D-tryptophan triggered epithelial-mesenchymal transition by activating TGF-β signaling pathway
2022-06-23ChongWangFangtingWangYanboWangLinglinFu
Chong Wang, Fangting Wang, Yanbo Wang, Linglin Fu*
Food Safety Key Laboratory of Zhejiang Province, School of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou 310018, China
Keywords:
D-tryptophan
Epithelial-mesenchymal transitions
Smad4
Transforming growth factor-β
A B S T R A C T
D-tryptophan is a special kind of nonprotein amino acid showing multiple physiological functions, but the detailed mechanisms are not fully revealed, impairing its further development and applications. This work was to investigate D-tryptophan physiological function and demonstrate the underlying mechanisms.D-tryptophan suppressed HaCaT cell proliferation but increased cell migration. Specifically, D-tryptophan decreased E-cadherin and increased Snail, Twist, and Slug expression, resulting in the development of an epithelial-mesenchymal transitions (EMT) phenomenon. Moreover, D-tryptophan promoted the expression of transforming growth factor-β (TGF-β) 1, and Smad4 knockout damages D-tryptophan’s ability. These results indicated that D-tryptophan stimulated HaCaT cells to produce TGF-β1 and thus activated the TGF-β/Samd pathway, resulting in the triggering of EMT. This study revealed the molecular mechanisms of D-tryptophan activity, provided D-tryptophan as a potential approach for cancer treatment, wound healing, organ development and other relevant applications.
1. Introduction
Epithelial-mesenchymal transition (EMT) is a biological event of epithelial cells, in which the cells lose their epithelial traits and shift toward the mesenchymal phenotype [1]. EMT plays a crucial role in embryonic development and wound healing, but the dysregulation of EMT will facilitate cancer invasion and metastasis [2]. EMT employed several important transcription factors including the members of the Snail, Twist, and Slug families. When activated by various signals, these transcription factors suppress the expression of epithelial phenotypic genes such asE-cadherin, claudins, occludin,and cytokeratins, and induce the expression of mesenchymal genes such asN-cadherin, vimentin, and fibronectin, and thus result in the EMT phenomenon [3-5].
Members of the transforming growth factor-β (TGF-β) family include multifunctional proteins that regulate various biological processes. Many studies have demonstrated that TGF-β1 could induce EMT [6]. In addition, some research also demonstrated that TGF-β1 could upregulate vimentin and fibronectin and downregulateE-cadherin, contributing to cell migration and invasion capabilities [7-9].TGF-β signaling acts as a tumor promoter in advanced epithelial tumors and drives metastasis by favoring EMT [10], proliferation,dissemination, angiogenesis, and tumor escape from immune surveillance. TGF-β ligands bind to the type II TGF-β receptor, in turn, activating the type I receptor. The type I receptor phosphorylates downstream effectors Smad2 and Smad3, which then associate with Smad4 [11]. Meanwhile, previous reports have revealed an indispensable role of Smad4 in TGF-β1-induced expression of a subset of target genes [12]. Thus, Smad4 is necessary for some TGF-β-induced biological responses, e.g. EMT. Impaired regulation of the TGF-β signaling pathway is important in the pathogenesis of cancer.
D-tryptophan is a non-protein amino acid widely used in the food industry and agriculture. Although does not participate in protein synthesis,D-tryptophan shows special physiological functions. In bacteria,D-amino acid synthesis is a strategy for adapting to changing environmental conditions [13]. It has been reported thatD-tryptophan suppressed bacteria growth especially in high NaCl concentration and high temperature [14]. Recently, the inhibitory effects ofD-amino acids, especiallyD-tryptophan on biofilm development have been reported in foodborne microorganisms such asPseudomonas aeruginosa,Bacillus subtilis, andStaphylococcus aureus[15-17].Further analysis indicated that inhibitory effects ofD-tryptophan on bio film development might be attributed to changes of initial adhesion between cells and the properties of the extracellular matrix [18].For example, exposure to 10 mmol/LD-tryptophan causes bio film disassembly inCronobacter sakazakiiby reducing the initial adhesion between cells and changing the properties of the extracellular matrix [18]. Therefore,D-tryptophan may be used as a novel preservative for controlling bacterial growth in foods, particularly in seafood, because of its high effectiveness in environments with high sodium chloride concentration [19].
Moreover,D-tryptophan can elicit chemotactic responses of neutrophils through G-coupled protein receptor GPR109B [20], and ameliorate allergic airway inflammation and hyperresponsiveness [21,22].However, the potential physiological role ofD-tryptophan was not fully revealed, and the regulation ofD-tryptophan on EMT has not been reported.
In the present study, we investigated the effects ofD-tryptophan on EMT and cell migration and the molecular mechanisms involved using HaCaT keratinocytes. Furthermore, the involvement TGF-β/Smad signaling pathways was revealed. These results deepened the understanding of the molecular mechanisms of EMT, provided potential targets and methods for cancer therapy, and promoted the development ofD-tryptophan as an indicator for cancer detection.
2. Materials and methods
2.1 Materials
Fetal bovine serum (FBS) was purchased from Gibco® (Thermo Fisher, USA). Cell Counting Kit-8 (CCK-8) and primers were purchased from Sangon Biotech (Shanghai, China).D-tryptophan was purchased from Aladdin® (Aladdin Biochemical Technology Co., Shanghai, China). Anti-Smad4 antibody was purchased from Abcam (Cambridge, USA).
2.2 Cell culture
WT HaCaT cell was originally from ATCC. Smad4 KO HaCaT cell was previously generated by the CRISPR system in our lab. The cells were maintained in DMEM (KeyGEN BioTECH, Nanjing, China)containing 10% fetal bovine serum and 1% Penicillin-Streptomycin Solution (Sangon Biotech, Shanghai, China), and cultured at 37 °C with 5% CO2.
2.3 CCK-8 cell proliferation assay
Cell proliferation was measured by CCK-8 assay. HaCaT cells were seeded in 96-well plates at a density of 1.0 × 104cell/well and incubated for 24 h. ForD-tryptophan treatment,D-tryptophan was dissolved in DMEM complete medium to a final concentration of 1.25 mmol/L. The culture medium was replaced with the 1.25 mmol/LD-tryptophan and incubate for 24 h. Then CCK-8 solution was added to each well and incubated at 37 °C for 1 h. The absorbance wavelength was then measured at 450 nm using a spectrophotometer.
2.4 Scratch wound healing assay
The effect ofD-tryptophan on HaCaT migration was assessed using a scratch wound healing assay. Cells were treated with 10% FBS complete medium for 24 h in 6-well plates, in which the cells reached 95% to 100% confluence. A linear scratch was generated in the cell monolayer using a sterile 10 μL plastic pipette tip, and the wells were washed with PBS to remove cell debris. ForD-tryptophan treatment,D-tryptophan was dissolved in DMEM medium without FBS to a final concentration of 1.25 mmol/L. The solution was used to replace the culture medium, and the cells were further incubated for indicated times. Images were obtained from the same fields immediately 0, 12, 24, and 48 h after scratching, using a Nikon ECLIPSE Ti-S microscope (Tokyo, Japan). The cell migration rate was determined by measuring the reduction of the cell-free area using Image J software.
2.5 RNA extraction, reverse transcription, and real-time PCR
Total RNA of cell samples was extracted using the E.Z.N.A.®Total RNA Kit II (Omega Bio-Tek, USA). The reverse transcription of RNA to cDNA was performed using the HiScript® II qRT Super kit (Vazyme Biotech, USA). The real-time quantitative PCR (qPCR)analysis was performed using the SYBR Green I kit (Vazyme Biotech,USA) with the LightCycler®480 II system (Roche, USA). The expression ofGAPDHserved as the internal standard for calibration.The qPCR primers are listed in Table 1.

Table 1Primers used for qPCR analysis.
2.6 Statistical analysis
The quantitive experiments were repeated at least three times.Data were expressed as mean ± SEM and analyzed by ANOVA and Dunn’s test by using GraphPad Prism software, and the difference was considered statistically significant when *P< 0.05, **P< 0.01,***P< 0.001, and ****P< 0.000 1.
3. Results
3.1 D-tryptophan inhibits HaCaT cell proliferation and promotes its migration
To invest the potential biological function ofD-tryptophan, we first examined the effect on the proliferation of human keratinocytes HaCaT cells. Cultured HaCaT cells were stimulated with 1.25 mmol/LD-tryptophan for up to 24 h, thereafter the proliferation of HaCaT was evaluated by CCK-8 assay (Fig. 1). Compared with the control group,D-tryptophan slightly suppressed HaCaT proliferation, and the difference was statistically significant.

Fig. 1 The effect of D-tryptophan on the proliferation of HaCaT keratinocytes.The results were represented as mean ± SD from three replicates. The statistical differences were calculated by student’s t-test. *P < 0.05.
Furthermore, the effect ofD-tryptophan on cell migration was also assessed using a scratch wound healing assay. After introduced a wound by scratching the HaCaT cell monolayer, the cells were cultured in the fresh sera-free medium for additional 0, 12, 24, and 48 h, and cell migration was evaluated by measuring the reduction of the cell-free area. As shown in Fig. 2, while migration was barely observed in the control group, it was quite significant in the presence of 1.25 mmol/LD-tryptophan and the wound was almost healed after 48 h.

Fig. 2 D-tryptophan stimulates the migration of HaCaT keratinocytes. (A) Scratch wound healing assay with the WT HaCaT cell monolayer.(B) Cell migration was determined by measuring the reduction of the cell-free area width. The results were represented as mean ± SD from three replicates. The statistical differences were calculated by student’s t-test. ***P < 0.001; ****P < 0.000 1.
3.2 D-tryptophan regulated the expression of EMT-related genes
EMT occur when cells migrate and proliferate, which may be a key factor in the occurrence and development of certain malignancies as well as the wound healing following injury [23]. Based on the cell migration results thanD-tryptophan stimulated the migration of keratinocytes, suggesting thatD-tryptophan promoted EMT.To further verify the hypothesis and understanding the underlying mechanisms, the expression of EMT-involved genes underD-tryptophan stimulation was quantified by qPCR. As shown in Fig. 3A, the expression ofE-cadherin, a marker protein gene of epithelial cells of HaCaT cells afterD-tryptophan stimulation for 24 h.The results showed that pretreating ofD-tryptophan for 24 h resulted in a significant decrease in the expression ofE-cadherin, an epithelial cell marker. Moreover,Snail,Twist, andSlug, the key transcription factors regulating EMT, were all significantly upregulated byD-tryptophan treatment (Figs. 3B-D).

Fig. 3 D-tryptophan regulated EMT-related gene expression. The mRNA expression of E-cadherin (A), Snail (B), Twist (C), and Slug (D) were measured by qPCR. The expression of the housekeeping gene GAPDH was served as the internal standard for calibration. The results were represented as mean ± SD from three replicates. The statistical differences were calculated by student’s t-test. *P < 0.05, **P < 0.01.
3.3 D-tryptophan activated TGF-β signaling pathway
It has been reported that the activation of TGF-β pathway can both suppress cell proliferation and promote EMT [24,25], which was consistent with the biological function ofD-tryptophan on HaCaT cells.
Interestingly, qPCR results showed that the expression ofTGF-β1was significantly upregulated byD-tryptophan stimulation (Fig. 4B,“WT” group), suggesting thatD-tryptophan activated TGF-β signaling by promoting TGF-β1 production, which may further mediateD-tryptophan functions.

Fig. 4 D-tryptophan upregulated TGF-β expression. (A) Validation of the Smad4 KO HaCaT cell line by Western blot with an anti-Smad4 antibody.(B) WT and Smad4 KO HaCaT cells were treated with 1.25 mmol/L D-tryptophan for 24 h, then the mRNA expression of TGF-β1 were measured by qPCR. An equal volume of medium was used as the negative control, and the expression of housekeeping gene GAPDH was served as the internal standard for calibration. The results were represented as mean ± SD from three replicates. The statistical differences were calculated by student’s t-test. *P < 0.05, ****P < 0.000 1.
Smad4 is an indispensable member in TGF-β signaling involving the expression of a subset of target genes [26,27]. To further investigated the relationship betweenD-tryptophan and TGF-β signaling, a Smad4 KO HaCaT cell line was established by the CRISPR-Cas9 system (Fig. 4A), in which the TGF-β signaling was blocked. Based on this, the cells were treated byD-tryptophan, and the expression ofTGF-β1was measured by qPCR (Fig. 4B). Noticeably,D-tryptophan significantly promoted the expression ofTGF-β1in both WT and Smad4 KO cells, indicating thatD-tryptophan-induced TGF-β1 production was upstream of Smad4-dependent TGF-β signaling.
3.4 D-tryptophan regulates cell migration and proliferation by activating TGF-β/Smad4 signaling
SinceD-tryptophan and TGF-β show similar biological functions on suppressing cell proliferation and promoting EMT, andD-tryptophan can activate TGF-β signaling, it was suggested that the biological activities ofD-tryptophan were mediated by the TGF-β pathway. To further validate the hypothesis, WT and Smad4 KO HaCaT cells were stimulated byD-tryptophan for 24 h, thereafter the cell proliferation was evaluated by CCK-8 assay. As shown in Fig. 5, whileD-tryptophan has a significant inhibitory effect on the proliferation of WT cells, the effect was blocked in Smad4 KO cells.
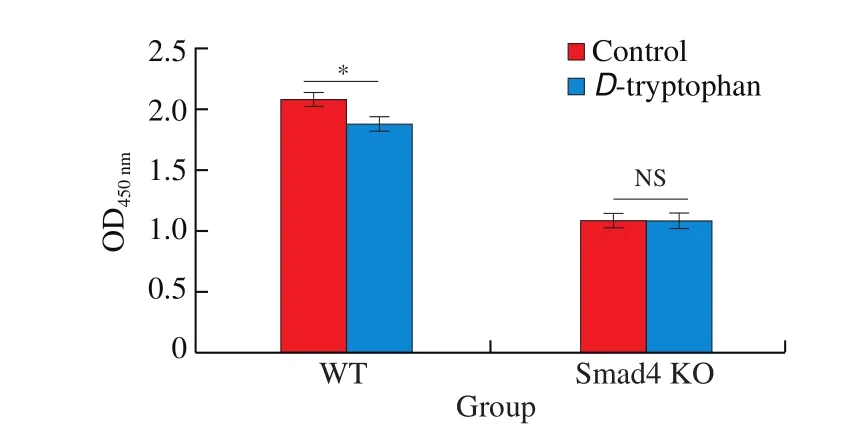
Fig. 5 Smad4 was required for D-tryptophan suppressed cell proliferation.WT and Smad4 KO cells were incubated with the 1.25 mmol/L D-tryptophan in 1% FBS medium. At the indicated time points, cell proliferation was determined by CCK-8 assay. The results were represented as mean ± SD from three replicates. The statistical difference was calculated by ANOVA. *P < 0.05,NS: not significant.
Furthermore, the effect ofD-tryptophan on Smad4 KO cells migration was also assessed using a scratch wound healing assay. Compared with WT cells that significantly migrated afterD-tryptophan stimulation, Smad4 KO cells did not migrate to fill the gap in the scratched area over time (Fig. 6A). Quantified results further revealed thatD-tryptophan dramatically promoted WT HaCaT cell migration but did not show significant effects on Smad4 KO cells (Fig. 6B). These results demonstrated that the biological functions ofD-tryptophan on suppressing cell proliferation and promoting cell migration were mediated by the activation of TGF-β/Smad4 signaling.

Fig. 6 Smad4 was required for D-tryptophan-promoted cell migration. (A) Scratch wound healing assay with the Smad4 KO HaCaT cell monolayer.(B) Cell migration. The results were represented as mean ± SD from three replicates. The statistical differences were calculated by student’s t-test. ***P < 0.001,****P < 0.000 1.
3.5 D-tryptophan regulated EMT-related genes expression by stimulating TGF-β/Smad4 signaling
To investigate the underlying mechanisms of the biological functions ofD-tryptophan, especially the involvement of EMT, the expression of EMT-related genes (includingE-cadherin,Snail,Twist,andSlug) were measured in both WT and Smad4 KO HaCaT cells treated withD-tryptophan. As shown in Fig. 7, althoughE-cadherinwas downregulated byD-tryptophan in WT HaCaT cells, the effect was blocked in Smad4 KO cells (Fig. 7A). Similarly, whileD-tryptophan enhanced the expressions ofSnail,Twist, andSlugin WT HaCaT cells, no significant difference was observed in Smad4 KO cells (Figs. 7B-D). These results indicating that Smad4 was indispensable for the effect ofD-tryptophan on EMT-related genes.

Fig. 7 Smad4 was required for D-tryptophan-induced EMT. The mRNA expression of E-cadherin (A), Snail (B), Twist (C), and Slug (D) were measured by qPCR. An equal volume of medium was used as the negative control, and the expression of housekeeping gene GAPDH was served as the internal standard for calibration. The results were represented as mean ± SD from three replicates. The statistical differences were calculated by student’s t-test. *P < 0.05, **P < 0.01,NS: not significant.
4. Discussion
D-tryptophan is a special nonprotein amino acid having various physiological functions [28]. In this study, we evaluated the role ofD-tryptophan in stimulating EMT cells and investigated its underlying mechanisms. We found that the TGF-β1 pathway, a crucial signaling in regulating cell progression and EMT-related cancers, was activated byD-tryptophan.
We first discovered thatD-tryptophan significantly inhibited HaCaT cell proliferation and promoted its migration. These two effects antagonize each other in oncogenesis, while the former may suppress tumorigenesis, the latter may facilitate cancer spreading around the body. Previous studies also linkedD-tryptophan to cancer. It has been shown that the metabolites of the kynurenine pathway involved inD-tryptophan are related to the occurrence of some cancers [29].This is because indoleamine 2,3-dioxygenase (IDO) catalyzes the first rate-limiting step in the breakdown of tryptophan. Tryptophan depletion from tissues, as it results from IDO activation, alters inflammation as well as T cell-mediated immune responses implicated in a variety of pathological settings including cancer [30,31].Therefore,1-methyl-D-tryptophan (1-MT) is extensively utilized in preclinical trials to deplete IDO activity and kynurenine pathway [32].Whether the kynurenine pathway mediated the activity ofD-tryptophan on cancer requires far more investigations.
EMT is a phenomenon present in both physiological and pathological processes and contributes to organ fibrosis, promotes carcinoma progression and wound healing [5]. During EMT,the epithelial cells transferred to mesenchymal cells with higher migration ability, which is similar to theD-tryptophan-treated HaCaT cells in this study. To test whetherD-tryptophan induced EMT, we first applied qPCR to evaluate the expression of EMT-related genes.E-cadherin is an important component of extracellular connections and a specific mesenchymal marker, playing as a key protein in EMT, and facilitating cell migration and invasion [33]. In this study,withD-tryptophan stimulation, the gene expression ofE-cadherindecreased. Consistently, the EMT transcription factorsSnail,Twist,andSlugwere all upregulated. Therefore, theD-tryptophan-treated HaCaT cells lost epithelial features and obtained mesenchymal characteristics, demonstrating thatD-tryptophan triggered EMT in HaCaT cells.
It has been reported that TGF-β signaling can suppress cell proliferation, promote cell migration, and trigger EMT [34], which is quite similar to the function ofD-tryptophan in this study.Interestingly, we found thatD-tryptophan directly upregulated the expression of theTGF-β1gene, suggesting the direct activation of TGF-β signaling byD-tryptophan. Smad4 is regarded as one of the most important molecules in the TGF-β signaling pathway, and the deficiency of Smad4 will impair TGF-β signaling [26]. Based on this, we established a Smad4 KO HaCaT cell line to block the TGF-β pathway and investigate the influence onD-tryptophan activity. As expected, in this cell line, the effects ofD-tryptophan on both TGF-β downstream genes and EMT events were all impaired.Therefore, these loss-of-function results all demonstrated that Smad4,as well as TGF-β pathway, were required forD-tryptophan function on regulating EMT. All these results indicated thatD-tryptophan stimulates HaCaT cells to produce TGF-β1, thereby activating the TGF-β pathway and causing EMT. However, howD-tryptophan regulates the production of TGF-β1 cytokines in HaCaT cells remained to be an interesting problem and required further study.
The results of this study shed light on the further applications ofD-tryptophan. On the one hand, as the dysregulated proliferation is the primary feature of tumor cells, the suppression of cell proliferation led to a potential anti-tumor activity ofD-tryptophan. On the other hand, the EMT participates in various important physiological events such as embryonic development and wound healing, thereforeD-tryptophan could be a promising candidate for facilitating these events. Moreover, since the TGF-β signaling pathway plays a crucial role in human bodies, the regulating of the TGF-β pathway byD-tryptophan would have potential applications in relevant aspects.However, it should be noted that EMT facilitates the deterioration of cancer, therefore the activity ofD-tryptophan on oncogenesis should be completely investigated before practical applications, and the application ofD-tryptophan in the food industry should consider the potential risks. Noticeably, this work focused on the cellular activity and underlying mechanisms ofD-tryptophan, to make the practical application of these findings possible, further physiological research such as mouse model study and cohort study is required. On the other hand, this work detected the gene expression of TGF-β and EMT-related genes, although the physiological function of these genes-encoded proteins was observed (including the activation of the TGF-β pathway and the EMT phenotype), protein-expression evidence would be required for further validation and mechanical investigation.
5. Conclusion
In summary, this study revealed the detailed molecular mechanism of the regulatory activity ofD-tryptophan on stimulating EMT (Fig. 8).D-tryptophan promoted the production of TGF-β1 cytokines, thereby activated the TGF-β signaling pathway, regulated the downstream target gene expression, and finally resulted in the physiological events especially EMT. This study revealed the molecular mechanisms ofD-tryptophan activity, providedD-tryptophan as a potential regulator for TGF-β signaling and EMT, which would be wildly applied in cancer treatment, wound healing, organ development, and other relevant aspects.

Fig. 8 The mechanism of D-tryptophan triggered EMT. D-tryptophan promotes the expression of TGF-β1, which further activates the TGF-β signaling pathway in a Smad4 dependent way. Activated TGF-β pathway downregulates E-cadherin and upregulates Snail, Twist, and Slug transcription,and finally triggers EMT.
conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was financially supported by the National Key R&D Program of China (2018YFD0401200), the National Natural Science Foundation of China (31801452) and the Zhejiang Provincial Science and Technology Foundation of China (2018C02025 and 2019C02069).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
