Hypoglycemic effects of naturally processed Polygonum multi florum extract in KK CgAy/J mice and its mechanism of action
2022-06-23WenpingTangJianfengZhanShimingLiYueLiuChiTangHo
Wenping Tang, Jianfeng Zhan, Shiming Li*, Yue Liu, Chi-Tang Ho*
a Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resources Comprehensive Utilization, Huanggang Normal University, Huanggang 438000, China
b Department of Food Science, Rutgers University, New Brunswick 08901, USA
c Department of Chemical Biology, Rutgers University, Piscataway 08901, USA
Keywords:
Diabetes
Stilbene glycoside
Polygonum multi florum
Hypoglycemic activity
Homeostatic model assessment
A B S T R A C T
In 2021, there are approximately 537 million adults ageing 20-79 years affected by diabetes worldwide and the number is rising rapidly, hence it is important to manage and control diabetes mellitus and its associated complications. Food is one of the key factors in preventing and combating diseases such as diabetes. Both as a food and an herbal medicine, Polygonum multiflorum (PM) has been used as an anti-aging tonic, for hair darkening in traditional Chinese medicine for several centuries. The recent research effort of PM has been focused on antioxidant, anti-ageing and anti-tumor properties. In the present study, we utilized the traditional processing of harvested raw PM, and identified several stilbene components and then evaluated the potential anti-diabetic effects of the processed PM extract (PME). PME (0.075%) was given to diabetic mice (KK CgAy/J)in drinking water and after 7 weeks, PME-treated mice had significantly lower glucose levels than mice in the diabetic control group (P < 0.01). The mechanism was explored with ELISA and Western blotting and results suggested that the effect was through maintaining β-cell function.
1. Introduction
Diabetes mellitus is one of the prevalent diseases leading to high morbidity and mortality resulting from its complications in the world. There are approximately 537 million people (20-79 years of age) affected by diabetes in 2021 and will reach 783 million by 2045, according to the International Diabetes Federation [1]. Type 2 diabetes (T2D) is characterized with insulin resistance, accounting for more than 90% of diabetes patients. Over-nutrition, physical inactivity and ageing are the main factors contributing to T2D aside from genetic inheritance [2,3].
Grown in Southeast Asia over the past centuries,Polygonum multi florum(PM) is used in traditional Chinese medicine and also as a tonic food for hair darkening, aging, and energy enhancing among others [4,5]. PM extract (PME) and individual phytochemicals in PM possess antioxidant, immune regulation, anti-inflammatory and hypoglycemic effects [6-8]. Phytochemicals currently identified in PME includecis-2,3,5,4-tetrahydroxystilbene-2-O-β-D-glucoside(cis-THSG),trans-2,3,5,4-tetrahydroxystilbene-2-O-β-D-glucoside(trans-THSG), emodin-8-O-β-D-glucoside, physcion-8-O-β-D-glucoside, emodin, chrysophanol, rhaponticoside, torachrysone-8-O-β-D-glucoside, chrysophanol-8-O-β-D-glucoside, physcion and others. The identification and characterization of stilbene glycoside,emodin and physcion in this medicinal plant has been previously carried out by high performance liquid chromatography (HPLC)and HPLC coupled with mass spectroscopy (LC-MS) [9-11].Two major groups of bioactive constituents in PM are stilbene glycosides and anthraquinone derivates. Recent research has provided evidence that stilbene compounds have hypoglycemic,anti-aging and anti-atherosclerotic effects and it also reduced brain pathologic atrophy and promoted learning and memory ability in aging rats [5,12-14].
Whiletrans-THSG is a dominant stilbene compound in PM and its stereoisomer,cis-THSG, exists in a very low percentage in nature, but increases in the process of repetitive steaming and drying.The structure oftrans-THSG (Fig. 1) has a 1,2-diphenylethylene skeleton, reminiscent of common stilbene compounds such as resveratrol and pterostilbene. Comparing to resveratrol,trans-THSG has an extra hydroxyl at 2-position and a 2-O-glycoside group at the phenyl group. It is postulated that the additional hydroxyl group at the 2-position after the loss of the glucose moietyin vivo, i.e.trans-2,3,5,4’-tetrahydroxystilbene, has increased the antioxidant activity dramatically comparing to resveratrol. Indeed,trans-THSG demonstrated a strong protective effect against age-related oxidative stress [15].Trans-THSG also exerted anti-inflammatory activity in a mouse ear edema model [8], lowered blood glucose level in CF-1 diabetic mice [5], and efficiently inhibited the formation of advanced glycation end products, which plays a major pathogenic role in diabetes complications and other diseases [16].

Fig. 1 Chemical structures of cis-THSG and trans-THSG.
In the investigation of anti-diabetic agents and their mechanisms in T2D, there are three categories of animal models: (i) spontaneous or genetically derived diabetic animals such asob/ob,db/db,KK, and KK/Ay among others; (ii) experimentally induced non-spontaneous diabetes models such as chemical, surgical,transgenic/knock-out animals; and (iii) diet/nutrition induced non-spontaneous diabetes animals [17]. Spontaneously diabetic animals of T2D can be obtained from the animals with one or several genetic mutations transmitted from generation to generation. These animals generally inherit diabetes either as single or multi-gene defects. The metabolic disorders result from single gene defect (monogenic) which may be due to a dominant gene (e.g. KK/Ay mouse) or recessive gene(db/db,ob/obmice, Zucker fatty rats) or it can be of polygenic origin(e.g. New Zealand obese mice) [18]. Advantages of genetically derived animal models include characteristics resembling human T2D,less variability, small sample size, homogeneous genetic background and others.
In the present study, we treated KK/Ay diabetic mice with natural PME, prepared from PM purchased from the market without purification or enrichment of certain constituents of PM, to better represent natural PM. This study was aimed at investigating the potential anti-diabetic effect of PM as a functional food.
2. Materials and methods
2.1 Chemistry
2.1.1 Preparation of PME
Root powder of dry PM was purchased from Anguo Mayway Herb Company Ltd. (Anguo City, Hebei Province, China),and followed the referenced extraction procedure with slight modification [19]. Briefly, the powder of PM roots was extracted with 60% aqueous ethanol, at a ratio of solvent to solid of 1:9 (V/m),at room temperature (25 °C) for two days while stirring. The mixture was filtered off, washed with 60% of aqueous ethanol and repeated two more times to extract the root powder. Then the ethanolic extracts were combined and concentratedin vacuo. The resulted residue was lyophilized to yield the PME.
2.1.2 Purification of pure trans-THSG
PME was subjected to a column chromatography packed with macroporous resin (Diaion HP-20, styrene-divynilbenzene resin,Millipore-Sigma, St. Louis, MO, USA). The column was eluted stepwise with each of three different concentrations of aqueous ethanol (30%, 40%, and 50%). The 40% aqueous-ethanol fraction was collected, concentratedin vacuoand lyophilized to yield white powdertrans-THSG, which was characterized with MS and NMR as well as HPLC program utilized were described in detail in a previous publication [7].
2.2 Cell viability by MTT assay
The cytotoxicity oftrans-THSG was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay [19]. Raw cells were seeded onto a 96-well plate (1 × 105cells/well) and cultured in 100 μL of DMEM for 24 h in a CO2incubator. When cells had reached 70%–80% confluence, the spent medium was replaced with culture medium containing THSG solution concentrations from 2.5 μmol/L to 80 μmol/L. After 24 h incubation at 37 °C, MTT assay was applied to detect the cell survival and proliferation using a plate reader (Synergy2; Bio tek, Winooski,VT, USA) at a wavelength of 570 nm.The expression of cell viability was the percentage of absorbance relative to control, the control comprising cells only exposed to 0.05% DMSO.
2.3 Anti-diabetic effects of raw PME animal models
2.3.1 Female KK CgAy/J diabetic mice study with enriched PME
Transgenic female KK CgAy/J diabetic mice were purchased from Jackson Labs (Barr Harbor, ME, USA) and were housed in stainless steel wire-bottomed cages and acclimatized under laboratory conditions (19-23 °C, humidity 60%, 12 h light/dark cycle). The mice were divided into two groups with 10 mice in each group, and fed with Western high fat diet: total fat 21% by weight (> 60% saturated fatty acids) and sucrose 34%, composed of 20% kcal of protein, 20% kcal of carbohydrate, and 60% kcal of fat (from butter). Group 1 is the diabetic control group, which had free access to regular drinking water; group 2 is the PM-treatment group which had free access to drinking water with 0.075% of PME. Body weight, food and water uptake of the mice were recorded on a regular basis. After 7 weeks,all the experimental mice were sacrificed. Body weight, blood glucose level and lipid profile were measured and recorded. The weight of parametrium fat, retro-peritoneal fat and brown fat was recorded. Liver,spleen and kidney were removed and weighed as well.
2.3.2 Biochemical assays
2.3.2.1 Blood glucose level, insulin level, organ weight and lipid profile
Blood samples were collected and centrifuged at 12 500 r/min for 1 h. Blood glucose was measured with a Contour blood glucose meter (Bayer, Tarrytown, New York, USA) and lipid profile was measured with PTS Panels test strips (PTS Diagnostics, Whitestown,IN, USA). Serum insulin level was measured with a commercial kit(Cayman Chemical, Detroit, MI, USA), and performed according to the protocol. Liver and fat tissues were homogenized, lysed with lysis buffer (0.5% sodium lauryl sarkosinate + 10 mmol/L EDTA + 0.5 mg/mL proteinase K + 0.1 mg/mL RNase A in 50 mmol/L Tris-Base (pH 8.0)), centrifuged and protein of homogenates quantified using a BCA protein assay kit (Pierce Chemical, Rockford, IL, USA).
2.3.2.2 ELISA assay
The levels of proinflammatory cytokines (IL-1β, IL-6 and TNF-α)in the liver homogenates of control and experimental groups of KK CgAy/J mice were determined by specific ELISA kits according to the manufacturer’s instructions (Camarillo, CA, USA). The capture antibody, diluted with PBS, was used to coat a 96-well plate overnight at room temperature. The plate was then washed,blocked (1% BSA, 5% sucrose in PBS with 0.05% NaN3), and washed again. The standards were added to the plate leaving at least one zero concentration well and one blank well. The diluted samples (1:5-1:20) were then added to the plate. After incubating for 2 h,the plates were washed and the detection antibody was added.After incubating for another 2 h the plates were washed and Streptavidin-HRP was added. After 20 min incubation, the plates were washed, and substrate (H2O2) and tetramethylbenzidine were added.After 20 min incubation, the stop solution (1 mol/L H2SO4) was added and then, plates were read with a microplate reader at a wavelength of 450 nm. Standard plots were constructed by using standard cytokines and the concentrations for unknown samples were calculated from the standard plot.
2.3.2.3 Western blotting
The samples of liver or fat homogenates (60 μg of protein) in 4 × loading buffer were denatured at 95 °C for 5 min, and subjected to SDS-polyacrylamide gel (4%–10%) electrophoresis. The gel then was transferred onto a polyvinylidene di fluoride membrane (Bio-Rad,Hercules, CA, USA), and the membrane was blocked with TBS-T (20 mmol/L Tris-HCl, 150 mmol/L NaCl, and 0.1% Tween 20,pH 7.4), containing 5%-7% nonfat dried milk. The blocked membrane was incubated at 4 °C overnight with 1:500 dilution of monoclonal antibody for insulin receptor-α (IR-α), insulin receptor substrates-1 (IRS-1, liver) and glucose transporter type 4 (GLUT-4,fat, (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The immunoblotted membrane was incubated at room temperature for 2 h with secondary anti-rabbit or anti-mouse IgG antibodies conjugated with horseradish peroxidase and then exposed on X-ray film with ECL detection reagent (Amersham Pharmacia Biotech, Piscataway,NJ, USA). Bands were quantified using the Adobe Photoshop program with scanning process.
2.4 Statistics
All experiments and analyses were performed at least in triplicate.Results are expressed as means ± SE. Statistical analyses were performed using the Student’st-test. * denotes the difference was statistically significant (P< 0.05).
3. Results
3.1 Analysis of PME
Standard compounds oftrans-THSG andcis-THSG were prepared according to previous procedures [5]. The PME was analyzed with HPLC (Fig. 2) and the peaks were compared with standard compounds oftrans- andcis-THSG, which were prepared in house and characterized with MS and NMR. The total percentage of the two stilbene compounds (THSG) was 35% and thecis/transratio was 1:2.
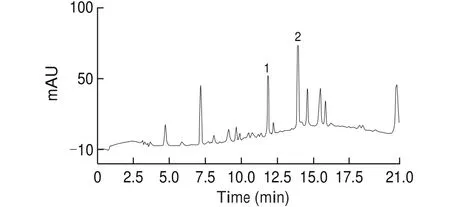
Fig. 2 HPLC chromatograms of PME (Peak 1, 11.9 min, cis-THSG; Peak 2,13.9 min, trans-THSG).
3.2 Hypoglycemic effect of enriched PME in KK CgAy/J diabetic mice and possible mechanism
After 7 weeks of PME administration in drinking water, body weights of the mice was comparable between control group and treatment group. Triglyceride level is higher but not statistically significant in the treatment group, but the other lipid parameters, such as HDL, LDL and total cholesterol almost stayed the same in the two groups. Weights of the liver and kidney also showed no difference in the two groups, but the spleen showed a significant weight loss. At the same time, the blood glucose in the treatment group mice was almost completely reverted to normal by the treatment of PME, illustrated by the glucose level of 233.6 mg/dLvs. 121.6 mg/dL in the control group and treatment group, respectively (Table 1). That indicates that PME has a strong hypoglycemic effect.

Table 1Antidiabetic effects of PME on female KK CgAy/J mice.
3.3 Proinflammatory cytokine levels in the liver
Proinflammatory cytokine (IL-6, IL-1β, and TNF-α) levels in the liver of both groups were measured with commercial ELISA kits after 7 weeks. From Fig. 3, levels of IL-6 and IL-1β had no significant difference between diabetic control group and PM treament group.However, for TNF-α the level slightly increased in PME treated group (P< 0.05). The level of IL-6 was the highest among all 3 cytokines, at around 200 pg/mg.

Fig. 3 Effect of PME on levels of IL-6, IL-1β, and TNF-α in liver samples.After 7 weeks of treatment, mice were sacrificed and liver samples were homogenized with homogenate buffer. Protein of liver homogenate was quantified and the levels of IL-6, IL-1β and TNF-α were measured with ELISA described before. *P < 0.05 versus control.
3.4 Markers for insulin signaling pathway
The levels of selected markers from insulin signaling pathway including IR-α and IRS-1 from the liver and GLUT-4 from the fat, did not show any significant difference from diabetic control and PME treament group (Fig. 4), which is consistent with previous findings. Taken together, these results suggest that the hypoglycemic effect of PME was not mediated through modulation of insulin signaling pathway.
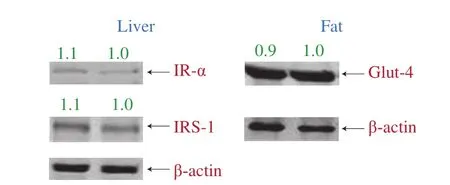
Fig. 4 Effect of PME on selected markers from insulin signaling pathway.Liver and fat tissues from mice in diabetic control and in PME group were homogenized, lysed, centrifuged, quantified with protein content, and applied to Western blotting as described before. For liver sample antibodies IR-α and IRS-1 were used, and for fat sample antibody GLUT-4 was used. Left band shows PME group while right band shows diabetic control.
3.5 Serum insulin levels
Serum insulin level significantly increased in the PM treament group compared to the diabetic control group (7 μU/mLvs. 1 μU/mL) (Fig. 5),indicating that PME had a potent effect in stimulating insulin secretion.
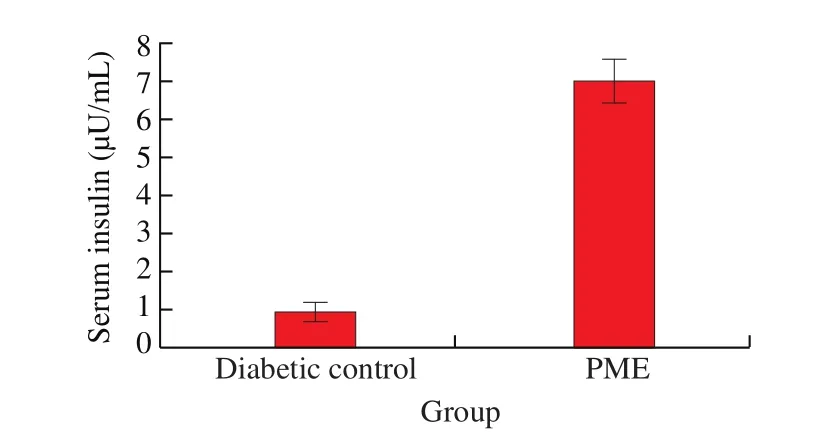
Fig. 5 Effect of PME on serum insulin levels of KK CgAy/J mice. After 7 weeks of treatment, blood samples were collected and centrifuged for 1 h at 12 500 r/min. Serum insulin was measured for with a commercial insulin kit.
3.6 HOMA-β and HOMA-IR
Based on the calculation from homeostatic model assessment (HOMA) model, HOMA-IR = Glucose × Insulin/405 and HOMA-β = (360 × Insulin)/(Glucose-63); with fasting blood glucose and fasting blood insulin level, insulin resistance increased slightly in PME group though not significantly (Fig. 6), but the more dramatic change is in the significant increase in β-cell function, which improved by more than 20 folds from 2 to 45 after PME treatment.
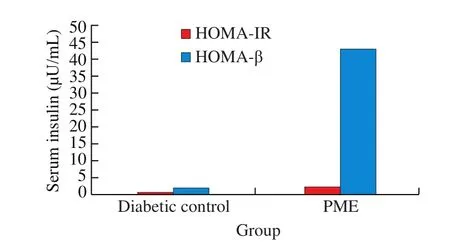
Fig. 6 Approximated insulin resistance (HOMA-IR) and β-cell function(HOMA-β) from HOMA model.
4. Discussion
PM has been used in traditional Chinese medicine for thousands of years. It is considered a tonic food to increase vitality and energy,strengthen the blood, kidneys and liver. There is evidence that PM can lower serum cholesterol, decrease hardening of the arteries,and improve immune function [20-22]. In recent years, PM has attracted a lot of interest and has become extensively studied due to its strong antioxidant activity. From previous studies, the 40% ethanol extract of PM roots has high 2,2-diphenyl-1-picrylhydrazyl(DPPH)-radical scavenging effect [23]. Lv et al. [15]showed that the antioxidant capacity of relatively impure PM (40% aqueous-ethanol fraction eluted from macroporous resin column) has strong protective effect against age-related oxidative stress. Since oxidative stress is currently suggested as a mechanism underlying diabetes and diabetic complications [24], in this study we utilized PME for the antidiabetic study.
Chronic inflammation is closely associated with diabetes mellitus [24].The in filtration and activation of macrophages result in the secretion of pro-inflammatory cytokines and chemokines, leading to the release of TNF-α, IL-1β and IL-6 [1,25]. Thus, the plasma levels of TNF-α, IL-1β and IL-6 are elevated among individuals with features of the insulin resistance syndrome and with clinically overt T2D mellitus [25]. Thereby, the inhibition of inflammation could be a potential pathway in treating diabetes. The strong antioxidant activity oftrans-THSG was confirmed previously in a rat model for aging [13,14], and the anti-inflammatory effects oftrans-THSG were revealed in an ear edema study of CD-1 mice, in which topical application of 2 μmol/L oftrans-THSG could effectively suppress persistent inflammation induced by TPA, and also reversed the up-regulation of cytokine levels of IL-6, IL-1β and TNF-α [7]. In RAW264.7 cell,trans-THSG suppressed NO production induced by LPS, manifesting thattrans-THSG has significant anti-inflammatory activities [7].
Sincetrans-THSG is one of the major phytochemicals in the PME, we postulated that the PME containing THSG may possess hypoglycemic effect. In addition,cis- andtrans-THSG exist in an approximate ratio of 1:2.Cis-THSG and is a stronger hypoglycemic agent thantrans-THSG in our previous study [7]. Hence, in this study we further examined the anti-diabetic effect of PME because it represents the natural PM as a food product in the model study of bioactive ingredients against T2D [4,7].
In this KK CgAy/J diabetic animal study, the high glucose level in the transgenic KKCg/Ay mice was almost completely normalized by 7-week treatment of 0.075% PME in drinking water. The strong hypoglycemic effect of PME was demonstrated for the first time in a T2D mouse model. The model used in the current study, KK Cg/Ay mouse is a transgenic animal model of T2D, characterized by hyperglycaemia, hyperinsulinemia and glucose intolerance.
Several cytokines, including IL-6, IL-1β and TNF-α, have been shown to cause insulin resistance and one mechanism by which these agents could cause insulin resistance is by inducing the expression of cellular proteins that inhibit insulin receptor signaling [27]. Since pro-inflammatory cytokines are closely related to insulin resistance,the mechanism of anti-diabetic effect of PME was studied first by evaluating if PME can suppress production of cytokine protein levels in the liver, especially those cytokines most related to diabetes. No significant difference was found in the levels of IL-6 and IL-1β from liver between diabetic control and PME group, while TNF-α increased slightly in PME group. Since TNF-α could interfere with insulin signaling pathway, we next evaluated if the pathway was affected by this increase in TNF-α. Specific markers were selected from insulin signaling pathway, including IR-α and IRS-1 from the liver and GLUT-4 from the fat. Consistent with previous findings,these markers were not significantly different in the two groups,suggesting that both insulin resistance and anti-inflammation was not the pathway by which the hypoglycemic effect of PME was mediated.
After measuring the insulin levels in the serum of the two groups,it was found that there was a significant increase in the level of insulin from the PME-treated group. With fasting glucose and fasting insulin levels, we calculated the insulin resistance and β-cell function for the two groups based on a model called HOMA, which is based on the assumption that the relationship between glucose and insulin in the basal state reflects the balance between hepatic glucose output and insulin secretion [28], and it has been shown to correlate well with experimental method [29]. From the results, insulin resistance increased slightly in PME group, but β-cell function improved by more than 20-fold, which was of great significant. Based on the above findings, a possible mechanism for PME’s hypoglycemic effect was proposed: in the diabetic mice, insulin resistance did not decrease,however, β-cells failure was postulated as either prevented or delayed,which should be further con firmed by pancreas pathology study. By the time that the mice were sacrificed there was still a considerable amount of insulin in the blood to compensate for insulin resistance; as a result, blood glucose levels are lowered.
5. Conclusion
Previous study has illustrated that single stilbene molecules,cis- and ortrans-THSG in PM or THSG-enriched PME exerted hypoglycemic activity by inhibition of oxidative stress and inflammatory markers and reduction of insulin resistance in diet-induced rodent models. In this study, we have revealed that the extract of regularly processed PM containing bothtrans- andcis-THSG as dominant stilbenes has demonstrated strong hypoglycemic activity in a KK Cg/Ay mouse model of genetically derived diabetic animals, manifesting the effectiveness of natural PM as a functional food combating diabetes.
conflict of interest
The authors declare that there is no conflict of interests.
Acknowledgement
This work was supported by Hubei Science and Technology Plan key Project, Hubei Province, China (2019ABA100).
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
