as evidenced by the significantly increased observed species and Chao1 indexes in HFD-MBC group as compared to the HFD group (P< 0.05, Fig. 3A). However, no significant differences were observed in the microbial community diversity (represented by PD whole tree and Shannon indexes, Fig. 3B)between the HFD and HFD-MBC groups.β-Diversity analysis,including PCoA and sample hierarchical clustering tree, was employed to learn more about the effects of MBC supplementation on the overall structural changes in the gut microbiota. Bray-Curtis-metrics-based PCoA displayed a distinct clustering of microbial community for the three groups, which was con firmed by the ANOSIM test (R2= 0.573 7,P= 0.001 < 0.05, Fig. 3C). The HFD-MBC group evidently clustered separately from the HFD group, indicating that MBC supplementation could significantly modulate the gut microbial structure of HFD-fed mice. In addition, the same result was also observed in the UPGMA(unweighted pair-group method with arithmetic means) tree, showing that the three groups separately clustered from each other (Fig. 3D).It was noted that the HFD group was far away from the NCD group,while the HFD-MBC group was much closer to the NCD group.

Fig. 3 Effects of MBC on the overall structure of the gut microbiota. (A, B) α-Diversity analysis. (C) PCoA score plot of the fecal microbial communities based on based on Bray-Curtis. (D) Hierarchical clustering based on the unweighted-unifrac distance matrix. &P < 0.05, &&P < 0.01, &&&P < 0.001 vs NCD. *P < 0.05,**P < 0.01, ***P < 0.001 vs HFD.
To further investigate the specific changes in gut microbiota composition caused by MBC supplementation, we analyzed the differences of taxonomic composition at three levels, including phylum, family, and genus. As shown in Fig. 4A, at the phylum level, the gut microbiota of mice in the three group mainly consists of Firmicutes and Bacteroidetes, followed by Verrucomicrobia and Actinobacteria. The taxonomic abundance presented a significant decrease in the Bacteroidetes in HFD-fed mice as compared to the NCD-fed mice, while MBC supplementation obviously increased the relative abundance of Bacteroidetes (Fig. S3A). However, no significant differences were observed in the relative abundance of Firmicutes among the three groups (Fig. S3A). Interestingly, the remarkably increased Firmicutes/Bacteroidetes (F/B) ratio caused by HFD was significantly reduced by MBC supplementation (P< 0.05,Fig. S3B). In addition, Verrucomicrobia showed a relatively higher abundance in the HFD-MBC group than that of the HFD group.There were no significant differences in the relative abundance of Actinobacteria between the HFD and HFD-MBC groups. At family level (Fig. 4B), the remarkable elevation of Ruminococcaceae and Desulfovibrionaceae and reduction of Akkermansiaceae,Muribaculaceae, and Rikenellaceae were observed in the HFD-fed mice when compared with those in the NCD group. Nevertheless,MBC supplementation could markedly restore the relative abundance of these microorganism in HFD-fed mice to a level similar to that of the NCD group (P< 0.05, Fig. S3C). Notably, the relative abundance of Lachnospiraceae was much higher in the HFD-MBC group than in another two groups (P< 0.05).

Fig. 4 Modulation of MBC on gut microbial community in HFD-fed mice. (A, B) Relative abundance of the fecal microbiota at the phylum and family levels.(C) Heatmap of the 50 OTUs altered by HFD responding to MBC supplementation. (D, E) The relative abundance of key genera. The color of spots in the left panel represents the relative abundance of OTU in each group. The white circles (○) and black dots (●) represent higher and lower relative abundance of OTUs in NCD or HFD-MBC groups than in HFD group, respectively, while the black stars (★) represent OTUs whose relative abundance in NCD group was altered by HFD and then reversed by MBC. &P < 0.05, &&P < 0.01, &&&P < 0.001 vs NCD. *P < 0.05, **P < 0.01, ***P < 0.001 vs HFD.
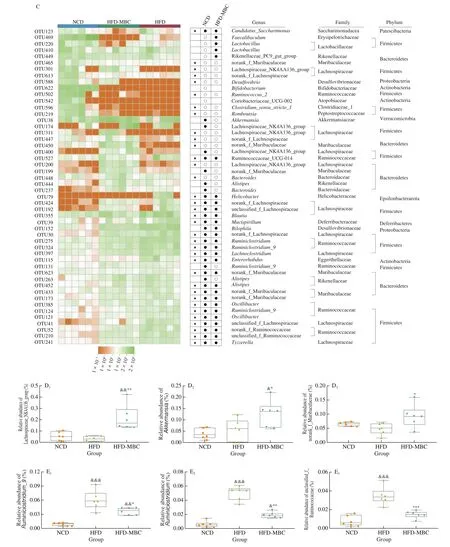
Fig. 4 (Continued)
We further identify the specific bacterial genera that responded to HFD feeding and MBC supplementation. Considering that the different OTUs in the same genus might show different responses to dietary intervention, the changes of gut microbiota at OTU levels were assessed to excavate the key microbiota. Based on this, a total of 50 OTUs with most relative abundances were selected to find the specific bacterial genera (Fig. 4C). Of these altered 50 OTUs, HFD feeding significantly increased 32 OTUs and decreased 18 OTUs as compared with those of the NCD group. MBC supplementation prevented 35 OTUs abundance change, of which 12 increased and 23 decreased(Fig. 4C, highlighted with black stars). Detailed analysis of the altered 34 OTUs indicated that MBC supplementation significantly increased the relative abundance of Lachnospiraceae_NK4A136_group, norank_f__Muribaculaceae, andAkkermansia(Fig. 4D) and decreased the relative abundance ofRuminiclostridium_9,Ruminiclostridium, and unclassified_f__Ruminococcaceae (Fig. 4E) in the HFD-fed mice.The bacteria genera norank_f__Muribaculaceae and Akkermansia belonged to the phyla Bacteroides and Verrucomicrobia, respectively.Furthermore, the results were consistent with the discriminant analysis (LDA) scores derived from LEfSe analysis, showing that the three bacterial genera (Lachnospiraceae_NK4A136_group, norank_f__Muribaculaceae, andAkkermansia) were the key phylotypes of gut microbiota in response to MBC supplementation (Fig. S4).
In addition, no significant differences were observed in the relative abundance ofLactobacillus, Rikenellaceae_RC9_gut_group,Bifidobacterium, Coriobacteriaceae_UCG-002,Bacteroides,Bilophila, andOscillibacterbetween the HFD and HFD-MBC groups(Fig. 4C). Among these bacteria genera, the changes in the relative abundance ofBifidobacteriumwere consistent with our previous results [14]. In our previous study, whole mung bean supplementation could significantly increase the relative abundances ofAkkermansiaandBifidobacteriumin HFD and streptozotocin-induced prediabetic mice, while decorticated mung bean only significantly promoted the relative abundance ofBifidobacterium. These results indicated that MBC could alter the relative abundance of some HFD-dependent taxa to a certain extent.
3.4 MBC altered the fecal metabolite profiles in HFD-fed mice
A potential mechanism of gut microbiota involved in the host metabolism is strongly associated with the metabolites generated by microbial fermentation in the gut. To assess the metabolic alterations in response to the changes of gut microbiota induced by MBC supplementation, fecal metabolites were analyzed using UHPLCQTOF-MS/MS-based untargeted metabolomics in both positive and negative modes. A total of 6 399 peaks were detected (2 536 and 3 863 valid peaks for the positive and negative ion modes, respectively) in the metabolomics data. As shown in the PCA plots (Fig. 5A), a distinct clustering of fecal samples was observed in the positive and negative modes among the three groups, indicating significant differences in the fecal metabolites composition. Subsequently, OPLS-DA model was conducted to evaluate the data quality and identify the potential biomarkers. The OPLS-DA score scatter plots presented a clear separation between the HFD and HFD-MBC groups in both positive and negative ion modes (Fig. 5B). Significant separations were also observed between the NCD and HFD groups (Fig. S5). Furthermore,the results of permutation test showed that the interpretation ability parameters (R2Y) were close to 1.0 and the prediction ability parameters (Q2) were all greater than 0.5, hinting good reliability and high predictability of the model (Fig. 5C, Table S3)in explaining the metabolites variations between the two groups.

Fig. 5 MBC modulated the metabolic profiles of the fecal samples. (A) PCA score plots of fecal samples from different groups; (B) Scores plots of OPLS-DA between the HFD and HFD-MBC groups; (C) Permutation tests conducted with 200 random permutations in the OPLS-DA model. 1 and 2 are in the positive and negative modes, respectively.
A total of 373 metabolites (182 in the positive ion mode and 191 in the negative ion mode) with definite information were identified among the three groups based on the VIP values > 1.0, fold change > 1.0 or < 1.0, andPvalues < 0.05. Volcano plot (Fig. 6A) showed 227 significantly altered metabolites after 12 weeks of MBC supplementation as compared with the HFD, of which 177 were up-regulated and 50 were down regulated. Specifically, heat map analysis exhibited the top 50 significantly altered metabolites between the HFD and HFD-MBC groups (Fig. 6B), contributing to their metabolic distinctions. They are mainly carboxylic acids, organic oxygen compounds, lipids and lipid-like molecules, and benzene.Based on the matched differential metabolites, we next analyzed the underlying metabolic pathways. It was found that, according to the KEGG classification, the differential metabolites identified between the two groups (HFD and HFD-MBC) were involved in seven categories of KEGG metabolic pathways, including organismal systems, metabolism, human diseases, drug development, cellular processes, genetic and environmental information processing(Fig. 6C). Interestingly, the lipid metabolism was significantly enriched in the metabolism category (Fig. 6C).
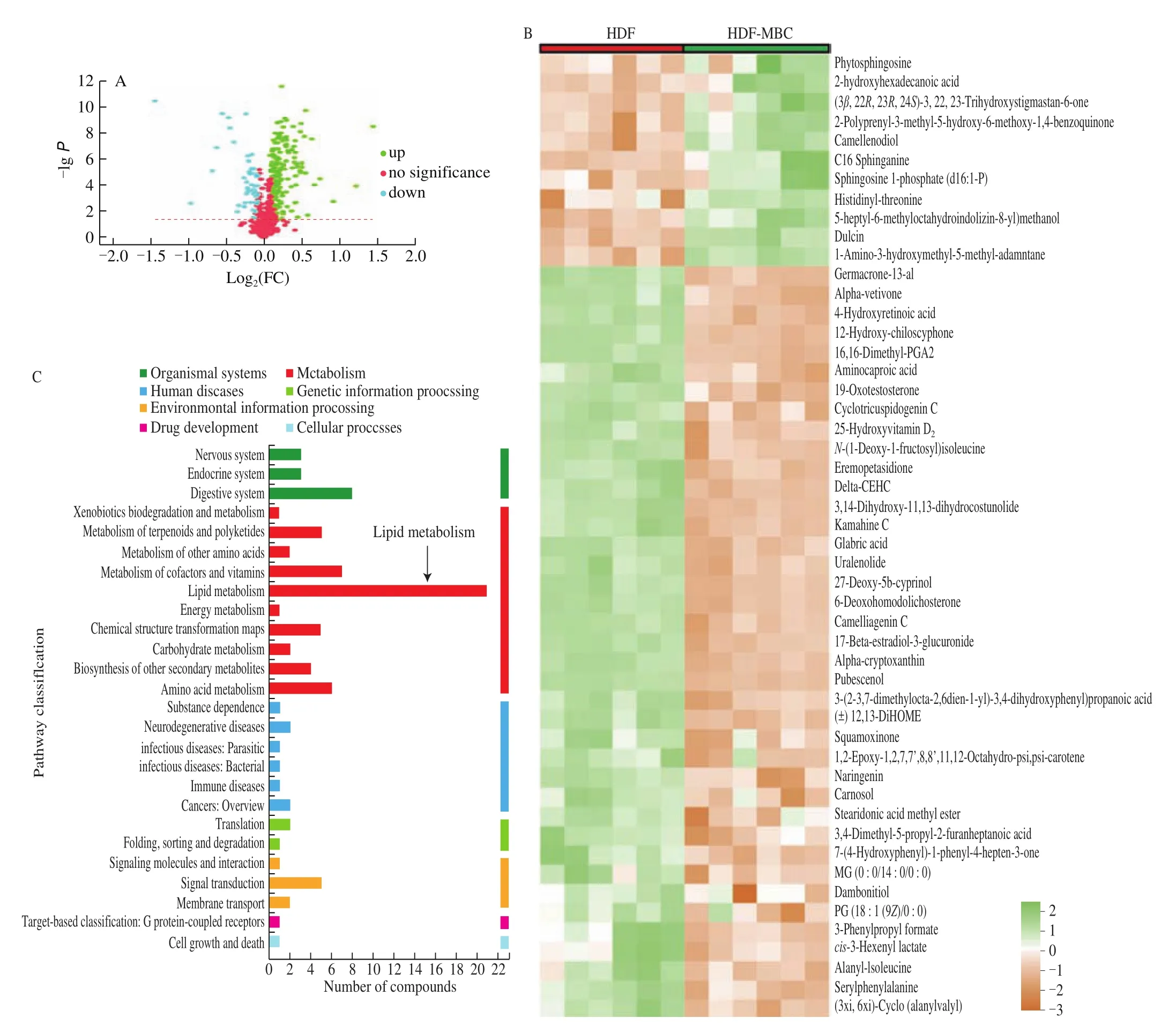
Fig. 6 MBC supplementation regulates fecal metabolite profiles in HFD-fed mice. (A) Volcano plot of fecal metabolomics of mice showing the significantly changed metabolites in HFD-MBC group compared to HFD group. (B) Heatmap of 50 significantly altered metabolites levels between the HFD and HFDMBC groups. Blocks in green and orange denote high and low z-score values, respectively. (C) KEGG annotation analysis of the changed metabolites based the differences between the HFD and HFD-MBC groups.
Based on the differential metabolites of HFD and HFD-MBC groups, pathway analysis was conducted via the KEGG topology analysis (Fig. 7). KEGG topology analysis found that ten metabolic pathways were enriched. Among them,α-linolenic acid metabolism,sphingolipid metabolism, tryptophan metabolism, arginine and proline metabolism, arginine biosynthesis, and tyrosine metabolism were the main metabolic pathways altered by MBC supplementation, which might be related to the prevention of HFD-induced NAFLD. The detailed information about pathway enrichment results are displayed in Table 1. Notably, sphingolipid metabolism (impact value =0.201 219 512,P= 0.000 755 183) andα-linolenic acid metabolism(impact value = 0.232 067 511,P= 0.001 941 061) were filtered out as the mainly metabolic pathways (P< 0.05) between the HFD and HFD-MBC groups. The three metabolites phytosphingosine,sphingosine, and sphinganine were identified to be related with the sphingolipid metabolism. The metabolites, including jasmonic acid,12-OPDA, andα-linolenic acid, were involved in theα-linolenic acid metabolism (Table 1).
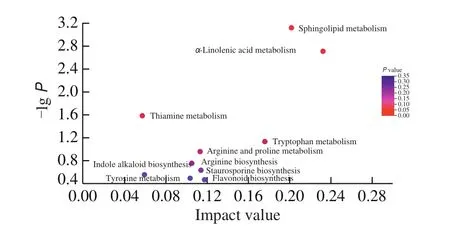
Fig. 7 Pathway impact in topology analysis. The size and color of each circle was based on the pathway impact value and P-value, respectively.

Table 1Effect of MBC on KEGG pathways.
3.5 MBC affected the concentrations of SCFAs
Next, we determined the concentrations of SCFAs using GC/MS method, considering that the untargeted metabolomics conducted with LC/MS was not suitable to measure the SCFAs due to their strong volatility. The concentrations of seven main SCFAs and total SCFAs were shown in the Fig. 8. Compared with the NCD group,HFD feeding significantly decreased the propionic acid and caproic acid contents, whereas had no effects on the concentrations of other SCFAs and the total SCFA. However, higher levels of acetic acid,propionic acid, butyric acid, valeric acid, and total SCFAs were observed in the HFD-MBC group than in the HFD group (Fig. 8).Conversely, MBC supplemention reduced the isobutyric acid content in HFD-fed mice. There was no significant difference in the caproic acid content between the HFD and HFD-MBC groups.
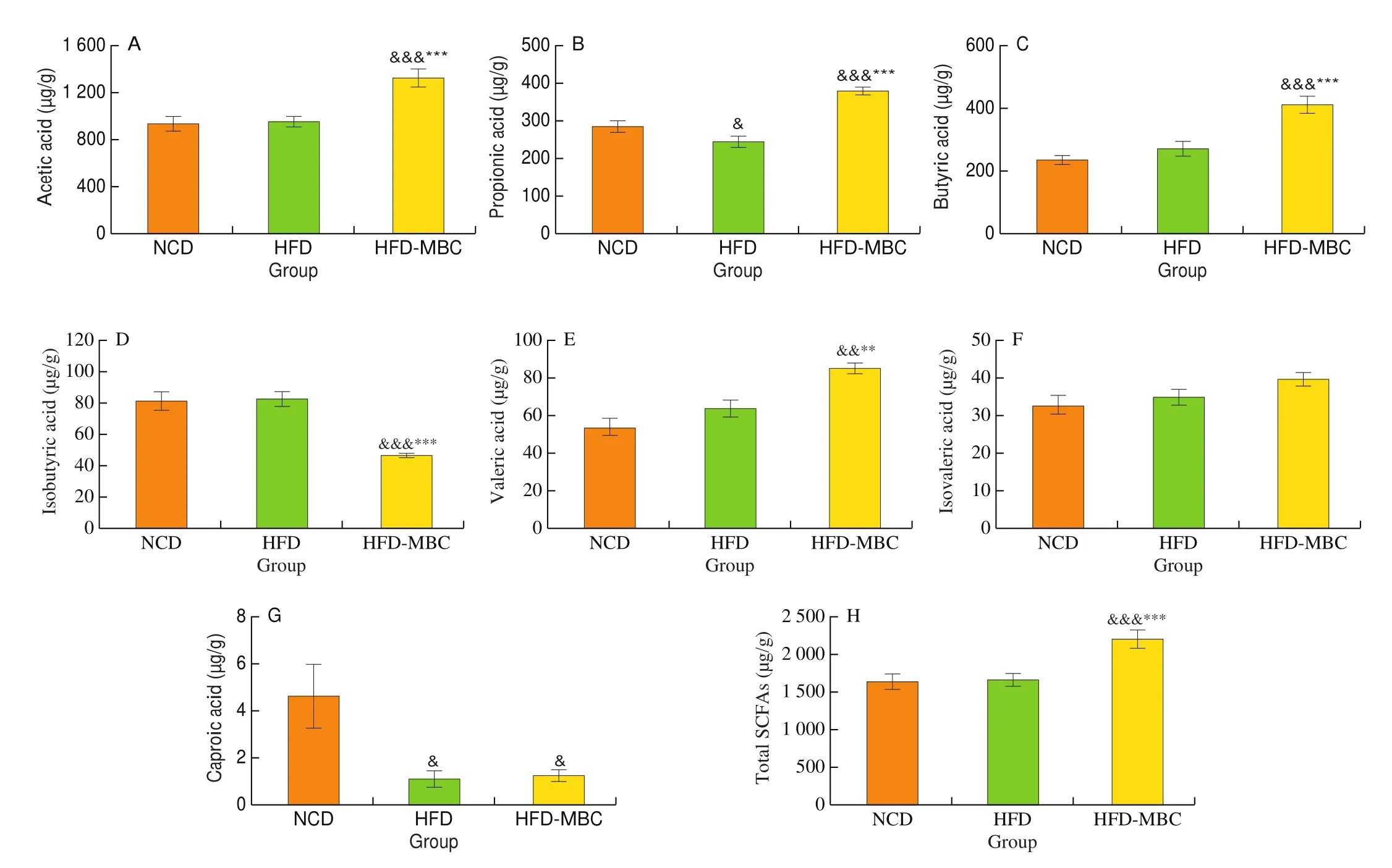
Fig. 8 SCFAs concentration of colonic content in different groups. &P < 0.05, &&P < 0.01, &&&P < 0.001 vs NCD. *P < 0.05, **P < 0.01, ***P < 0.001 vs HFD.
4. Discussion
NAFLD has become a primary public health concern worldwide.Without timely treatment, it eventually increases the risk of several chronic diseases, such as cardiovascular diseases and type 2 diabetes.Legumes diet intervention has been demonstrated to be an effective nutritional strategy that can prevent the development of NAFLD,due to their relatively low energy density and high nutrients [26].Previous studies have indicated that mung bean can protect against obesity-related diseases [27], and the MBC contributes greatly to its health benefits [15]. The abundant dietary fibre and potential health-promoting phenolic compounds are all deemed to be effective constituents contributing to the hypothetical function of MBC [28].However, the protective effect and potential mechanism of the MBC on NAFLD remain to be explored.
In this study, it is found that MBC could effectively decrease HFD-induced overweight (Figs. 1B-D). Furthermore, the histopathological observation indicated obvious lipid accumulation in the liver tissues of HFD-fed mice, while dietary supplementation of MBC significantly caused a potent improvement of hepatic steatosis as evidenced by the significant reduction of serum AST and ALT,and hepatic TC and TG (Figs. 1G,H). Obviously, MBC also exhibited the serum lipid profiles lowering effects, including TC, TG, and LDL-C (Fig. S1A), which could reflect the status of lipid adsorption and metabolism in the liver [29]. The insulin resistance, which can disrupt the synthesis and transport of intracellular triglycerides, was considered to be closely linked to the development of NAFLD [30].Intriguingly, MBC supplementation significantly attenuated the HFD-induced insulin resistance (Fig. S1B), contributing to the amelioration of hepatic steatosis. The oxidative stress and inflammatory response have emerged as the two primary causes involved in the pathogenesis of NAFLD [31]. Following the accumulation of hepatic TG, excess fatty acid oxidation increases the production of reactive oxygen species, thus resulting in hepatic oxidative stress. The activities of SOD and CAT could indirectly reflect the capacity to remove oxygen-free radicals during oxidative stress, whereas MDA, as a lipid peroxidation product, is an indicator of hepatic cell oxidative damage. In the present study, MBC significantly diminished oxidative stress via enhancing the activities of antioxidant enzymes (SOD and CAT) and reducing the MDA level in HFD-fed mice. On the other hand, the HFD-fed mice presented significantly higher hepatic TNF-α and IL-6 levels, and the changes were reversed by MBC.These results indicated that the dietary intervention by MBC was efficient to improve insulin resistance and reduce oxidative stress and inflammatory response, thus protecting against NAFLD.
Notably, MBC is rich in dietary fiber and polyphenols, and there is still contradiction between their poor bioavailability and biological functions [32]. Thus, our study hypothesized that these observations can be, at least partially, attributed to the modulation of gut microbiota composition and its metabolites. In the previous studies,germ free (GF) mice have been proven to resistant to high-fat diet(HFD)-induced obesity, despite a higher food intake [8]. Furthermore,GF mice that received fecal microbiota transplantation from an obese donor resulted in significantly greater increases in the body weight and total body fat than those receiving it from a lean donor [33,34].Notably, gut microbiota has been considered as the novel therapeutic target for NAFLD [35,36]. Thus, based on the above evidences, it was reasonable to believe that there is a direct relationship between the phenotype and gut microbiota. Accumulating evidence has revealed that dysbiosis of gut microbiota could disturb the hepatic carbohydrate and lipid metabolism, and affect the inflammatory response balance in the liver, thus resulting in the development of NAFLD [11,37].Therefore, normalizing gut microbiota through the dietary intervention could be an effective therapeutic target for treating NAFLD. In the present study, our results showed that MBC could effectively restore the gut microbiota structure and composition altered by HFD and improve the relative abundance of some key bacterial genera in mice fed with HFD. As expected, a decreased bacterial richness was observed previously in the HFD fed mice [38], which was consistent with our results, showing a reduction of observed species and Chao1 indexes in the HFD group. Interestingly, supplementation with MBC led to an increase in microbial community richness. Besides,β-diversity showed significant differences among the NCD, HFD,and HFD-MBC groups, suggesting that MBC could modulate the altered structure and composition of gut microbiota induced by HFD.Notably, at the phylum level, the increased F/B ratio in the HFD-fed mice was significantly decreased by MBC. In the last decade, the F/B ratio has been frequently deemed as a possible hallmark for HFD-induced gut microbiota dysbiosis [33,39]. However, the great amount of contradictory results has been reported in the recent literatures [40,41].The results of many studies did not observe any modifications of this parameter or even reported a decrease in the ratio of F/B in obese animals and humans [6,42]. Actually, in some studies, the obese individuals presented lesser bacterial diversity than that of the lean subjects [43,44], suggesting other compositional changes at the family or genus level might be more relevant than the F/B ratio. Therefore,although our study supported a causal relationship between the decreased F/B ratio caused by MBC and the improvement of hepatic steatosis in HFD-induced obese mice, further accurate study needs to be conducted to con firm the results.
Moreover, there is compelling evidence supporting a strong relationship between the specific bacterial genera and specific biological effects [45]. At the genus level, we observed that the relative abundance ofAkkermansia, Lachnospiraceae_NK4A136_group, and norank_f_Muribaculaceae were significantly enriched in the HFD-MBC group as compared to the HFD group. Currently,Akkermansia, as the microbial genus with potential anti-hepatic steatosis effects, appears to be most unanimously supported by the published reports [46,47]. Furthermore, oral administration of viableAkkermansiahas also been demonstrated to be effective in reversing HFD-induced metabolic disorders in mice [48], including lipid accumulation and insulin resistance, suggesting that targeting some particular advantageous bacteria might be a potential treatment for NAFLD. In addition, among the commonly and consistently reported findings, Lachnospiraceae_NK4A136_group and norank_f_Muribaculaceae were also found to be positively associated with a reduction in hepatic steatosis. For example, the preventive effect of many functional foods or bioactive components on NAFLD, such as whole mung bean [27], flavonoids from whole-grain oat [49], and Fu instant tea [50], were deemed to be closely related with the increased relative abundance of Lachnospiraceae_NK4A136_group or norank_f_Muribaculaceae in HFD-fed mice. In addition to the promotion of the beneficial bacteria genera, MBC supplementation also suppressed the bloom of some HFD-dependent taxa, such asRuminiclostridium_9,andRuminiclostridium, unclassified_f_Ruminococcaceae, which has been proven to be positively associated with the lipid accumulation in previous studies [51,52]. Notably,Akkermansia, Lachnospiraceae_NK4A136_group and norank_f_Muribaculaceae were important SCFAs-producing bacteria [53,54]. In summary, the improvement effect of MBC on the phenotype of HFD-fed mice was at least partially attributed to the modulation of gut microbiota structure and composition. SCFAs have been identified as intermediaries of dietinduced crosstalk between the gut microbiota and host physiology,due to their critical anti-inflammatory properties and homeostatic efficacy of balancing glycolipid metabolism [55]. Although some of the studies suggested that elevated SCFAs may contribute to the increased calorie intake and thus had opposing effects on the obesity and obesity related phenotypes including liver diseases [33],the SCFAs have positive effects on the improvement of NAFLD among the commonly and consistently reported findings [23,56]. As reported, SCFAs could suppress colon inflammation by activating the receptor GPR43, thereby protecting liver from the insults of microbial cell components coming from the portal vein [57]. Furthermore,SCFAs/GPR43 may also down-regulate insulin signaling in adipose tissue to alleviate the features of NAFLD [58]. In detail, the acetate was found to increase liver fat oxidation by improving mitochondrial modifications and the activity of AMP-activated protein kinase [59]while propionate could stimulate leptin secretion that inhibitedde novolipogenesis [60,61]. For the butyrate, it is an energy source for colonic epithelial cells, facilitating the reduction of liver inflammation by protecting the intestinal barrier [62]. In our study, consumption of MBC significantly enhanced the production of SCFAs, especially acetic acid, propionic acid, butyric acid, and total SCFAs.
To further explore the gut microbiota-mediated effects of MBC on HFD-induced NAFLD, the fecal non-targeted metabolites were detected by using UHPLC-MS/MS. The results of fecal metabolic profiling indicated that the significantly different metabolites among these groups, including carboxylic acids, organic oxygen compounds,lipids and lipid-like molecules, and benzene, were closely associated with the lipid metabolism. Furthermore, based on the KEGG topology analysis, it was found that these significantly different metabolites between HFD and HFD-MBC groups were mainly involved in the sphingolipid metabolism andα-linolenic acid metabolism (Fig. 7). The sphingolipids are both structural lipids and signaling molecules, and their synthesis and degradation play important roles in maintaining homeostasis [63]. Lipidomic analyses revealed that there was a strong correlation between sphingolipid content and triglycerides in the liver [64]. The differential metabolites between HFD and HFDMBC groups, such as sphingosine and sphinganine (Table 1),were the major elements of sphingolipid metabolism pathway [65],which might play important roles in the improvement of NAFLD.Besides,α-linolenic acid metabolism was also found to be significantly enriched after MBC supplementation (Table 1).α-Linolenic acid is an essential fatty acid indispensable to the human body, and it has a significant regulatory effect on liver homeostasis [66]. MBC supplementation significantly promoted the relative abundance ofα-linolenic acid. In the previous study,the untargeted metabolomic analysis showed that nuciferine could alleviate HFD-induced hepatic steatosis via regulating theα-linolenic acid metabolism [67]. Moreover, the correlations between the improved multi-tissue homeostasis ofα-linolenic acid and gut microbiota in HFD-fed mice have been confirmed [68].These results suggested that MBC could be metabolized by gut microbiota into small molecules, thus activating multiple metabolic pathways to alleviate hepatic steatosis.
5. Conclusions
In summary, MBC presented a potential protective effect on HFD-induced NAFLD, as evidenced by the reduced hepatic steatosis,the decreased serum lipid levels, and the improved insulin resistance.Further microbiome and metabolomics studies demonstrated that MBC favorably modulated the structural and composition changes of gut microbiota with a promotion in the relative abundance ofAkkermansia, Lachnospiraceae_NK4A136_group, and norank_f_Muribaculaceae and a reduction in the relative abundance ofRuminiclostridium_9, andRuminiclostridium, unclassified_f_Ruminococcaceae, thus mediating the alterations in SCFAs and a wide range of metabolites. Furthermore, MBC effectively altered the relative abundance of some specific metabolites beneficial for the preventive of NAFLD, which were involved in the multiple metabolic pathways to regulate the lipid metabolism in liver. The specific alterations within the microbes-metabolic axis may be responsible for the protective effects of MBC on NAFLD. As such, MBC, as a by-products of mung bean processing, could be a potent candidate in functional foods used for the management of hepatic steatosis.However, future explorations of the serum metabolomes and the validation of metabolic signaling pathways in the further study will be conducted based on the present findings.
conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was financially supported by National Key Research and Development Program of China (2021YFD1600604), China Agriculture Research System of MOF and MARA-Food Legumes(CARS-08-G19), Research Foundation for Youth Scholars of Beijing Technology and Business University (QNJJ2022-18).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.04.023.
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
