Relook at DPP-4 inhibitors in the era of SGLT-2 inhibitors
2022-06-21AwadheshKumarSinghRituSingh
TO THE EDlTOR
We read with interest a minireview by Florentin
[1] putting their arguments in favor of DPP-4 inhibitors (DPP-4Is) as a second-line drug after metformin in people with type 2 diabetes mellitus (T2DM) in particular who are elderly and have chronic kidney disease (CKD) stage 3A or lower. This wonderfully written minireview discusses the role of DPP-4Is in the era of two other novel anti-diabetic agents such as SGLT-2 inhibitors (SGLT-2Is) and GLP-1 receptor agonists (GLP-1RAs) that have shown a remarkably beneficial effect on cardiovascular (CV) and renal endpoints making them an ideal second or arguably even first-line drug in people with T2DM having established CV disease (CVD), heart failure (HF) and CKD. While authors have discussed the pharmacological differences amongst different DPP-4Is and put a perspective on the CV outcome trials in the era of SGLT-2Is and GLP-1RAs, few vital details seem to be missing and some of the statements appear rather ambiguous that need clarification. The most important area that is surprisingly missing in this review is the efficacy comparison between DPP-4Is
SGLT-2Is or GLP-1RAs. Expectedly, the HbA1c lowering effect of DPP-4Is would be inferior to GLP-1RAs owing to their mechanism of action that causes a physiological
pharmacological rise of GLP-1 respectively and indeed, several head-to-head studies of long-acting GLP-1RAs have shown a superior HbA1c lowering beside a significant reduction in weight and systolic blood pressure (SBP) when compared with DPP-4Is. However, the HbA1c lowering effect of DPP-4Is is not clinically meaningful different from SGLT-2Is. To this end, several studies have evaluated the HbA1c lowering effect of SGLT-2Is
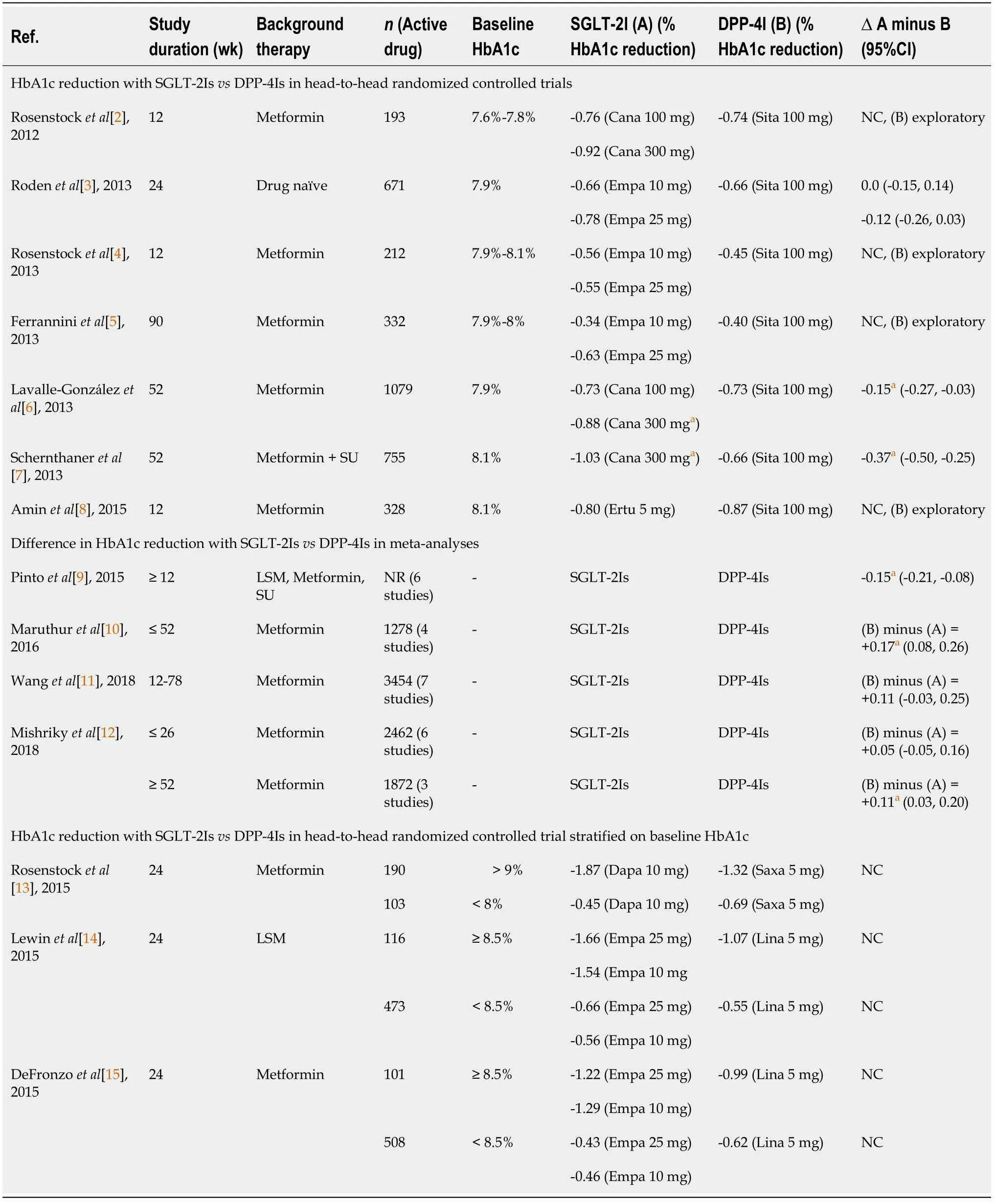
DPP4Is in the past decade[2-8]. Although in most of these SGLT-2Is head-to-head studies with DPP-4Is, HbA1c reduction was similar between the two drug classes; DPP-4Is were used as an open-label active comparator arm only for exploratory analysis. One study that compared empagliflozin 10 and 25 mg with 100 mg sitagliptin as an active comparator in a double-blind randomized fashion found no difference in HbA1c lowering[3]. However, two studies that compared canagliflozin 100 and 300 mg with sitagliptin 100 mg as an active comparator in a double-blind randomized fashion, found 300 mg canagliflozin to be superior to 100 mg sitagliptin in HbA1c lowering, though no difference was noted with 100 mg canagliflozin (Table 1)[6,7]. Meta-analyses that compared HbA1c lowering with DPP-4Is
SGLT-2Is yielded discordant results[9-12]. While some found no difference in HbA1c lowering, others showed a small but significant HbA1c lowering with SGLT-2Is compared to DPP-4Is (Table 1). Notably, weight and SBP reduction were consistently superior with SGLT-2I
DPP-4I in all these head-to-head studies including meta-analyses. Another interesting piece of missing information that needs discussion is the differential HbA1c lowering effect of DPP-4Is vs. SGLT-2Is stratified on baseline HbA1c. While the SGLT-2Is appear to lower the HbA1c more favorably compared with DPP-4Is in the background of higher baseline value (HbA1c 8.5%-9.0%), DPP-4Is lowered HbA1c more favorably compared with SGLT-2I in people having a modest baseline HbA1c value (< 8%-8.5%) (Table 1)[13-15]. This finding suggests DPP-4Is may have a favorable effect on HbA1c lowering compared to SGLT-2Is in people with T2DM having a modest baseline HbA1c, in absence of high CV risk. Although a reduction in HbA1c is always larger when baseline HbA1c is high, we do not know exactly why DPP-4Is reduce HbA1c larger compared to the SGLT-2Is when the baseline value is modest. Since SGLT-2Is HbA1c lowering ability is dependent on the renal threshold of glucose excretion (RT
), modest baseline HbA1c may not produce further lowering of RT
.
It all began when my stepchildren came for a visit shortly after their father and I were married. Cheryl was 8, and Chuck was 10. Our small apartment soon became an obstacle course littered with stuffed animals, toys, and games.
Nevertheless, we humbly disagree with the author’s conclusion about “the lack of evidence with SGLT-2Is and GLP-1RAs in elderly patients with diabetes as well as the contraindication of SGLT-2Is in patients with CKD, grade 3A and lower, make DPP-4Is a safe choice in such populations.” Let us recall that:(1) About one-fourth patients population (24.2%) in HF trial of SGLT-2I dapagliflozin were elderly [≥ 75 years, median age 79 years (76-82 years)] and they benefitted equally [Hazard ratio (HR), 0.68; 95% Confidence interval (CI), 0.53-0.88] when compared to the overall population (HR, 0.74; 95%CI, 0.65-0.85) in terms of reduction of the primary composite endpoint of CV death or HF hospitalization (HHF) or urgent HF visits (
= 0.76)[16]; (2) Mean age of the population in CV-, HF- and renal-outcome trials of SGLT-2Is varied from as low as 62 years in renal outcome trial of dapagliflozin (DAPA-CKD) to as high as 72 years in HF trial of empagliflozin (EMPEROR-Preserved) that found a significantly beneficial renal and CV effect respectively[17]; (3) Current guidelines recommend using SGLT-2Is in patients with CKD if eGFR is ≥ 30 mL/min/1.73 m
and in addition, empagliflozin has been granted an additional label of use up to eGFR ≥ 20 mL/min/m
in patients with HF with reduced ejection fraction and CKD[18]; (4) The latest Kidney Disease:Improving Global Outcomes 2022 guideline which is currently under public review recommend using SGLT-2Is in patients with CKD if eGFR ≥ 20 mL/min/1.73 m
regardless of background HF. Moreover, once SGLT-2Is are initiated it is reasonable to continue even if the eGFR falls below 20 mL/min per 1.73 m
unless it is not tolerated or kidney replacement therapy is initiated[19]; (5) Although there are no head-to-head randomized controlled trials that compared CV outcomes between DPP-4Is
DPP-4Is, several large real-world, propensitymatched studies showed a significant reduction in HHF with SGLT-2Is compared with DPP-4Is in patients with T2DM, regardless of baseline high CV risk[20]; and (6) Finally, the 2011 European Diabetes Working Party for Older People clinical guideline that recommended DPP-4I as a second-line drug of choice in elderly were made before the US Federal Drug Administration approval of first SGLT-2I
canagliflozin in 2013 and first positive CV outcome with empagliflozin in 2015. These findings suggest author’s conclusion is discordant with the available evidence[21].
We need to live each moment wholeheartedly, with all our senses -- finding pleasure in the fragrance of a back-yard garden, the crayoned picture of a six-year-old, the enchanting beauty of a rainbow. It is such enthusiastic love of life that puts a sparkle in our eyes, a lilt in our steps and smooths the wrinkles from our souls.
A short walk from my house in Hampshire, on a hill overlooking the heathland, is a plaque1 marking the spot where Richard Pryce Jones deliberately2 crashed his Halifax bomber3 during the war. He could have parachuted to safety, but that would have meant crashing into the village. The epitaph(,) reads: He died that others might live.
Singh AK designed the research; Singh R performed the research, Singh AK and Singh R analyzed the data; Singh AK wrote the letter, and Singh R revised the manuscript.
India
The troll then showed him the three bushels of money which he had earned during the past year; they stood beside the other three, and all the six now belonged to him
The authors have nothing to disclose.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See:https://creativecommons.org/Licenses/by-nc/4.0/
Awadhesh Kumar Singh 0000-0002-8374-4536; Ritu Singh 0000-0003-1779-0638.
Chang KL
A
Chang KL
1 Florentin M, Kostapanos MS, Papazafiropoulou AK. Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment.
2022; 13:85-96 [PMID:35211246 DOI:10.4239/wjd.v13.i2.85]
2 Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W; Canagliflozin DIA 2001 Study Group. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes.
2012; 35:1232-1238 [PMID:22492586 DOI:10.2337/dc11-1926]
3 Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC; EMPA-REG MONO trial investigators.Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes:a randomised, doubleblind, placebo-controlled, phase 3 trial.
2013; 1:208-219 [PMID:24622369 DOI:10.1016/S2213-8587(13)70084-6]
4 Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, Woerle HJ. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia.
2013; 15:1154-1160 [PMID:23906374 DOI:10.1111/dom.12185]
5 Ferrannini E, Berk A, Hantel S, Pinnetti S, Hach T, Woerle HJ, Broedl UC. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin:an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes.
2013; 36:4015-4021 [PMID:24186878 DOI:10.2337/dc13-0663]
6 Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy:a randomised trial.
2013; 56:2582-2592 [PMID:24026211 DOI:10.1007/s00125-013-3039-1]
7 Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G.Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea:a 52-week randomized trial.
2013; 36:2508-2515 [PMID:23564919 DOI:10.2337/dc12-2491]
8 Amin NB, Wang X, Jain SM, Lee DS, Nucci G, Rusnak JM. Dose-ranging efficacy and safety study of ertugliflozin, a sodium-glucose co-transporter 2 inhibitor, in patients with type 2 diabetes on a background of metformin.
2015; 17:591-598 [PMID:25754396 DOI:10.1111/dom.12460]
9 Pinto LC, Rados DV, Remonti LR, Kramer CK, Leitao CB, Gross JL. Efficacy of SGLT2 inhibitors in glycemic control,weight loss and blood pressure reduction:a systematic review and meta-analysis.
2015; 7:A58[DOI:10.1186/1758-5996-7-s1-a58]
10 Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S.Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes:A Systematic Review and Meta-analysis.
2016; 164:740-751 [PMID:27088241 DOI:10.7326/M15-2650]
11 Wang Z, Sun J, Han R, Fan D, Dong X, Luan Z, Xiang R, Zhao M, Yang J. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors
dipeptidyl peptidase-4 inhibitors as monotherapy or add-on to metformin in patients with type 2 diabetes mellitus:A systematic review and meta-analysis.
2018; 20:113-120 [PMID:28656707 DOI:10.1111/dom.13047]
12 Mishriky BM, Tanenberg RJ, Sewell KA, Cummings DM. Comparing SGLT-2 inhibitors to DPP-4 inhibitors as an add-on therapy to metformin in patients with type 2 diabetes:A systematic review and meta-analysis.
2018; 44:112-120 [PMID:29477373 DOI:10.1016/j.diabet.2018.01.017]
13 Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, Iqbal N. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy:a randomized double-blind trial of saxagliptin plus dapagliflozin addition
single addition of saxagliptin or dapagliflozin to metformin.
2015; 38:376-383 [PMID:25352655 DOI:10.2337/dc14-1142]
14 Lewin A, DeFronzo RA, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes.
2015; 38:394-402 [PMID:25633662 DOI:10.2337/dc14-2365]
15 DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin.
2015; 38:384-393 [PMID:25583754 DOI:10.2337/dc14-2364]
16 Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L,Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz-Piasecka M, Bengtsson O, Sjöstrand M, Langkilde AM, Jhund PS, McMurray JJV. Efficacy and Safety of Dapagliflozin in Heart Failure With Reduced Ejection Fraction According to Age:Insights From DAPA-HF.
2020; 141:100-111 [PMID:31736328 DOI:10.1161/CIRCULATIONAHA.119.044133]
17 Singh AK, Singh R. Similarities and differences in cardio-renal outcome trials with SGLT-2 inhibitors:call for pharmacogenomic studies?
2022 [DOI:10.21037/prpm-22-2]
18 Kidney Disease:Improving Global Outcomes (KDIGO) Diabetes Work Group. . KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease.
2020; 98:S1-S115 [PMID:32998798 DOI:10.1016/j.kint.2020.06.019]
19 KDIGO 2022 Clinical Practice Guidelines for Diabetes Management in Chronic Kidney Disease. [Accessed March 5,2022]. Available from:https://kdigo.org
20 Singh AK, Singh R. Heart failure hospitalization with SGLT-2 inhibitors:a systematic review and meta-analysis of randomized controlled and observational studies.
2019; 12:299-308 [PMID:30817235 DOI:10.1080/17512433.2019.1588110]
21 Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary.
2011; 37 Suppl 3:S27-S38 [PMID:22183418 DOI:10.1016/S1262-3636(11)70962-4]
