lmpact of stopping smoking on metabolic parameters in diabetes mellitus:A scoping review
2022-06-21MagdalenaWalickaCristinaRussoMichaelBaxterIsaacJohnGraziaCaciRiccardoPolosa
lNTRODUCTlON
Approximately 1.3 billion people worldwide use tobacco, most commonly in the form of tobacco smoking, and more than 7 million people die every year as a result of smoking related conditions[1,2]. Smoking is the main cause of lung cancer, chronic obstructive pulmonary disease, and cardiovascular disease[3,4].
Exposure to cigarette smoke is associated with vascular damage, endothelial dysfunction, and activation of oxidative stress, inflammatory pathways, coagulation, and fibrinolysis[5,6]. A similar mechanism of endothelial dysfunction is described for people with diabetes. It is therefore not surprising that smoking enhances the combined harmful effects of elevated blood glucose levels, accelerating vascular damage in diabetic patients who smoke[7,8].
One day her mother, having made some cakes, said to her, Go, my dear, and see how your grandmother is doing3, for I hear she has been very ill. Take her a cake, and this little pot of butter. 4
Smokers with diabetes [both type 1 diabetes (T1D) and type 2 diabetes (T2D)] may be at a higher risk due to the direct effect of vascular damage as well as the indirect adverse effect that smoking has on glycemic control and lipid levels[9].
Although the Van der Veldes tried to locate Carl Meier, he was never found. But the family did find a way to honor his courage and kindness. When my mother s little brother was born the following year, the grateful family named him Karel, the Dutch version of Carl .
The risk of cardiovascular events in diabetic patients is reduced with smoking cessation[10]. In the Action in Diabetes and Vascular Disease:Preterax and Diamicron MR Controlled Evaluation study, smoking cessation in those with diabetes was associated with a 30% reduction in all-cause mortality[11]. A comprehensive evaluation of predicted coronary heart disease (CHD) among current and exsmokers who had T2D in Spain found that ex-smokers had approximately 20% lower CHD risk at 10 years compared to current smokers[12].
Although there is evidence that patients with diabetes can reduce the risk of macrovascular complications by giving up smoking, there is no conclusive evidence for the impact on the risk of microvascular complications[9,13,14]. The impact of quitting smoking on microvascular complications of diabetes and its metabolic indices is unclear. Furthermore, stopping smoking is known to cause weight gain which in turn may have unpredictable metabolic effect in patients with diabetes.
To the best of our knowledge, there have been no published systematic reviews to quantify the health benefits of smoking cessation in the diabetes population to date. The purpose of this scoping review is to create a single narrative describing the impact of smoking cessation in people with diabetes on glycemic control, insulin resistance and insulin secretion, and lipid abnormalities as well as biochemical parameters of nephropathy.
He gave a loud knock at the door, and an old woman s voice answered from within, but as she did not seem to be hurrying herself to open it he redoubled his blows, and demanded to be let in imperiously, quite forgetting that he was no longer in his own kingdom
SEARCH METHODS
The published literature on the impact of stopping smoking on metabolic indices, including glycemic control, insulin resistance, and lipid abnormalities was systematically reviewed in September and October 2021. The studies on biochemical parameters of nephropathy were also included. The literature search was conducted using the following databases:PubMed, Embase, ScienceDirect library, Database of
s of Reviews of Effects, Scopus, and Google Scholar, using medical subject headings. We also used an artificial intelligence technology-based open multidisciplinary citation analysis database named Reference Citation Analysis. Search queries were developed by a trained librarian experienced in developing search strategies for reviews and were based on diabetes, smoking cessation, fasting plasma glucose (FPG) levels, hemoglobin A1c (HbA1c), insulin resistance, insulin secretion, lipids [total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoproteins (VLDL)], microalbuminuria, creatinine. More specifically, search terms included (“smoking cessation” OR “former smokers” OR “ex-smokers” OR “stop smoking” OR “quitting”) AND (“diabetes”) AND (“glucose” OR “glycemi*” OR “HbA1c” OR “insulin resistance” OR “HOMA” OR “insulin secretion” OR “total cholesterol” OR “HDL” OR “LDL” OR “VLDL” OR “microalbuminuria” OR “albuminuria” OR “creatinine” OR “GFR”). Search results were filtered to include only human studies and published from 1980. The titles, abstracts, and full texts of the search results were sequentially and independently screened by MW and GC for inclusion. A few studies were identified, including cross-sectional, case-control, and cohort studies, randomized clinical trials, and observational clinical studies, as well as systematic reviews and meta-analyses. The references of relevant studies were also manually reviewed for additional eligible citations.
SMOKlNG CESSATlON AND lNClDENCE OF DlABETES
According to a meta-analysis conducted by Pan
[15], recent quitters are at higher risk for developing diabetes, although this risk progressively declines with time[15].
It is often found that giving up smoking leads to a significant increase in weight[16,17]. According to a large prospective United Kingdom study, smoking abstenance was associated with an average weight gain of 8.79 kg at eight years, while continuing smokers gained only 2.24 kg[17]. This has been confirmed in a meta-analysis showing that quitting smoking is associated with a bodyweight gain of 4-5 kg after 12 mo of abstinence, with most of the weight gain occurring between the third and the sixth month after quitting[18]. As nicotine (in tobacco cigarettes) suppresses appetite and increases resting metabolic rate[19], people who stop smoking gain weight because they have diminished resting energy expenditure and increased appetite. Moreover, quitters often substitute smoking with excessive eating/snacking, as shown in several studies of eating behaviors[20].
Oh, how gladly she would have shaken off all this grandeur39, and laid aside the heavy wreath! The red flowers in her own garden would have suited her much better, but she could not help herself: so she said, “Farewell,” and rose as lightly as a bubble to the surface of the water
It is likely that the weight gain associated with stopping smoking is responsible for the initial increase in risk of developing T2D. The increase in the risk of T2D after quitting was directly proportional to weight gain, but not increased among quitters without weight gain[21].
He gave, however, as good as he got, and they became so enraged33 that they tore up trees and beat each other with them, till they both fell dead at once on the ground
SMOKlNG CESSATlON AND GLYCAEMlC CONTROL
Patients with diabetes can become more insulin resistant with worsening glycemic control when they gain weight. Pani
[22], examining the predictors of diabetes progression (defined as HbA1c³ 7% or the initiation of hypoglycemic therapy), found that weight gain was an independent predictor. Each extra pound of weight that is gained increases the risk of developing diabetes by 2%.
In patients with diabetes, quitting smoking may cause increased appetite, caloric intake, and weight gain, which would predictably lead to the worsening of glycemic control. In contrast, stopping smoking appears to have a beneficial effect on carbohydrate metabolism in the long run which may potentially mitigate the initial adverse metabolic effects of smoking cessation[23].
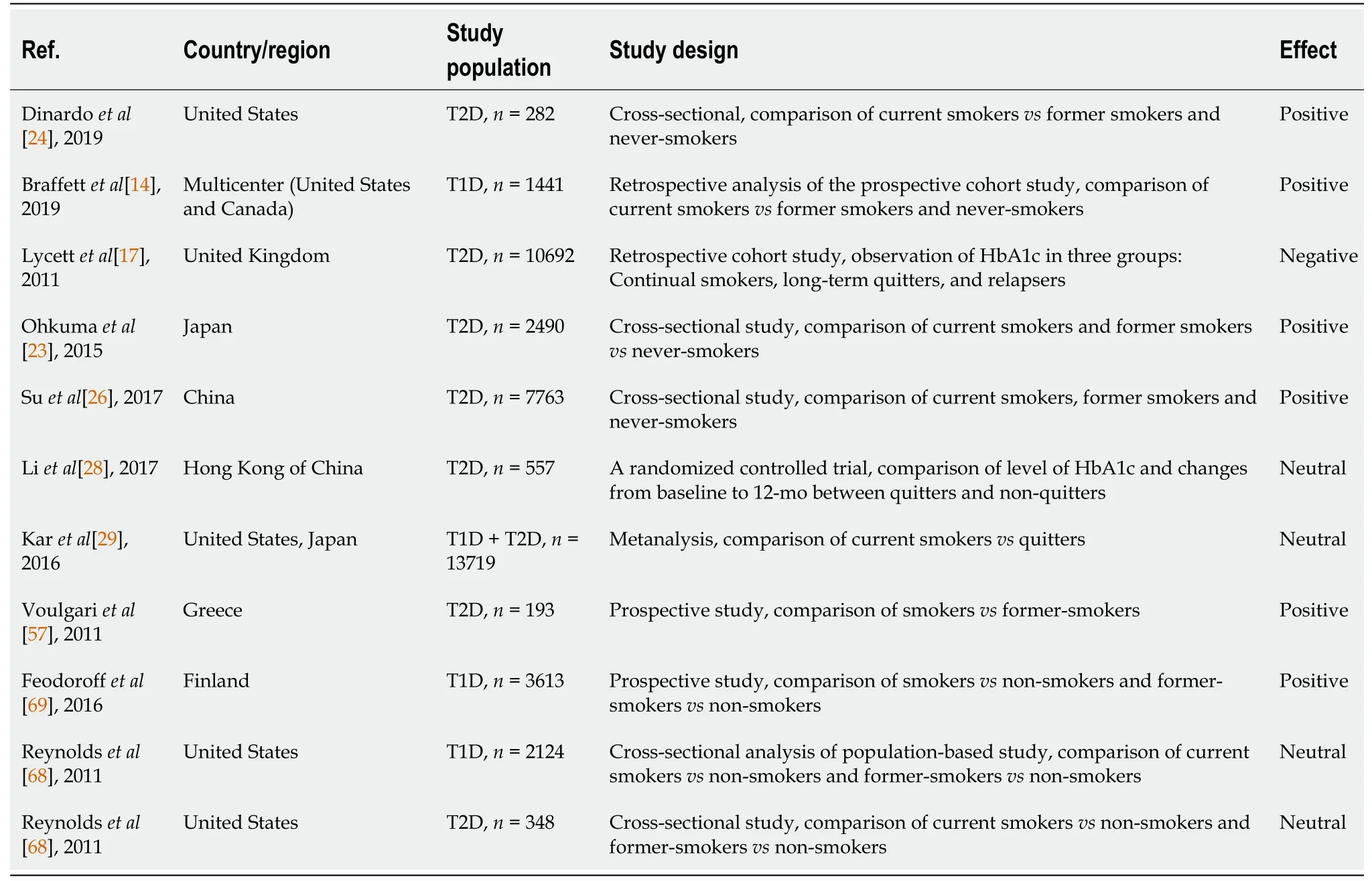
Considering the complex interplay among factors that affect glycemic control, some uncertainty in the findings of studies that investigate the impact of stopping smoking on glycemic control might be anticipated. Studies comparing smokers to ex-smokers, both with T1D and T2D, demonstrated that active smoking is associated with worse glycemic control. In the study of Dinardo
[24] current smokers (with an average smoking history of 30 years, an average daily habit of one pack of cigarettes
day) had higher mean HbA1c in comparison with former smokers. In the multiple linear regression analysis, current smoking was independently and significantly associated with higher HbA1c. Braffett
[14], using the data of a well-characterized cohort group with T1D from the Diabetes Control and Complications Trial (1983-1993), showed that in comparison to former smokers (subjects who previously smoked but quit > 3 mo prior to baseline), current smokers (subjects who currently smoked or quit < 3 mo prior to baseline) had higher mean HbA1c levels (average difference of 0.31%) over an average of 6.5 years of follow-up. The mean HbA1c levels for former smokers were similar to those of whom have never smoked. In relation to not only the current smoking status but also to its lifetime intensity and duration, the mean HbA1c levels were higher (average difference 0.22%) for current smokers with more than 10 pack-years in comparison to former smokers with less than 10 pack-years.
In an observational study of 10692 adult smokers with T2D, 29% of patients who had quit smoking and remained abstinent for at least 1 year revealed an increase in HbA1c of 0.21% with the need to intensify glucose-lowering treatment[25]. In further observation, HbA1c level decreased as abstinence continued, and became comparable to this in people who continued to smoke after a 3-year follow-up. Patients who stopped smoking gained weight (4.68 kg on average), but the results suggested that the change in weight was not directly related to the increase in HbA1c.
In Asiatic patients quitting smoking is generally associated with an improvement in glycemic control. In a study of 2490 male Japanese patients with T2D, HbA1c decreased linearly with the years after stopping smoking; however, there was no correlation with FPG[23]. Similarly, in a study of 7763 Chinese men with T2D, the HbA1c level decreased progressively with each year that the patients had stopped smoking; in this study, FPG levels decreased[26]. In a smaller retrospective cohort study comprising 241 Taiwanese patients with T2D, the group completing the smoking cessation program showed a significant decrease in FPG and HbA1c levels at 3-mo follow-up compared to baseline. Due to the fact that the analyses of cardiometabolic factors were carried out before and after participation in the smoking cessation program in the whole group (regardless of the outcome of the smoking cessation program), it is difficult to interpret these results[27].
In contrast, there are a number of studies on Asian patients failing to show improvement in glycemic control after stopping smoking. In a randomized controlled trial conducted in China, results of quitting smoking did not affect HbA1c levels at 1-year follow-up. The study included 557 smokers with T2D[28].
In the meta-analysis published by Kar
[29] there was no statistically significant difference in HbA1c between smokers and quitters. However, when the meta-analysis was reanalyzed including studies comparing nonsmokers and active smokers, a statistically significant difference was demonstrated and this was positively associated with smoking duration; increasing as the years of smoking increased.
In addition to reducing overall and cardiovascular mortality, stopping smoking may provide significant additional health benefits to people with diabetes. It is important to note, however, that weight gain experienced after stopping smoking may attenuate some of these health benefits[79].
A summary of the studies evaluating smoking cessation's effect on HbA1c is shown in Table 1. The table also includes HbA1c data from studies of smokers with diabetic nephropathy.
SMOKlNG CESSATlON AND lNSULlN RESlSTANCE AND lNSULlN SECRETlON
The pathogenic mechanisms underlying T2D are a balance between insulin resistance and beta-cell dysfunction. Smoking has been shown to influence both insulin resistance and insulin secretion. Studies on animals have shown that cigarette smoke can impair insulin production and secretion in addition to reducing beta-cell viability and proliferation[30].
Now the Princess happened to walk that way; and when she heard the tune13, shestood quite still, and seemed pleased; for she could play Lieber Augustine ;it was the only piece she knew; and she played it with one finger.
However, the direct effect of nicotine on insulin resistance is not supported in studies looking at the use of snus. Snus is an oral tobacco product that delivers significant levels of nicotine without producing any toxic combustion byproducts[34]. Since the 1980s, snus consumption has been growing in popularity in Sweden, gradually replacing cigarette smoking[35,36].
With the exception of one study, which has methodological issues including a flawed cross-sectional design and the lack of adjustment for smoking history in snus users[37], there is clear evidence that snus use does not produce a significant rise in diabetes risk[38-41]. Moreover, there was no association between snus use and insulin levels or glucose tolerance in a large study involving 1266 subjects and primarily focused on cardiovascular risk factors[42]. Insignificant relative risks for T2D were reported in a meta-analysis for never-smoking current, former and ever-snus users[43]. In addition, impaired glucose tolerance and related endpoints were not associated in any significant way.
It has been demonstrated that smokers have greater waist-to-hip circumference ratios[44,45]. Waistto-hip circumference ratio is one of the most pragmatic clinical measures of central obesity. One of the major contributing factors in obesity-related metabolic complications is fat distribution. The visceral abdominal depot (abdominal obesity) is linked to metabolic dysfunction (cardiovascular disease, insulin resistance, T2D). Conversely, lower body adiposity (gluteofemoral obesity) is associated with improved cardiovascular and metabolic profiles[46]. The abdomen adipose tissue is characterized by the rapid uptake of diet-derived fat and a high lipid turnover that is easily stimulated by stress hormones[46]. Increased release of free fatty acids and abnormalities in adipokine secretion observed in people with abdominal obesity promote insulin resistance[47].
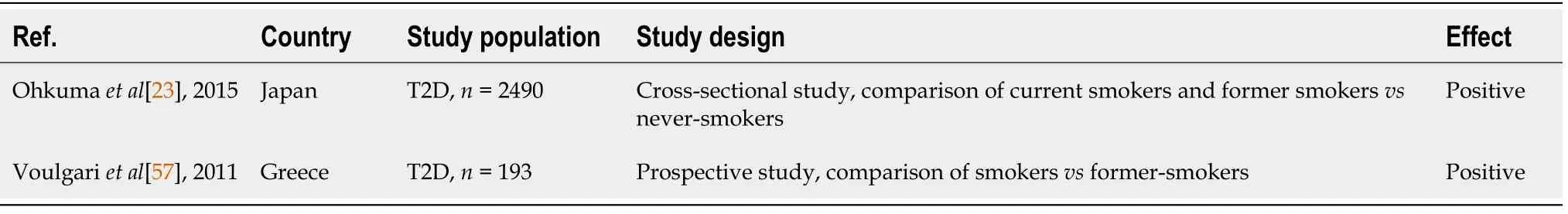
There is no clear impact of stopping smoking on characteristics associated with diabetic nephropathy. The effect of smoking cessation on microalbuminuria was investigated by Voulgari
[57] in subjects with newly diagnosed T2D mellitus. Within a year of stopping smoking, the prevalence of those with microalbuminuria markedly declined compared to those who continued smoking. However, eGFR was comparable between the two study groups. Smokers who stopped smoking had a lower microalbuminuria rate irrespective of the effect of drug therapy (antihypertensive, hypolipidemic, and antidiabetic).
He had been travelling for many days, and had left his fatherland behind him, when close to the road he came upon a huge tree, and on its topmost bough11 an eagle was sitting shaking the branches with all his might
However, studies assessing insulin resistance indicators in quitters do not have consistent results. There was a statistically significant negative correlation between homeostasis model assessmentestimated insulin resistance (HOMA-IR) values among asymptomatic, Korean male ex-smokers without diabetes[52]. In contrast, other studies (also in the groups without diabetes) showed that quitting smoking was associated with greater insulin resistance as measured by Quicki or HOMA-IR[53,54].
I am the raven-mother; I am the raven-mother, each ravencroaked, and Anne Lisbeth felt that the name also applied to her;and she fancied she should be transformed into a black bird, andhave to cry as they cried, if she did not dig the grave
Smoking cessation may be associated with worsening fat distribution. In a population-based study (Inter99 Study) performed in Copenhagen, the mean increase in waist circumference after quitting at the one-year follow-up was 3.88 cm (42% of the quitters had increased their waist circumference by ≥ 5 cm). Quitters with high baseline tobacco consumption were more likely to have substantially increased waist circumference. In this study, abstinence from smoking was the most important predictor of substantial weight gain and a substantial increase in waist circumference[49]. Likewise, a study with the use of computed tomography showed that both current and former smoking is associated with increased visceral adipose tissue[50]. In a population-based study performed in Norway, former smokers compared with current smokers had a lower waist to hip ratio (additionally among women, waist circumference was lower)[51].
The observed discordance amongst the insulin sensitivity findings is likely to be due to a change in body weight. After stopping smoking, insulin sensitivity is likely to change because of weight fluctuations. It was shown that the HOMA-IR index after quitting significantly increases in weight gainers, but not in weight maintainers[55]. In the study by Heggen
[56], no differences were found in HOMA-IR between quitters and smokers but the findings must be interpreted within the context of similar modest body weight changes in quitters and smokers at 3-mo follow-up.
These studies have most commonly included people without diabetes. The only study investigating the relationship between insulin resistance and smoking cessation among patients with diabetes is that of Ohkuma
[23]. The authors found that smoking cessation has a time-dependent link with insulin resistance in Japanese patients with T2D; HOMA-IR levels decreased in ex-smokers over time relative to current smokers. HOMA-IR was also assessed in the prospective study, evaluating the effect of smoking on the progression of microalbuminuria in T2D. Smoking cessation was associated with the amelioration of insulin resistance parameters in spite of the small but significant increase in body mass index. This observation may be explained by the fact that many quitters increased their physical activity[57].
In an era of evidence-based medicine, the lack of data regarding the metabolic effects of smoking and smoking cessation in diabetes is very disappointing and needs to be addressed. Diabetes is one of the major population health issues, the consequence of which appears to be amplified by smoking. The lack of good quality research on the impact of smoking cessation on metabolic parameters in this population hampers clinicians' ability to give informed advice on the effectiveness and management of stopping smoking. This is a complex medical and sociological issue that demands a greater research focus to better inform people with diabetes and assist healthcare providers to implement the most effective interventions.
Now it happened that the cat and dog met each other on their travels, and though they had not been the best of friends at home, they were quite glad to meet among strangers
Now listen towhat I tell you! You can snore; you are snoring the whole night, and Ihardly a quarter of an hour! And the blood rose to the head of theexcited criminal; he threw himself upon his comrade, and beat him with his clenced fist in the face
The search for publications on quitting and insulin secretion in patients with diabetes was unproductive. In the population without diabetes, Morimoto
[58] found that the risk of impaired insulin secretion in an ex-smoker is similar to that in never-smokers, where the risk is almost twice as high in current smokers when compared with never smokers, with the magnitude of this increase being dose-dependent (
increasing with a number of pack-years). Stadler
[54] showed a 31% increase in beta-cell secretion (as measured by insulinogenic index 140) after > 3 mo of not smoking.
SMOKlNG CESSATlON AND LlPlDS ABNORMALlTlES
Patients with T2D characteristically have abnormal plasma lipids profiles which are marked by hypertriglyceridemia, reduced HDL cholesterol levels, and increased concentration of small dense LDL. These abnormalities are a result of a multifactorial process, including abdominal obesity, insulin resistance, increased free fatty acid flux, and inflammation[59]. Cigarette smoke has been shown to increase the atherogenic nature of the lipid profile[60]. Smoking is associated with increased triglycerides (TG), total cholesterol, and LDL, as well as reduced levels of cardioprotective HDL[61]. In a prospective study of 808 young Asian adults, smokers were three times more likely to have low HDL cholesterol and were 2.6 times more likely to develop hypertriglyceridemia[62]. There is a clear assumption in healthcare messaging that stopping smoking may correct dyslipidemia, which is especially relevant in smokers with diabetes. Studies, conducted on patients without diabetes, indicate that quitting smoking increases HDL levels[63,64]. The increase in HDL has frequently been observed in spite of weight gain experienced after cessation of smoking[63]. Evidence also indicates that smokers may have improved HDL function (increased cholesterol efflux capacity and decreased HDL inflammatory index) after quitting smoking[65].
Data on TG levels are conflicting. Some studies performed in the group without diabetes showed that smoking cessation is associated with a reduction of this lipid fraction[66], however, others studies have failed to confirm this[64].
Data on LDL is also limited, but evidence seems to suggest that smoking cessation does not affect LDL levels or LDL size[63,67].
There has also been speculation that nicotine in tobacco smoke could play a significant role in promoting insulin resistance. Although chronic exposure to nicotine may be necessary to impact insulin sensitivity in nicotine naive subjects, acute exposure to nicotine can cause negative effects on insulin sensitivity in individuals with pre-existing insulin resistance[31-33].
A few studies have tested diabetic patients' lipid profiles after quitting smoking. Results are inconsistent. In Reynolds
[68], 3466 youth who had T1D (
= 2887) or T2D (
= 579) and were smokers were examined for prevalence of tobacco use and the coexistence of cardiovascular risk factors. Compared to patients who were non-smokers, past smokers with T1D had significantly higher odds of having high LDL cholesterol levels, and those who were current smokers had significantly higher chances of having high TG levels. Patients with T2D did not exhibit these relationships, but the smaller numbers of patients included in the study could have influenced the statistical significance of the results.
In the study of Luque-Ramírez
[12] patients with T2D who smoke had lower HDL and higher TG levels compared to their nonsmoking counterparts.
Lipid parameters were examined in two studies in patients with diabetic nephropathy. After stopping smoking for at least 1 year, patients had significantly lower total cholesterol, LDL, and HDL levels than those who continued to smoke[57]. In a similar study of patients with T1D, total cholesterol, TG, and LDL levels of current and former smokers were higher than those of non-smokers, whereas lower HDL levels were observed in current smokers[69].
Horns! so that was what he promised me! Let someone find the plum-seller at once and bring him to me! Let his nose and ears be cut off! Let him be flayed53 alive, or burnt at a slow fire and his ashes scattered54 to the winds! Oh, I shall die of shame and despair! Her women ran at the sound of her screams, and tried to wrench13 off the horns, but it was of no use, and they only gave her a violent headache
A summary of the studies evaluating smoking cessation's effect on lipid parameters is shown in Table 3.

SMOKlNG CESSATlON AND BlOCHEMlCAL PARAMETERS OF NEPHROPATHY
It is well known that chronic kidney disease (CKD) and end-stage renal disease (ESRD) can complicate diabetes mellitus. Diabetic nephropathy is characterized by proteinuria and/or the decline of renal function [
reduced glomerular filtration rate (GFR)][70]. Aside from high blood sugar levels, other risk factors that contribute to the development and progression of diabetic kidney disease include high blood pressure, dyslipidemia, and genetic predisposition[71]. Smoking may also be a factor in the development and progression of kidney failure possibly through a mechanism of progressive arteriolar damage, increased renovascular resistance, and increased intraglomerular capillary pressure[72-75]. While many studies have examined the relationship between cigarette smoking and kidney disease with conflicting results, a meta-analysis of 15 prospective cohort studies with 65064 incident cases of CKD suggests that smoking is as an independent risk factor in the general population[76].
Compared with nonsmokers, smokers are characterized by greater insulin resistance and hyperinsulinemia[48]. However, little research has been conducted on the impact of smoking cessation on insulin resistance and insulin secretion.
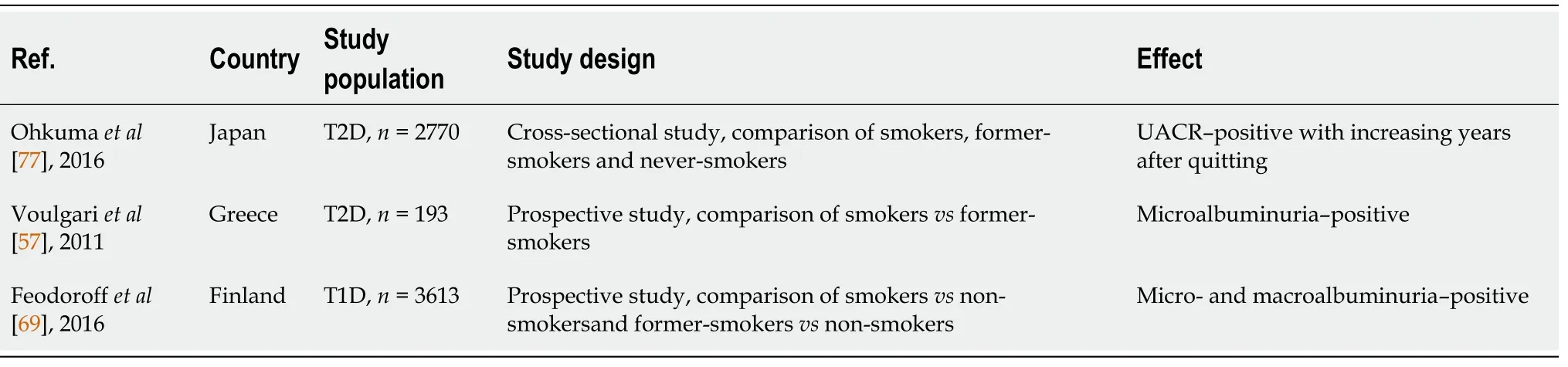
In a study of 2770 patients with T2D, Ohkuma
[77] investigated the association of smoking and its abstinence with parameters of CKD. In comparison to non-smokers, former and current smokers had higher urinary albumin-creatinine ratios. In the former smokers, this ratio decreases linearly with increasing years after quitting smoking. Furthermore, current smokers' risk is related to how many cigarettes
day they smoke. After quitting smoking, age-adjusted creatinine-based GFR declined compared to the never-smokers but increased in parallel with increasing cigarette consumption. The increased eGFR of smokers may be related to glomerular hyperfiltration which is implicated as a mechanism for the progression of diabetic nephropathy[78].
Progressive kidney damage can result from glomerular hyperfiltration over time. According to Ohkuma's study, the proportion of smokers with CKD increased with the number of cigarettes they smoked
day (compared with never-smokers). However, as the years passed since quitting, the proportion of patients with CKD decreased. There was a significant increased HbA1c level for current smokers and a greater proportion of hypertension for ex-smokers compared with never smokers and current smokers with respect to the other risk factors for nephropathy in this study[77].
Using data from the Finnish Diabetic Nephropathy Study, which included 3613 T1D patients, the 12-year cumulative risk of microalbuminuria, macroalbuminuria, and ESRD by smoking status was calculated. Current and former smokers were more likely to have micro- and macro-albuminuria (ESRD for current smokers only) than non-smokers. There were no statistically significant differences in the 12-year cumulative risk of microalbuminuria and macroalbuminuria between former smokers and never smokers. There were significantly poorer glycemic control and lipid parameters for smokers compared to nonsmokers. Adjusting for HbA1c and lipid variables, the increased risk of diabetic nephropathy progression among current and former smokers was attenuated. Smoking-related changes in lipids and glucose control may account for the majority of nephropathic changes due to diabetes[69]. This observation suggests that poor glucose control and lipid alterations in smokers are the main drivers of nephropathic changes in diabetes.
A summary of the studies evaluating smoking cessation's effect on characteristics associated with diabetic nephropathy is shown in Table 4.
CONCLUSlON
But one day the Princess, wandering sadly by the river, spied Prince Featherhead fast asleep in the shade of a tree, and stole nearer to enjoy the delight of gazing at his dear face unobserved
When considering the potential impact of stopping smoking on metabolic parameters in patients with diabetes, the benefits of cessation of smoking are less clear because the expected outcomes have not been consistently demonstrated. Studies have shown both improvements and temporary deterioration in glycemic control after quitting smoking. Only a few available studies have investigated the effect of quitting smoking on insulin resistance and lipid parameters in diabetic patients. These studies also report inconsistent results. Smoking cessation appears to have a clear beneficial effect on markers of nephropathy, particularly after longer periods of smoking abstinence.
The review of the published literature found only a few studies, many of which had design and methodological shortcomings, and as such-need to be interpreted with caution. A major issue for this area of study is the lack of randomized controlled trials that have been carried out to date.
A summary of the studies evaluating smoking cessation's effect on HOMA-IR is shown in Table 2.
The authors would like to thank Ms. Erika Anastasi for her help with the literature search. The authors' thoughts and prayers are with all the victims of the Russian-Ukrainian conflict.
Walicka M contributed to the conceptualization, literature search, and screening, writing, review, editing; Russo C, Baxter M and John I contributed to the writing, reviewing, editing; Caci G performed the literature search and screening, writing, reviewing, editing; Polosa R contributed to the conceptualization, writing, reviewing, editing, revising, supervising; all author read and approved the final version of the manuscript.
Department of Clinical and Experimental Medicine of the University of Catania, No. 6C813202024/1_3_02_07_01/2020.
When he arrived I was sitting on my car hood1()eager with anxiety tinged2() anticipation(,). He approached with a big handsome smile and bright eyes and took my hand and said lets walk. I slid off the hood(,) and proceeded to my parents driveway with him.
RP is full tenure professor of Internal Medicine at the University of Catania (Italy). RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. RP has also received grants from European Commission initiatives (U-BIOPRED and AIRPROM) and from the Integral Rheumatology & Immunology Specialists Network (IRIS) initiative. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., Health Diplomats, and Sermo Inc. RP has served on the Scientific Advisory Board of Cordex Pharma, Inc., CV Therapeutics, Duska Therapeutics Inc, Pfizer, and PharmaCielo. RP is also founder of the Center for Tobacco prevention and treatment (CPCT) at the University of Catania and of the Center of Excellence for the acceleration of HArm Reduction (CoEHAR) at the same University, which has received support from FSFW to conduct 8 independent investigatorinitiated research projects on harm reduction. RP has filed a patent application concerning an app tracker for smoking behaviour developed for ECLAT Srl. RP is involved in the following pro bono activities:scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA) and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). All other authors have no declared relevant conflict of interest to declare in relation to this study.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See:https://creativecommons.org/Licenses/by-nc/4.0/
United Kingdom
Magdalena Walicka 0000-0001-8072-5279; Cristina Russo 0000-0003-2357-7147; Michael Baxter 0000-0002-7653-2244; Isaac John 0000-0003-2856-6180; Grazia Caci 0000-0003-1325-0466; Riccardo Polosa 0000-0002-8450-5721.
Fan JR
A
Fan JR
1 World Health Organization. Newsroom. Tobacco. [cited 26 July 2021]. Available from:https://www.who.int/newsroom/fact-sheets/detail/tobacco
2 GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories,1990-2015:a systematic analysis from the Global Burden of Disease Study 2015.
2017; 389:1885-1906 [PMID:28390697 DOI:10.1016/S0140-6736(17)30819-X]
3 Institute for Health Metrics and Evaluation. Findings from the Global Burden of Disease Study 2017. Seattle, WA:IHME, 2018. [cited 26 July 2021]. Available from:https://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Booklet.pdf
4 IHME. Institute for Health Metrics and Evaluation FHM GBD Compare-Viz Hub. 2020. [cited 26 July 2021]. Available from:https://vizhub.healthdata.org/gbd-compare/
5 Guarino F, Cantarella G, Caruso M, Russo C, Mancuso S, Arcidiacono G, Cacciola RR, Bernardini R, Polosa R.Endothelial activation and injury by cigarette smoke exposure.
2011; 25:259-268 [PMID:21880215]
6 Caponnetto P, Russo C, Di Maria A, Morjaria JB, Barton S, Guarino F, Basile E, Proiti M, Bertino G, Cacciola RR, Polosa R. Circulating endothelial-coagulative activation markers after smoking cessation:a 12-month observational study.
2011; 41:616-626 [PMID:21198559 DOI:10.1111/j.1365-2362.2010.02449.x]
7 Kondo T, Nakano Y, Adachi S, Murohara T. Effects of Tobacco Smoking on Cardiovascular Disease.
2019; 83:1980-1985 [PMID:31462607 DOI:10.1253/circj.CJ-19-0323]
8 Knapp M, Tu X, Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy.
2019; 40:1-8 [PMID:29867137 DOI:10.1038/s41401-018-0042-6]
9 Molla GJ, Ismail-Beigi F, Larijani B, Khaloo P, Moosaie F, Alemi H, Mansournia MA, Ghadimi T, Ghaemi F, Nakhjavani M, Esteghamati A. Smoking and Diabetes Control in Adults With Type 1 and Type 2 Diabetes:A Nationwide Study From the 2018 National Program for Prevention and Control of Diabetes of Iran.
2020; 44:246-252 [PMID:31494031 DOI:10.1016/j.jcjd.2019.07.002]
10 Pan A, Wang Y, Talaei M, Hu FB. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus:A Meta-Analysis and Systematic Review.
2015; 132:1795-1804 [PMID:26311724 DOI:10.1161/CIRCULATIONAHA.115.017926]
11 Blomster JI, Woodward M, Zoungas S, Hillis GS, Harrap S, Neal B, Poulter N, Mancia G, Chalmers J, Huxley R. The harms of smoking and benefits of smoking cessation in women compared with men with type 2 diabetes:an observational analysis of the ADVANCE (Action in Diabetes and Vascular Disease:Preterax and Diamicron modified release Controlled Evaluation) trial.
2016; 6:e009668 [PMID:26747037 DOI:10.1136/bmjopen-2015-009668]
12 Luque-Ramírez M, Sanz de Burgoa V; en nombre de los participantes del estudio DIABETES. Impact of smoking cessation on estimated cardiovascular risk in Spanish type 2 diabetes mellitus patients:The DIABETES study.
2018; 218:391-398 [PMID:29891175 DOI:10.1016/j.rce.2018.04.014]
13 Cai X, Chen Y, Yang W, Gao X, Han X, Ji L. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes:a meta-analysis.
2018; 62:299-306 [PMID:30128962 DOI:10.1007/s12020-018-1697-y]
14 Braffett BH, Rice MM, Young HA, Lachin JM. Mediation of the association of smoking and microvascular complications by glycemic control in type 1 diabetes.
2019; 14:e0210367 [PMID:30615671 DOI:10.1371/journal.pone.0210367]
15 Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes:a systematic review and meta-analysis.
2015; 3:958-967 [PMID:26388413 DOI:10.1016/S2213-8587(15)00316-2]
16 Eisenberg D, Quinn BC. Estimating the effect of smoking cessation on weight gain:an instrumental variable approach.
2006; 41:2255-2266 [PMID:17116119 DOI:10.1111/j.1475-6773.2006.00594.x]
17 Lycett D, Munafò M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers.
2011; 106:188-196 [PMID:20925685 DOI:10.1111/j.1360-0443.2010.03136.x]
18 Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes:meta-analysis.
2012; 345:e4439 [PMID:22782848 DOI:10.1136/bmj.e4439]
19 Zoli M, Picciotto MR. Nicotinic regulation of energy homeostasis.
2012; 14:1270-1290 [PMID:22990212 DOI:10.1093/ntr/nts159]
20 Killi AE, Baspinar MM, Basat O. Association between post-cessation weight gain and eating behavior changes.
2020; 7:153-160 [PMID:32259037 DOI:10.14744/nci.2019.58712]
21 Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, Willett WC, Manson JE, Hu FB, Sun Q. Smoking Cessation, Weight Change, Type 2 Diabetes, and Mortality.
2018; 379:623-632 [PMID:30110591 DOI:10.1056/NEJMoa1803626]
22 Pani LN, Nathan DM, Grant RW. Clinical predictors of disease progression and medication initiation in untreated patients with type 2 diabetes and A1C less than 7%.
2008; 31:386-390 [PMID:18083790 DOI:10.2337/dc07-1934]
23 Ohkuma T, Iwase M, Fujii H, Kaizu S, Ide H, Jodai T, Kikuchi Y, Idewaki Y, Hirakawa Y, Nakamura U, Kitazono T.Dose- and time-dependent association of smoking and its cessation with glycemic control and insulin resistance in male patients with type 2 diabetes mellitus:the Fukuoka Diabetes Registry.
2015; 10:e0122023 [PMID:25822499 DOI:10.1371/journal.pone.0122023]
24 Dinardo MM, Sereika SM, Korytkowski M, Baniak LM, Weinzierl VA, Hoenstine AL, Chasens ER. Current Smoking:An Independent Predictor of Elevated A1C in Persons With Type 2 Diabetes.
2019; 45:146-154 [PMID:30755104 DOI:10.1177/0145721719829068]
25 Lycett D, Nichols L, Ryan R, Farley A, Roalfe A, Mohammed MA, Szatkowski L, Coleman T, Morris R, Farmer A,Aveyard P. The association between smoking cessation and glycaemic control in patients with type 2 diabetes:a THIN database cohort study.
2015; 3:423-430 [PMID:25935880 DOI:10.1016/S2213-8587(15)00082-0]
26 Su J, Qin Y, Shen C, Gao Y, Pan EC, Pan XQ, Tao R, Zhang YQ, Wu M. [Association between smoking/smoking cessation and glycemic control in male patients with type 2 diabetes].
2017; 38:1454-1459 [PMID:29141328 DOI:10.3760/cma.j.issn.0254-6450.2017.11.003]
27 Chen HJ, Huang WH, Chan HL, Hwang LC. Improvement in Cardiometabolic Risk Factors During Smoking Cessation Treatment in Patients with Type 2 Diabetes:A Retrospective Cohort Study.
2021; 14:1695-1702 [PMID:33889004 DOI:10.2147/DMSO.S303446]
28 Li WH, Wang MP, Lam TH, Cheung YT, Cheung DY, Suen YN, Ho KY, Tan KC, Chan SS. Brief intervention to promote smoking cessation and improve glycemic control in smokers with type 2 diabetes:a randomized controlled trial.
2017; 7:45902 [PMID:28378764 DOI:10.1038/srep45902]
29 Kar D, Gillies C, Zaccardi F, Webb D, Seidu S, Tesfaye S, Davies M, Khunti K. Relationship of cardiometabolic parameters in non-smokers, current smokers, and quitters in diabetes:a systematic review and meta-analysis.
2016; 15:158 [PMID:27881170 DOI:10.1186/s12933-016-0475-5]
30 Tong X, Chaudhry Z, Lee CC, Bone RN, Kanojia S, Maddatu J, Sohn P, Weaver SA, Robertson MA, Petrache I, Evans-Molina C, Kono T. Cigarette smoke exposure impairs β-cell function through activation of oxidative stress and ceramide accumulation.
2020; 37:100975 [PMID:32283079 DOI:10.1016/j.molmet.2020.100975]
31 Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance.
1996; 94:878-881 [PMID:8790020 DOI:10.1161/01.cir.94.5.878]
32 Epifano L, Di Vincenzo A, Fanelli C, Porcellati F, Perriello G, De Feo P, Motolese M, Brunetti P, Bolli GB. Effect of cigarette smoking and of a transdermal nicotine delivery system on glucoregulation in type 2 diabetes mellitus.
1992; 43:257-263 [PMID:1425888 DOI:10.1007/BF02333019]
33 Axelsson T, Jansson PA, Smith U, Eliasson B. Nicotine infusion acutely impairs insulin sensitivity in type 2 diabetic patients but not in healthy subjects.
2001; 249:539-544 [PMID:11422660 DOI:10.1046/j.1365-2796.2001.00840.x]
34 Lunell E, Fagerström K, Hughes J, Pendrill R. Pharmacokinetic Comparison of a Novel Non-tobacco-Based Nicotine Pouch (ZYN) With Conventional, Tobacco-Based Swedish Snus and American Moist Snuff.
2020; 22:1757-1763 [PMID:32319528 DOI:10.1093/ntr/ntaa068]
35 The European Commission. Special Eurobarometer 458. Attitudes of Europeans towards tobacco and electronic cigarettes. 2017. [cited 26 July 2021]. Available from:http://ec.europa.eu/commfrontoffice/publicopinion/index.cfm/Survey/getSurveyDetail/instruments/SPECIAL/surveyKy/
36 Ramström L, Borland R, Wikmans T. Patterns of Smoking and Snus Use in Sweden:Implications for Public Health.
2016; 13 [PMID:27834883 DOI:10.3390/ijerph13111110]
37 Persson PG, Carlsson S, Svanström L, Ostenson CG, Efendic S, Grill V. Cigarette smoking, oral moist snuff use and glucose intolerance.
2000; 248:103-110 [PMID:10947888 DOI:10.1046/j.1365-2796.2000.00708.x]
38 Eliasson M, Asplund K, Nasic S, Rodu B. Influence of smoking and snus on the prevalence and incidence of type 2 diabetes amongst men:the northern Sweden MONICA study.
2004; 256:101-110 [PMID:15257722 DOI:10.1111/j.1365-2796.2004.01344.x]
39 Wändell PE, Bolinder G, de Faire U, Hellénius ML. Association between metabolic effects and tobacco use in 60-year-old Swedish men.
2008; 23:431-434 [PMID:18470624 DOI:10.1007/s10654-008-9260-4]
40 Neumann A, Norberg M, Schoffer O, Norström F, Johansson I, Klug SJ, Lindholm L. Risk equations for the development of worsened glucose status and type 2 diabetes mellitus in a Swedish intervention program.
2013; 13:1014 [PMID:24502249 DOI:10.1186/1471-2458-13-1014]
41 Rasouli B, Andersson T, Carlsson PO, Grill V, Groop L, Martinell M, Midthjell K, Storm P, Tuomi T, Carlsson S. Use of Swedish smokeless tobacco (snus) and the risk of Type 2 diabetes and latent autoimmune diabetes of adulthood (LADA).
2017; 34:514-521 [PMID:27353226 DOI:10.1111/dme.13179]
42 Eliasson M, Asplund K, Evrin PE, Lundblad D. Relationship of cigarette smoking and snuff dipping to plasma fibrinogen,fibrinolytic variables and serum insulin. The Northern Sweden MONICA Study.
1995; 113:41-53 [PMID:7755654 DOI:10.1016/0021-9150(94)05425-i]
43 Lee PN, Thornton AJ. The relationship of snus use to diabetes and allied conditions.
2017; 91:86-92 [PMID:29061372 DOI:10.1016/j.yrtph.2017.10.017]
44 Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, Khaw KT. Cigarette smoking and fat distribution in 21,828 British men and women:a population-based study.
2005; 13:1466-1475 [PMID:16129730 DOI:10.1038/oby.2005.177]
45 Troisi RJ, Heinold JW, Vokonas PS, Weiss ST. Cigarette smoking, dietary intake, and physical activity:effects on body fat distribution--the Normative Aging Study.
1991; 53:1104-1111 [PMID:1850574 DOI:10.1093/ajcn/53.5.1104]
46 Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes.
2015; 11:90-100 [PMID:25365922 DOI:10.1038/nrendo.2014.185]
47 Westphal SA. Obesity, abdominal obesity, and insulin resistance.
2008; 9:23-29; discussion 30 [PMID:19046737 DOI:10.1016/s1098-3597(08)60025-3]
48 Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking.
1992;339:1128-1130 [PMID:1349365 DOI:10.1016/0140-6736(92)90730-q]
49 Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population:the Inter99 study.
2007; 44:290-295 [PMID:17222450 DOI:10.1016/j.ypmed.2006.11.015]
50 Lee K, Lee CM, Kwon HT, Oh SW, Choi H, Park JH, Cho B. Associations of smoking and smoking cessation with CTmeasured visceral obesity in 4656 Korean men.
2012; 55:183-187 [PMID:22728048 DOI:10.1016/j.ypmed.2012.06.009]
51 Graff-Iversen S, Hewitt S, Forsén L, Grøtvedt L, Ariansen I. Associations of tobacco smoking with body mass distribution; a population-based study of 65,875 men and women in midlife.
2019; 19:1439 [PMID:31675936 DOI:10.1186/s12889-019-7807-9]
52 Kim KW, Kang SG, Song SW, Kim NR, Rho JS, Lee YA. Association between the Time of Length since Smoking Cessation and Insulin Resistance in Asymptomatic Korean Male Ex-Smokers.
2017; 2017:6074760 [PMID:28706954 DOI:10.1155/2017/6074760]
53 Ding R, Huang T, Han J. Diet/Lifestyle and risk of diabetes and glycemic traits:a Mendelian randomization study.
2018; 17:18 [PMID:29375034 DOI:10.1186/s12944-018-0666-z]
54 Stadler M, Tomann L, Storka A, Wolzt M, Peric S, Bieglmayer C, Pacini G, Dickson SL, Brath H, Bech P, Prager R,Korbonits M. Effects of smoking cessation on β-cell function, insulin sensitivity, body weight, and appetite.
2014; 170:219-217 [PMID:24179100 DOI:10.1530/EJE-13-0590]
55 Inoue K, Takeshima F, Kadota K, Yoda A, Tatsuta Y, Nagaura Y, Yoshioka S, Nakamichi S, Nakao K, Ozono Y. Early effects of smoking cessation and weight gain on plasma adiponectin levels and insulin resistance.
2011; 50:707-712 [PMID:21467702 DOI:10.2169/internalmedicine.50.4600]
56 Heggen E, Svendsen M, Tonstad S. Smoking cessation improves cardiometabolic risk in overweight and obese subjects treated with varenicline and dietary counseling.
2017; 27:335-341 [PMID:28216282 DOI:10.1016/j.numecd.2016.12.011]
57 Voulgari C, Katsilambros N, Tentolouris N. Smoking cessation predicts amelioration of microalbuminuria in newly diagnosed type 2 diabetes mellitus:a 1-year prospective study.
2011; 60:1456-1464 [PMID:21489578 DOI:10.1016/j.metabol.2011.02.014]
58 Morimoto A, Tatsumi Y, Deura K, Mizuno S, Ohno Y, Watanabe S. Impact of cigarette smoking on impaired insulin secretion and insulin resistance in Japanese men:The Saku Study.
2013; 4:274-280 [PMID:24843666 DOI:10.1111/jdi.12019]
59 Bahiru E, Hsiao R, Phillipson D, Watson KE. Mechanisms and Treatment of Dyslipidemia in Diabetes.
2021; 23:26 [PMID:33655372 DOI:10.1007/s11886-021-01455-w]
60 Gossett LK, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Smoking intensity and lipoprotein abnormalities in active smokers.
2009; 3:372-378 [PMID:20161531 DOI:10.1016/j.jacl.2009.10.008]
61 Chelland Campbell S, Moffatt RJ, Stamford BA. Smoking and smoking cessation -- the relationship between cardiovascular disease and lipoprotein metabolism:a review.
2008; 201:225-235 [PMID:18565528 DOI:10.1016/j.atherosclerosis.2008.04.046]
62 Kim SW, Kim HJ, Min K, Lee H, Lee SH, Kim S, Kim JS, Oh B. The relationship between smoking cigarettes and metabolic syndrome:A cross-sectional study with non-single residents of Seoul under 40 years old.
2021; 16:e0256257 [PMID:34411160 DOI:10.1371/journal.pone.0256257]
63 Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins:outcomes from a randomized clinical trial.
2011; 161:145-151 [PMID:21167347 DOI:10.1016/j.ahj.2010.09.023]
64 King CC, Piper ME, Gepner AD, Fiore MC, Baker TB, Stein JH. Longitudinal Impact of Smoking and Smoking Cessation on Inflammatory Markers of Cardiovascular Disease Risk.
2017; 37:374-379 [PMID:27932354 DOI:10.1161/ATVBAHA.116.308728]
65 Takata K, Imaizumi S, Kawachi E, Suematsu Y, Shimizu T, Abe S, Matsuo Y, Tsukahara H, Noda K, Yahiro E, Zhang B,Uehara Y, Miura S, Saku K. Impact of cigarette smoking cessation on high-density lipoprotein functionality.
2014;78:2955-2962 [PMID:25319317 DOI:10.1253/circj.cj-14-0638]
66 Attard R, Dingli P, Doggen CJM, Cassar K, Farrugia R, Wettinger SB. The impact of passive and active smoking on inflammation, lipid profile and the risk of myocardial infarction.
2017; 4:e000620 [PMID:28878948 DOI:10.1136/openhrt-2017-000620]
67 Nakamura M, Yamamoto Y, Imaoka W, Kuroshima T, Toragai R, Ito Y, Kanda E, J Schaefer E, Ai M. Relationships between Smoking Status, Cardiovascular Risk Factors, and Lipoproteins in a Large Japanese Population.
2021; 28:942-953 [PMID:33116031 DOI:10.5551/jat.56838]
68 Reynolds K, Liese AD, Anderson AM, Dabelea D, Standiford D, Daniels SR, Waitzfelder B, Case D, Loots B, Imperatore G, Lawrence JM. Prevalence of tobacco use and association between cardiometabolic risk factors and cigarette smoking in youth with type 1 or type 2 diabetes mellitus.
2011; 158:594-601.e1 [PMID:21129757 DOI:10.1016/j.jpeds.2010.10.011]
69 Feodoroff M, Harjutsalo V, Forsblom C, Thorn L, Wadén J, Tolonen N, Lithovius R, Groop PH. Smoking and progression of diabetic nephropathy in patients with type 1 diabetes.
2016; 53:525-533 [PMID:26668013 DOI:10.1007/s00592-015-0822-0]
70 Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988-2014.
2016; 316:602-610 [PMID:27532915 DOI:10.1001/jama.2016.10924]
71 Tziomalos K, Athyros VG. Diabetic Nephropathy:New Risk Factors and Improvements in Diagnosis.
2015; 12:110-118 [PMID:26676664 DOI:10.1900/RDS.2015.12.110]
72 Gambaro G, Verlato F, Budakovic A, Casara D, Saladini G, Del Prete D, Bertaglia G, Masiero M, Checchetto S, Baggio B. Renal impairment in chronic cigarette smokers.
1998; 9:562-567 [PMID:9555657 DOI:10.1681/ASN.V94562]
73 Ritz E, Benck U, Franek E, Keller C, Seyfarth M, Clorius J. Effects of smoking on renal hemodynamics in healthy volunteers and in patients with glomerular disease.
1998; 9:1798-1804 [PMID:9773780 DOI:10.1681/ASN.V9101798]
74 Orth SR. Effects of smoking on systemic and intrarenal hemodynamics:influence on renal function.
2004; 15 Suppl 1:S58-S63 [PMID:14684675 DOI:10.1097/01.asn.0000093461.36097.d5]
75 Elihimas Júnior UF, Elihimas HC, Lemos VM, Leão Mde A, Sá MP, França EE, Lemos A, Valente LM, Markman Filho B. Smoking as risk factor for chronic kidney disease:systematic review.
2014; 36:519-528 [PMID:25517282 DOI:10.5935/0101-2800.20140074]
76 Xia J, Wang L, Ma Z, Zhong L, Wang Y, Gao Y, He L, Su X. Cigarette smoking and chronic kidney disease in the general population:a systematic review and meta-analysis of prospective cohort studies.
2017; 32:475-487 [PMID:28339863 DOI:10.1093/ndt/gfw452]
77 Ohkuma T, Nakamura U, Iwase M, Ide H, Fujii H, Jodai T, Kaizu S, Kikuchi Y, Idewaki Y, Sumi A, Hirakawa Y,Kitazono T. Effects of smoking and its cessation on creatinine- and cystatin C-based estimated glomerular filtration rates and albuminuria in male patients with type 2 diabetes mellitus:the Fukuoka Diabetes Registry.
2016; 39:744-751 [PMID:27250568 DOI:10.1038/hr.2016.51]
78 Hammer Y, Cohen E, Levi A, Krause I. The Relationship between Cigarette Smoking and Renal Function:A Large Cohort Study.
2016; 18:553-556 [PMID:28471605]
79 Liu G, Hu Y, Zong G, Pan A, Manson JE, Rexrode KM, Rimm EB, Hu FB, Sun Q. Smoking cessation and weight change in relation to cardiovascular disease incidence and mortality in people with type 2 diabetes:a population-based cohort study.
2020; 8:125-133 [PMID:31924561 DOI:10.1016/S2213-8587(19)30413-9]
