宫颈原位腺癌的临床诊断及其治疗方式
2022-06-22何雪梅
何雪梅
[摘要] 目的 探讨宫颈原位腺癌(AIS)的临床诊断及治疗方式。 方法 选取2018年9月至2021年3月江苏省徐州市妇幼保健院收治的31例宫颈原位腺癌患者,分析其临床表现、诊断方法、治疗方式及病理情况。结果 患者平均年龄(41.48±7.60)岁。25例患者无异常症状;细胞学异常者13例;高危型HPV阳性者28例;阴道镜图像5例评估为AIS。多点活检病理发现AIS 11例,锥切术后病理发现20例。6例因生育要求仅行锥切术,25例进一步行全子宫切除术,1例病理升级为浸润性腺癌。结论 宫颈原位腺癌临床症状极少见,联合细胞学、HPV检测及阴道镜检查可提高诊断的敏感度,确诊依靠病理。完成生育后尽早行全子宫切除术,以降低宫颈腺癌的发生率。
[关键词] 宫颈原位腺癌;宫颈细胞学;HPV;阴道镜;宫颈活检;宫颈锥切
[中图分类号] R737.33 [文献标识码] B [文章编号] 1673-9701(2022)12-0054-03
[Abstract] Objective To investigate the clinical diagnosis and treatment of adenocarcinoma in situ (AIS) of cervix. Methods A total of 31 patients with adenocarcinoma in situ of cervix who were admitted to Xuzhou Maternal and Child Health Care Hospital in Jiangsu Province from September 2018 to March 2021 were selected to analyze their clinical manifestations, diagnostic methods, treatment methods and pathological conditions. Results The mean age of the patients was (41.48±7.60) years, and 25 patients had no abnormal symptoms. Cytological abnormalities were observed in 13 patients. A total of 28 patients were high-risk HPV positive. Colposcopic images in 5 patients were assessed as AIS. The number of patients who were diagnosed as AIS is 11 by multi-point biopsy and 20 by athological examination after conization. Conization was performed only in 6 patients due to fertility requirements; total hysterectomy was performed in 25 patients; invasive adenocarcinoma was developed in 1 patient. Conclusion Clinical symptoms of adenocarcinoma in situ of cervix are rare. The cytology, HPV test combined colposcopy can improve the sensitivity of diagnosis. The diagnosis of AIS depends on pathological examination. Complete hysterectomy should be performed as soon as possible after completion of fertility requirements to reduce the incidence of cervical adenocarcinoma.
[Key words] Adenocarcinoma in situ of cervix; Cervical cytology; HPV; Colposcopy; Cervical biopsy; Conization of cervix
宫颈癌是最常见的女性恶性肿瘤之一,其死亡率高居女性恶性肿瘤第二位,严重威胁女性健康及家庭安定[1]。其中,鳞状细胞癌(squamous cell carcinoma,SCC)占宫颈癌的75%~80%,腺癌(cervicail adenocarcinoma,AC)占20%~25%[2]。宫颈原位腺癌(adenocarcinoma in situ,AIS)是浸润性腺癌的前期病变,指部分或全部宫颈腺体被异型腺上皮替代,具有恶性细胞的特征,有进展为浸润性腺癌的风险[3],其分布具有多灶累及和跳跃性[4],经典的环形电切术(loop electrosurgical excision procedure,LEEP)或冷刀锥切术(cold-knife conization,CKC)治疗难彻底,易复发[5],常常需行全子宫切除术,无法兼顾女性的生育要求。
近年来,随着宫颈细胞学检查、人乳头瘤病毒(human papilloma virus,HPV)检测和阴道镜检查技术的提高和普及,通过早期筛查,宫颈鳞状细胞上皮内病变(cervical intraepithelial neoplasia,CIN)能够达到早发现和早处理。然而,由于宫颈原位腺癌临床表现不典型,宫颈细胞学检查和阴道镜检查不敏感,且病理活检受取材位置影响,临床上宫颈原位腺癌误诊率高,難以做到早发现、早治疗[3]。因此,宫颈鳞癌的发病率呈现逐年下降的趋势,宫颈腺癌及原位腺癌的发病率却不断升高,且发病年龄趋向年轻化[6]。临床研究发现,AIS临床诊断到早期浸润性腺癌有约5年的窗口期,因此对AIS进行早期诊断及治疗,能大大降低浸润性腺癌的发病率,对患者的预后有非常重要的意义[7]。本文通过对宫颈原位腺癌的临床表现、诊断方法、治疗方式、术后病理情况进行分析,以期为临床早期诊断、早期治疗提供依据,现报道如下。
1 资料与方法
1.1 一般资料
选取2018年9月至2021年3月江苏省徐州市妇幼保健院收治的宫颈原位腺癌患者31例。患者年龄28~56岁,平均(41.48±7.60)岁。
1.2 纳入和排除标准
纳入标准:所有患者经阴道镜下宫颈活检病理或锥切术后病理确诊为宫颈原位腺癌。排除标准:①合并妇科其他恶性肿瘤者;②有宫颈手术史者;③肝肾功能异常或其他全身严重疾病不能耐受手术者。
1.3 研究方法
回顾性分析31例患者的临床症状、细胞学、HPV结果、阴道镜检查影像、活检及锥切病理、治疗方法、术后病理情况。31例患者的阴道镜检查均于江苏省徐州市妇幼保健院宫颈疾病科阴道镜室完成,所有患者均行LEEP锥切。有25例进一步行全子宫切除术,其中4例在外院行全子宫切除。
2 结果
2.1 临床表现
31 例患者中,有5例出现异常阴道流血:其中4例表现为同房出血,1例表现为月经紊乱;1例出现阴道排液;其余25例患者均无明显临床症状。
2.2 宫颈细胞学
31 例患者中有13例宫颈细胞学结果显示异常:9例为不能明确意义的非典型鳞状细胞(atipycal squamons cells of undermined singnifiecation,ASCUS),1例为低度鳞状上皮内病变(low-grade squamous intraepithelial lesion,LSIL),2例为高度鳞状上皮内病变(high-grade squamous intraepithelial lesion,HSIL),1例为非典型腺细胞(atypical glandular cell,AGC);其余18例无异常。
2.3 HPV检测结果
31 例患者中,高危型HPV阳性者28例:其中单纯HPV16阳性者10例,合并其他高危HPV阳性者4例;单纯HPV18阳性者5例,合并其他高危HPV阳性者6例;非16/18高危型HPV阳性者3例;HPV阴性者3例。
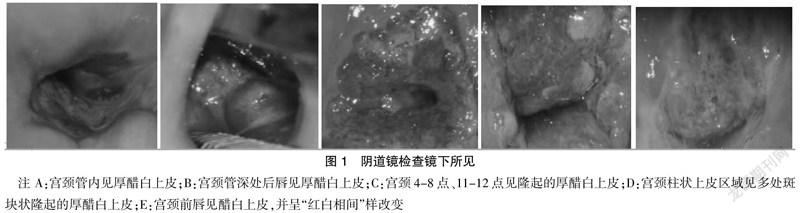
2.4 阴道镜图像
31 例患者均行光电一体阴道镜检查,其中有5例评估为AIS:2例在颈管内见厚醋白上皮,2例在柱状上皮表面见多个隆起的厚醋白上皮,1例在转化区见厚醋白上皮、呈红白相间样改变;其余26例均评估为CIN。见图1。
2.5 病理诊断情况
活检病理发现AIS 11例;因CIN行LEEP锥切,术后病理发现AIS 18例,2例患者因细胞学为HSIL、HPV16阳性,按照ASCCP指南“即时即治”的原则,直接行诊断性锥切病理诊断。其中对8例病理可疑AIS者通过免疫组化检测Ki-67、p16的表达,最终诊断为AIS。31例患者中,6例为单纯性AIS,25例为AIS合并CIN,其中21例合并HSIL,4例合并LSIL。
2.6 治疗后结果
31 例患者均行LEEP锥切术,术后3例患者切缘阳性;6例因有生育要求选择保守治疗,仅行LEEP锥切手术,术后病理示:标本切缘均为阴性;剩余25例进一步行腹腔镜下全子宫切除术,其中4例在外院行全子宫切除,术后1例病理升级为浸润性腺癌。
3 讨论
原位腺癌是宫颈腺癌的癌前病变,30~40岁女性是该病的高危人群,患者平均年龄37岁[8]。原位腺癌患者大多数没有临床症状,少数患者表现为阴道流血及排液[7],本研究中仅5例患者出现异常阴道流血,1例出现阴道排液。
宫颈细胞学检测是宫颈癌早期筛查的主要手段。宫颈腺癌及原位腺癌中仅有30%出现细胞学形态异常,且专业的细胞学阅片医生缺乏,阅片质量参差不齐,因此,临床上原位腺癌的宫颈细胞学诊断的敏感性不高[9]。本研究中仅有13例患者细胞学出现异常,其原因主要有:①AIS病变优先延伸到宫颈管内,并发生在转化区下方的宫颈裂隙的较深部分,而细胞学检查常常无法检测出腺体病变,其诊断准确率仅有50%[10-11];②细胞学样品中异常腺细胞稀少,易误诊为无上皮内病变(NILM),而不是非典型腺细胞(AGC),因此导致AIS的漏诊[12]。
AIS同鳞癌一样,主要由高危型HPV持续感染引起,以HPV16型和HPV18型多见,极少与HPV感染无关[13]。文献报道,HPV16和HPV18阳性率分别为49%和52%[14],本研究显示,31例患者中,HPV阳性率为90.32%,其中HPV16和HPV18阳性率分别为45.16%和35.48%,提示HPV16在AIS中的检出率更高,是目前引起HPV相关肿瘤最常见的类型[15],因此,对于持续性HPV16/18阳性者应高度重视,转诊阴道镜。阴道镜作为宫颈癌筛查三阶梯的第2步,其作用越来越重要。既往阴道镜医师大多满足于发现CIN,当AIS和CIN共存时,CIN病变镜下表现明显更易于发现,尤其是当CIN病变范围较广时,或腺性病变可能与鳞状病变相邻,或夹在鳞状病变之间,位于鳞状病变的下方或上方时,腺上皮病变则易被忽视,特别是宫颈管内腺上皮病变。据文献报道,阴道镜检出AIS的敏感度仅为9.8%[16]。随着检查技术的进步及阴道镜医师能力的不断提高,阴道镜下AIS的诊断率越来越高。本研究中有5例阴道镜下评估为AIS,阴道镜下诊断率达16.13%。
AIS最终确诊依靠病理诊断,有研究表明,病理上有时很难将AIS与CIN病变区别开,尤其是小灶AIS经常被误诊为HSIL[17],部分患者需要进一步行免疫组化或原位杂交确诊。本文资料显示有8例患者在病理的基础上进一步行免疫组化确诊;31例患者中有6例为单纯性AIS,25例为AIS合并CIN,与文献报道的大约60%的患者伴有CIN病变结果相一致[18]。
2019 年ASCCP指南指出AIS的首选治疗方法为全子宫切除术,但术前须得到阴性切缘的标本,对于有生育要求的育龄女性,锥切治疗是可接受的,但前提是手术标本的切缘亦為阴性,且按要求定期随访[19]。目前对AIS的锥切方式,LEEP相较于CKC具有手术创伤小、花费低、且能减轻患者经济负担的优势,应用越来越广[20]。本研究中,31例患者均行LEEP锥切,术后仅3例患者切缘阳性;有25例进一步行全子宫切除术,术后1例病理升级为浸润性腺癌。提示AIS病变呈跳跃性特点,即使锥切切缘阴性,也可能存在病变残留及升级的风险。文献报告,约54%的患者在锥切术后子宫切除术标本中残留了病变[7]。
综上所述,鉴于宫颈原位腺癌临床症状不特异,AIS大多起源于宫颈管内膜,并向颈管深处延伸,且病变具有多发性和不连续性,故单纯依靠细胞学、HPV检测或阴道镜检查中的一项,难以高效诊断AIS,三种检测手段联合筛查可大大提高AIS诊断的敏感性及准确性。对于AIS应首选全子宫切除,有生育要求的女性完成生育后,尽早行全子宫切除术,以降低宫颈腺癌的发生率。
[参考文献]
[1] 刘萍.中国大陆13年宫颈癌临床流行病学大数据评价[J].中国实用妇科与产科杂志,2018,34(1):41-45.
[2] 谢幸,孔北华,段涛.妇产科学(第九版)[M].北京:人民卫生出版社,2018:298-299.
[3] Teoh D,Musa F,Salani R,et al. Diagnosis and management of adenocarcinoma in situ:A society of gynecologic oncology evidence-based review and recommendations[J].Obstetrics and Gynecology,2020,135(4):869-878.
[4] 杨德诗,闵敏.宫颈原位腺癌合并高级别鳞状上皮内病变2例临床病理特征[J].实用妇科内分泌电子杂志,2020, 7(9):108-109.
[5] Upadhyay Baskota S,Wang T,Zhao C. Follow-up findings in postconservative treatment surveillance for women with cervical adenocarcinoma in situ[J].Journal of Lower Genital Tract Disease,2021,25(1):38-42.
[6] Ferlay J,Colombet M,Soerjomataram I,et al. Estimating the global cancer incidence and mortality in 2018:GLOBOCAN sources and methods[J].International Journal of Cancer,2019,144(8):1941-1953.
[7] Srisomboon S,Tantipalakorn C,Charoenkwan K,et al. Cervical screening results leading to detection of adenocarcinoma in situ of the uterine cervix[J].Asian Pacific Journal of Cancer Prevention,2019,20(2):377-382.
[8] Wang X,Bi Y,Wu H,et al. Oncologic and obstetric outcomes after conization for adenocarcinoma in situ or stage IA1 cervical cancer[J].Scientific Reports,2020,10(1):19 920.
[9] 寧莉,朱亚飞,彭琴.宫颈腺癌病例2例及文献复习[J].赣南医学院学报,2021,41(7):694-697.
[10] Zhong P,Yin C,Jin Y,et al. More focus on atypical glandular cells in cervical screening: Risk of significant abnormalities and low histological follow-up rate[J].Cyto Journal,2020,17:22.
[11] Vahedpoor Z,Behrashi M,Khamehchian T,et al. Comparison of the diagnostic value of the visual inspection with acetic acid (VIA) and pap smear in cervical cancer screening[J].Taiwanese Journal of Obstetrics & Gynecology,2019,58(3):345-348.
[12] Conrad RD,Liu AH,Wentzensen N,et al. Cytologic patterns of cervical adenocarcinomas with emphasis on factors associated with underdiagnosis[J].Cancer Cytopa-thology,2018,126(11):950-958.
[13] 潘蕾,李娟,刘媛,等.宫颈细胞学和高危型人乳头瘤病毒联合检测在宫颈腺癌中的辅助诊断价值[J].上海交通大学学报(医学版),2018,38(11):1366-1369.
[14] Bel Haj Rhouma R,Ardhaoui M,El Fehri E,et al. Distribution of human papillomavirus in precancerous and cancerous cervical neoplasia in Tunisian women[J].Infectious Agents and Cancer,2021,16(1):52.
[15] Schiffman M,Doorbar J,Wentzensen N,et al. Carcinogenic human papillomavirus infection[J].Nature Reviews Disease Primers,2016,2:16 086.
[16] Ullal A,Roberts M,Bulmer JN,et al. The role of cervical cytology and colposcopy in detecting cervical glandular neoplasia[J].Cytopathology:Official Journal of the British Society for Clinical Cytology,2009,20(6):359-366.
[17] Umezawa T,Umemori M,Horiguchi A,et al. Cytological variations and typical diagnostic features of endocervical adenocarcinoma in situ:A retrospective study of 74 cases[J].Cyto Journal,2015,12:8.
[18] Song T,Lee YY,Choi CH,et al. The effect of coexisting squamous cell lesions on prognosis in patients with cervical adenocarcinoma in situ[J].European Journal of Obstetrics,Gynecology,and Reproductive Biology,2015, 190:26-30.
[19] Perkins RB,Guido RS,Castle PE,et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors[J].Journal of Lower Genital Tract Disease,2020,24(2):102-131.
[20] 黃伟娟,高雁荣,郭舟群.冷刀锥切术与宫颈环形电切术治疗宫颈鳞状上皮内瘤变的疗效比较[J].实用癌症杂志,2020,35(11):1853-1856.
(收稿日期:2021-08-23)
