Unraveling the core functional bacteria and their succession throughout three fermentation stages of broad bean paste with chili
2022-06-20SongfengYuJiSongToHuJunWngXiojingLiuYuZhengLeiShiShoupengWnMinWng
Songfeng Yu, Ji Song, To Hu, Jun Wng, Xiojing Liu,Yu Zheng,,*, Lei Shi, Shoupeng Wn, Min Wng,*
a State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education,College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China
b College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou 310058, China
c Tianjin Limin Condiment Limited Company, Tianjin 300308, China
Keywords:
Broad bean paste with chili
Canonical correspondence analysis
Flavor compounds
Microbial community
Core bacteria
A B S T R A C T
The entire fermentation process of traditional Chinese broad bean paste with chili comprises three individual stages: Tianbanzi, chili pei, and paste fermentation (Tianbanzi-chili pei mixture). Three stages share average 77.53% of all bacteria (89 genera), indicating that the similar environment leads to the similar bacterial communities. One, one, and three genera are exclusive to Tianbanzi, chili pei, and paste stages, respectively,due to the special physical and chemical properties for each stage. Total acidity, pH, and NaCl are important endogenous factors that promote the succession of bacterial communities. According to the dynamics of organic acids, reducing sugars, amino acids, and volatile compounds, 60-, 210-, and 180-day are considered the best fermentation periods for Tianbanzi, chili pei, and paste, respectively, to balance time cost and product quality.Three (Tetragenococcus, Lactobacillus, and Pseudomonas), four (Tetragenococcus, Lactobacillus, Bacillus, and Pseudomonas), and five (Tetragenococcus, Lactobacillus, Bacillus, Pseudomonas, and Pediococcus) genera are considered the core functional bacteria of Tianbanzi, chili pei, and paste fermentation, respectively.
1. Introduction
Broad bean paste with chili (BBPC) is an important condiment and coloring agent for food preparation and has been widely used in diet in Asian countries for hundreds years [1,2]. BBPC features a range of biological functions, including anti-inflammatory [3]and anti-obesity activities [4]. The multifaceted characteristics of BBPC make it popular in the food market, with an approximately 800 000 t been consumed each year in China.
Similar to traditional fermented foods, such as vinegar, miso, and kimchi [5], Chinese BBPC (CBBPC) is produced by spontaneous fermentation technology. Fermentation is generally divided into three individual stages includingTianbanzi, chilipei, and paste fermentation (mixture ofTianbanzi, and chilipei) and spans at least several months to achieve the desirable flavor and taste. ForTianbanzifermentation, the broad bean pasted with wheat flour is scattered with fungal spores (Aspergillus oryzae) and incubated under certain conditions for several days to obtainQu. TheQuis mixed with brine to form a mixture known asTianbanzi, which is spontaneously fermented for more than 2 months at room temperature. For chilipeifermentation, fresh chili is chopped and mixed with salt to yield chilipei, which is spontaneously fermented for more than 7 months. In the paste fermentation stage,Tianbanziand chilipeiare mixed, and the semi-solid mash is placed in fermentation tanks for more than 6 months to produce CBBPC. The production process of CBBPC is illustrated in Fig. 1.
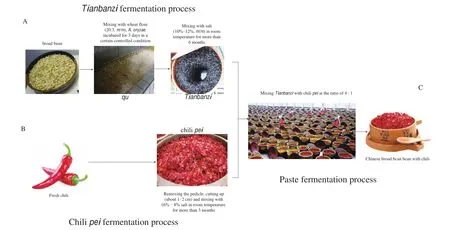
Fig. 1 The process diagram for broad bean paste with chili fermentation. (A) Tianbanzi fermentation stage, (B) chili pei fermentation stage, and (C) paste fermentation stage.
Microbiota affects the characteristics of fermented foods and contributes to their quality; it can break down macromolecules into small peptides and monosaccharides and produce various flavor components [6]. The microorganism community and overall characteristics of CBBPC continuously change throughout the stages of fermentation due to the growth and metabolism of microorganisms.A better understanding of microbial community succession serves as the basis to understand the process of fermentation [7]. Previous studies focused on the diversity and dynamics of the microbial community in different kinds of sauces through culture-dependent or culture-independent methods [8-10]. Bacteria are the dominant microorganisms for the fermentation of broad bean paste, in which the number of fungi gradually decreases or disappears during fermentation [9,11,12]. Although these studies have provided insights into microbial populations in fermented foods, knowledge regarding the structure of core microbial community remains limited. Currently,studies have focused on the structure of the core microbial community in fermentation of traditional foods [13,14]. Therefore, in the present study, the microbial succession patterns during the three stages of CBBPC fermentation were tracked. The factors that promoted the succession of bacterial communities were revealed by canonical correspondence analysis (CCA). And the contribution of core bacteria to flavor compounds was also evaluated.
2. Materials and methods
2.1 Sampling
Tianbanzi, chilipei, and paste samples were collected from a CBBPC manufacturing company located in Yinchuan city, China.Tianbanzisamples of T1 (0 day), T2 (7 days), T3 (60 days), T4 (120 days), and T5 (240 days), chilipeisamples of J1 (30 days), J2 (120 days), J3 (210 days), J4 (300 days), and J5 (390 days), and paste samples of D1 (7 days), D2 (90 days), D3 (180 days), D4 (270 days),and D5 (390 days) were periodically collected to monitor dynamic changes in microbial communities during fermentation. All samples were collected from 3 parallel fermentation tanks.
2.2 Physicochemical indicators analysis
Approximate 1 g of the sample was homogenized with 10 mL of distilled water. pH was determined using a pH meter (Mettler Toledo,USA). The contents of reducing sugars (g/100 g), NaCl (g/100 g),total acidity (g/100 g), and amino acid nitrogen (g/100 g) were determined according to a previous report [15].
2.3 Volatile compounds analysis
Headspace solid-phase microextraction (HS-SPME) combined with gas chromatography-mass spectrometry (GC-MS) was used to analyze volatile compounds. A total of 5 g of the samples were transferred into a 20 mL headspace vial and stirred in a 60 °C water bath. The carboxen/polydimethylsiloxane tip (Supelco-Analytical,Sigma-Aldrich®, USA) was inserted into the vial and incubated at 60 °C for 30 min. The tip was withdrawn and introduced into GC-MS,which was conducted on Agilent 6890N GC-5973 MS equipped with an HP-5 phenyl polysiloxane capillary column (30 m ×250 µm, 0.25 µm, Agilent, USA). Carrier gas (helium) was applied at a flow rate of 1 mL/min. The GC oven temperature was held at 40 °C for 5 min, increased from 40 °C to 85 °C at a rate of 6 °C/min,maintained at 85 °C for 1.5 min, increased from 85 °C to 148 °C at a rate of 2.5 °C/min, held at 148 °C for 1 min, increased from 148 °C to 280 °C at a rate of 20 °C/min, and maintained at 280 °C for 1 min. The ionization energy was 70 eV, and the range of scanned molecular weight was 25–450 u. Volatile compounds were positively identified by comparing the retention index (RI) and mass spectra of the reference standards in the mass spectral library of the National Institute of Standards and Technology (NIST). The integration reports were accepted if the RI value was above 800. Relative content was de fined by normalized peak areas [16].
Metabolite profiles were visualized using a heat map generated through Hierarchical Clustering Explorer (HCE) version 3.5 [17].
2.4 Analysis of bacterial community
For DNA extraction, each sample was mixed with phosphate buffered saline (PBS, pH 7.2) and vortexed. The samples were filtered with four layers of sterile cheesecloth, and the filtrate was centrifuged at 8 000 ×gfor 10 min at 4 °C. The pellet was then subjected to DNA extraction using the GMO Food DNA Extraction Kit (Tiangen,Beijing, China) after glass bead-beating to disrupt the microbial cell wall according to the manufacturer’s instruction.
Illumina MiSeq sequencing was performed at GeneWiz, Inc. (Suzhou, China). Universal primers 515F(5’-G T G C C A G C M G C C G C G G T A A-3’) a n d 8 0 6 R(5’-GGACTACHVGGGTWTCTAAT-3’) were used to amplify the V3–V4 hypervariable regions of the 16S rRNA gene for MiSeq sequencing [18].
The sequence data were demultiplexed, quality filtered, and analyzed using QIIME Pipeline version 1.7.0 [18]. High-quality sequences (length > 150 bp, without ambiguous base “N”, and average base quality score > 30) were used for analysis. Operational taxonomic units (OTUs) were classified at a 97% similarity threshold value of 16S rRNA sequences. The resulting tables of OTUs (BIOM format) were filtered for OTU reading of more than five times and were used for taxonomic assignment.
Predominant bacteria were defined as those with microbial relative abundance of more than 1%, and core functional bacteria were de fined as those associated with fermentation and formation offlavor compounds.
2.5 Multivariate statistical analysis
All possible Spearman’s rank correlations among the abundant genera (the first 30 genera according to abundance) were calculated using a species correlation network map showing the interactions among microbes during the entire fermentation. A network was created with Gephi (Web Atlas, Paris, France) to sort through and visualize the correlations [19].
CCA was conducted based on bacterial communities and main fermentation parameters (pH, total acidity, amino acid nitrogen,NaCl, and reducing sugar) via the vegan package in R program (http://vegan.r-forge.r-project.org/). To analyze the relationships among microbial communities, all possible Spearman’s rank correlations were calculated between each genus and the others (with average relative abundance of > 1%). Only significant correlations (P< 0.05,with false discovery rate correction) were considered valid. Data standardization was performed using IBM SPSS 19.0 software.
3. Results and discussion
3.1 Dynamic changes in physicochemical properties during the entire fermentation process
The entire fermentation process of CBBPC was analyzed.Tianbanzi, chilipei, and paste fermentation stages were monitored for 8, 13, and 13 months, respectively. As shown in Fig. 2, the dynamic changes in the physicochemical properties, including pH, total acidity,and the contents of amino acid nitrogen, NaCl, and reducing sugar.The total acidity gradually increased in the fermentation ofTianbanziand chilipeidue to fermentation of carbohydrates, whereas the pH continuously decreased. In paste fermentation, the total acidity rapidly increased and then decreased after 6 months (Figs. 2A, 2B). Although the total acidity dynamically changed, the content of reducing sugar fluctuated slightly from 3 g/100 g to 7 g/100 g during the three fermentation stages (Fig. 2D). This phenomenon can be due to the multilateral fermentation process of CBBPC, where saccharification of carbohydrates and formation of organic acids simultaneously occur because of the action of multi-microorganisms [21,22]. Starch and other polysaccharides will be decomposed by glucoamylase fromAspergillusto produce reducing sugars, which will then be consumed by microorganisms and the Maillard reaction [23]. Amino acid nitrogen is one of the most important indices that affect the quality of CBBPC products. The content of amino acid nitrogen rapidly increased in the fermentation ofTianbanziand paste (Fig. 2C) owing to the hydrolysis of protein substances from broad bean [24,25].Besides the growth and metabolism of microorganisms, Maillard reaction also consumes amino acid nitrogen [26]. These reactions result in changes inflavor and color. For chilipei, a negligible change in amino acid nitrogen content was observed (Fig. 2C). The NaCl contents in the three fermentation stages were relatively stable (Fig. 2E).
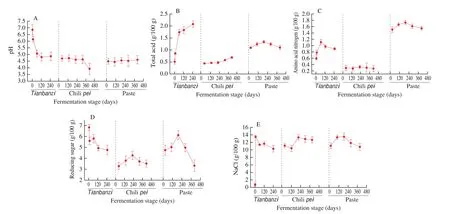
Fig. 2 Dynamic changes in physicochemical properties during the entire fermentation process. (A) pH, (B) total acidity, (C) amino acid nitrogen, (D) reducing sugar, and (E) NaCl.
Apart from organic acids, reducing sugars, amino acids, and other important flavor substances, volatile compounds have been reported and identified in sauces that typically use soybeans as the basic ingredient [27-29]. Microbial metabolites are important factors affecting the organoleptic properties and are known to represent a specific phenotype re flecting the microbial community [30]. Volatile flavor compounds throughout the entire fermentation process of CBBPC were analyzed to elucidate dynamic changes. A total of 100 volatile compounds were identified from 15 samples and included 8 alcohols, 31 esters, 8 phenols, 17 aldehydes, 11 alkanes, 15 terpenes,3 ketones, and 7 others compounds (Fig. 3). Heat mapping showed that esters, aldehydes, alcohols, and terpenes were the main volatile flavor compounds inTianbanzi, esters, alkanes, terpenes, and alcohols were the largest groups in chilipei, and esters, aldehydes, alkanes,and phenols were the dominant compounds in paste.
采用XRF法[15]检测样品(NaCl、城市固体废弃物模拟组分和厨余沼渣)中的Cl含量;取约3 g样品,分别将硼酸和样品用压片机(10 MPa下恒压30 s)压制成片放入 XRF仪器中,测定其中的 Cl含量。
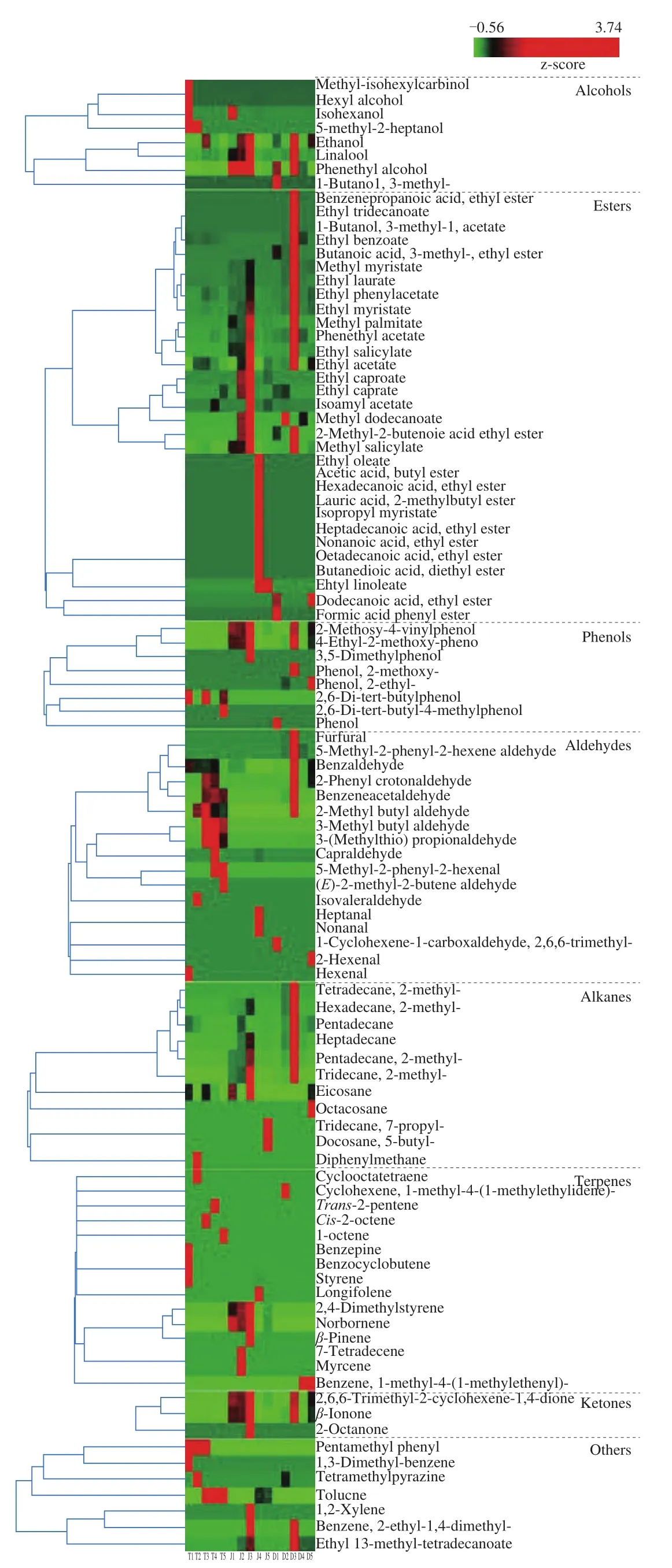
Fig. 3 Heatmap of volatile compounds in CBBPC during fermentation.
Esters were the largest group in each fermentation stage, reaching the highest content in samples of T3, J3, and D3, and most of the detected esters were ethyl esters (Fig. 3). Esters are one of the most important flavor compounds that endow fermented foods with honey and fruity flavors owing to their volatilization. Moreover, human nose is sensitive to esters [31]. The number of esters inTianbanziwas less than that in chilipeiand paste, and the concentration of esters was higher in paste than those in the other two stages. In many fermented foods the biosynthesis of esters proceeds through enzymatic reactions,such as esterification under the action of esterase. The fermentation conditions, including total acidity and amino acid nitrogen content,were different and varied in three stages (as described in Fig. 2).That might be the reason for the different number and content of esters. Moreover, because paste is achieved by mixingTianbanziand chilipei, the concentration of esters in paste would be higher than those in the other two stages [20]. In particular, dodecanoic acid ethyl ester, formic acid phenyl ester, 1-butanol, 3-methyl-, acetate,benzenepropanoic acid, ethyl ester, and ethyl tridecanoate were only detected in paste samples. The other esters were identified either inTianbanzior chilipei(Fig. 3). These results indicate that esters are produced after the mixture ofTianbanziand chilipei.
Alcohols give fermented foods pleasant aromas and sweet flavors [32].As shown in Fig. 3, eight kinds of alcohols were detected in the entire fermentation process. More kinds of alcohols were found inTianbanzithan in the other two stages. Ethanol, linalool, and phenethyl alcohol were commonly found in all the samples. Ethanol featured the highest content in all alcohols, which might be the reason for the highest concentration of ethyl esters. Alcohols and ethyl esters are regarded as the most important components that determin the flavor characteristics of broad bean paste [24]. Moreover, ethanol is important for stabilizing the quality of the soy sauce and also adding flavors [33]. Aldehydes are also important for contributing to various odors and are associated with flavor production as substrates in food. During theTianbanzifermentation stage, multiple kinds of aldehydes were observed, and their contents were higher than those in the other two stages. Benzaldehyde (cherry or almond-like odor)and benzacetaldehyde (rosy-like odor) were detected in the three fermentation stages, which are considered to enhance the flavor of BBPC. Furfural, 2-hexenal, 5-methyl-2-phenyl-2-hexenal aldehyde,and 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde were only found in paste. Hexanal, isovaleraldehyde, 3-methyl butyl aldehyde,3-(methylthio) propionaldehyde, 5-methyl-2-phenyl-2-hexene, and(E)-2-methyl-2-butene aldehyde were only detected inTianbanzi.Hexanal is a volatile compound that contributes a disagreeable green grass-like aroma, which declined inTianbanzifermentation process.Heptanal and nonanal were only detected in chilipei. Pyrazines and volatile phenols are important for the aroma of fermented soybean products [34]. Tetramethylpyrazine was the only pyrazine compound identified in the entire fermentation process (Fig. 3). Many phenolic compounds exhibit multiple biological activities and feature a positive effect on human health [35]. 4-Ethylphenol and 4-ethyl-2-methoxypheno, as two major odor contributors to the flavor of spicy,are commonly observed in fermented food. They are also considered to potentially enhance the flavor quality of soy sauce. As shown in Fig. 3, 4-ethyl-2-methoxy-phenol was commonly found and presented high content in all fermentation stages. 7-Propyl-tridecane and 5-butyl-docosane were only detected in chilipei, and octacosane and 2-methyl-tetradecane were only found in paste. The other alkanes were common in all the three fermentation stages. The main source of hydrocarbons is lipid auto-oxidation [36]. However, due to their low volatility and high perception threshold, their contribution to sample aromas would be negligible [37,38]. Most of the terpenes were detected inTianbanziand chilipeisamples. Additional miscellaneous compounds were also identified (Fig. 3), which might contribute to the aromatic complexity of the acceptable aroma character of CBBPC.
However, it should be mentioned that in this study HS-SPME was used to analyze the volatile components, and some flavor compounds with low volatility would be missed. Moreover, not all volatiles can be detected due to the specificity offibers [29].However, HS-SPME is a simple, fast and low-cost solvent-free method when compared with other extraction methods such as simultaneous distillation extraction (SDE) and solvent-assisted flavor evaporation (SAFE) [39].
For each stage, the sample with the most abundant and highest concentration of compounds was 60-dayTianbanzi(T3), 210-day chilipei(J3), and 180-day paste (D3) (Fig. 3). These results explain the important reason for the production of CBBPC by mixingTianbanzifermented for more than 2 months with chilipeifermented for more than 7 months. Moreover, paste samples fermented for 6 months exhibited the most abundant flavor composition and highest concentration, thus endowing the product with better aroma. This result indicates that the mid-late stage of fermentation is an important period for the generation and accumulation offlavor substances [24].However, a long fermentation process will lead to increased production cost. Therefore, a 6-month duration is the optimal period for balancing the time cost and product quality. This finding is also consistent with the actual production process.
3.2 Dynamics of bacterial community during the whole fermentation process
Microbial communities are critical to flavor formation during fermentation due to reactions catalyzed by their different enzymes [29].High throughput sequencing technology was employed to characterize bacterial community structures and their dynamic changes in the fermentation stages. The OTUs were clustered according to 97% identity, and taxonomic annotations were performed at different taxonomic levels (phylum, class, order, family, and genus). The relative abundance at the phylum and genus levels are shown in Fig. 4. At the phylum level, Firmicutes and Proteobacteria were predominant (average relative abundance > 1%) throughout the entire fermentation process (Fig. 4A). This result is similar to that of sauces [3,40-42]. The following dominant phyla were Bacteroidetes,Actinobacteria, and Cyanobacteria. The number of Firmicutes in paste samples was higher than that in the other samples. The abundance of bacterial community was relatively lower at the later period of each fermentation stage than that at the beginning because some bacteria are not resistant to the higher content of salt and the lower content of water in the fermentation environment (Fig. 4A and Fig. S1).

Fig. 4 Taxonomic composition of predominant bacterial communities in CBBPC during fermentation. (A) phylum level and (B) genus level. Relative abundance (%) was calculated from the copy of 16S rRNA gene sequences.
A total of 89 bacterial genera (75 bacterial genera inTianbanzisamples, 81 in chilipeisamples, and 86 in paste samples) were identified. More genera were found in paste samples than in the other samples based on the production process of CBBPC, whereTianbanziand chilipeiwere mixed for paste fermentation.
ForTianbanzifermentation, the bacterial community predominantly comprisedEnterococcus(19.5%, average relative abundance),Tetragenococcus(19.1%),Lactobacillus(17.8%),Staphylococcus(12.2%),Acinetobacter(8.1%), andPseudomonas(1.3%) at the genus level (Fig. 4B). Particularly,Enterococcus,Staphylococcus, and several unclassified bacteria were predominant in the initial fermentation period. However, the abundance ofTetragenococcusrapidly increased, followed byAcinetobacter,Lactobacillus, andPseudomonas. The numbers ofStaphylococcusand unclassified bacteria decreased and remained relatively stable until the end of this fermentation process. In chilipeifermentation,Staphylococcus(19.2%),Lactobacillus(14.4%),Tetragenococcus(10.7%),Bacillus(9.1%),Weissella(7.1%),Pseudomonas(4.3%),andProteus(2.1%) were the predominant bacterial communities.Tetragenococcus,Staphylococcus, and several unclassified bacteria were initially predominant, and then their abundance gradually decreased until the end of this fermentation process. The abundance ofLactobacillusrapidly increased, followed byBacillus. The numbers ofWeissellaandPseudomonasincreased from 30 days (J1) to 120 days (J2) and then remained relatively stable until the end of this fermentation process. For paste fermentation, the relative abundance ofEnterococcusandLactobacillusincreased from 7 days (D1) to 180 days (D3) and then remained relatively stable. Moreover, the abundance ofEnterococcusandLactobacilluswas higher than that of other bacteria throughout the entire paste fermentation process.Bacillusshowed the same trend withEnterococcusandLactobacillus.The abundance ofStaphylococcusincreased up to 180 days and then gradually decreased, whereas that ofTetragenococcusdecreased up to 180 days and then remained relatively stable.Enterobacter,Acinetobacter,Pseudomonas,Pediococcus, andWeissellawere relatively stable during the entire paste fermentation process. Overall,the bacterial communities in paste fermentation predominantly comprisedEnterococcus(39.2%),Lactobacillus(29.3%),Bacillus(6.2%),Tetragenococcus(5.5%),Staphylococcus(5.4%),Pediococcus(2.1%),Enterobacter(1.9%),Acinetobacter(1.7%),Pseudomonas(1.6%), andWeissella(1.3%).
3.3 Interactions among bacterial communities during fermentation
The production of CBBPC involves a spontaneous fermentation process, which is driven by microorganisms. Environmental microbiota will be an important source of fermentation microbiota.The bacteria generally shared amongTianbanzi, chilipei, and paste samples were analyzed via Venn analysis to elucidate whether a similar environment would lead to similar or different bacterial communities in the three fermentation stages. As shown in Fig. 5A,70 genera were shared between samplesTianbanzi(T) and chilipei(J),73 genera were shared betweenTianbanzi(T) and paste (D), 79 genera were shared between chilipei(J) and paste (D), and 69 genera(77.53% of all) were shared among all the samples in this study.Interestingly, OTU83 (Kocuria) was only detected inTianbanzisamples; OTU68 (Proteus Rosenbergiella) was only detected in chilipeisamples; and OTU54 (Pseudoxanthomonas), OTU56(Enterobacter), and OTU57 (Pediococcus) were only found in paste samples (Table S1 and S2). These results indicate that most kinds of bacteria are similar among the three fermentation stages because they were conducted under a similar environment. However, the construction and succession of bacterial communities differed due to varying raw materials and conditions for each fermentation stage,especially for paste fermentation.
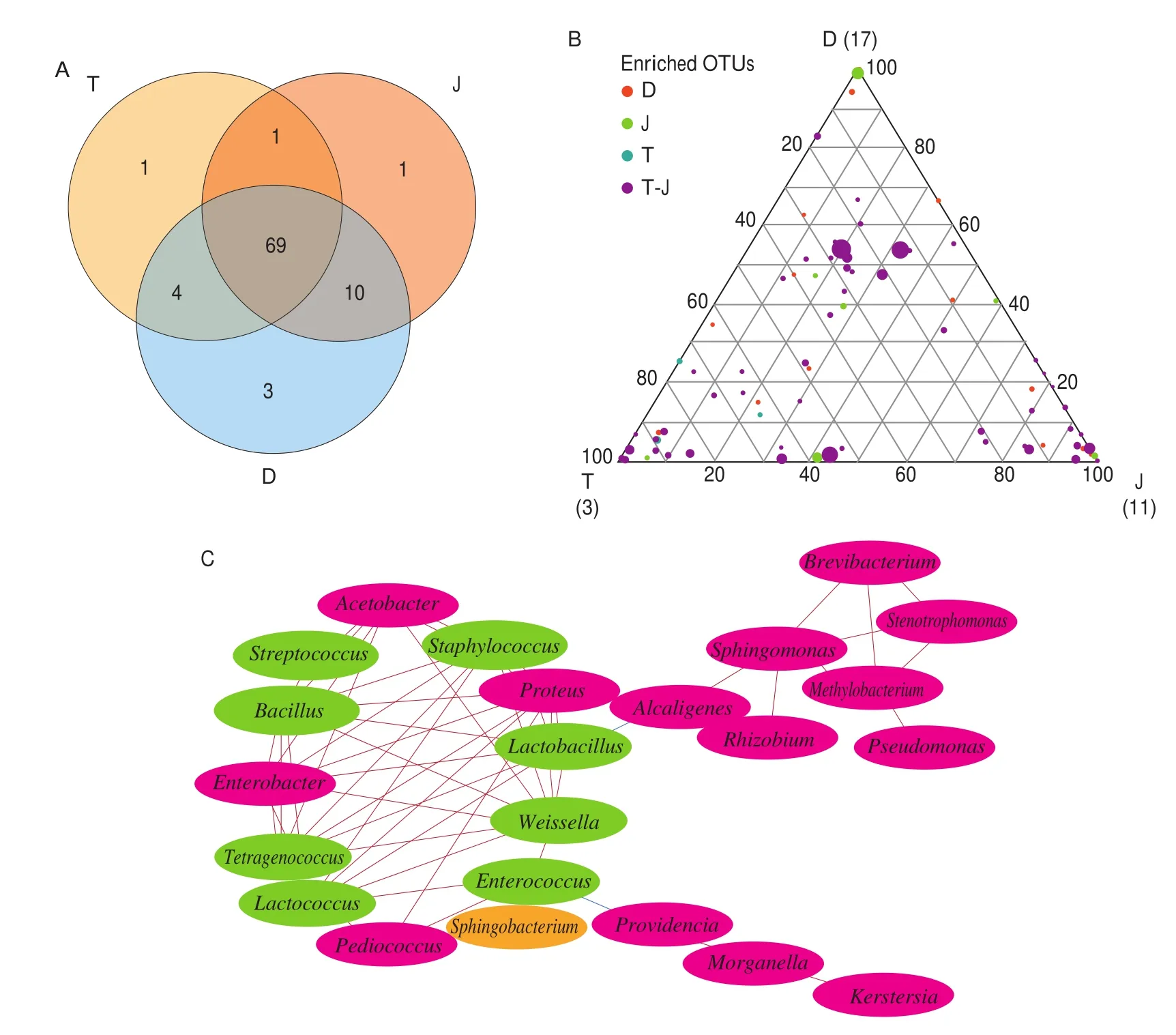
Fig. 5 Analysis of the interactions among microbes during the entire fermentation process. T, J, and D represent the OTU presented in samples of Tianbanzi, chili pei, and pastes, respectively. T-J represents the OTU presented in both Tianbanzi and chili pei samples. (A) Distribution of bacterial communities in fermentedTianbanzi, chili pei, and paste samples at the genus level. The Venn diagram shows the number of genera unique in a single sample type or shared by two or three sample types. (B) Ternary plots of all OTUs detected in the data set with relative abundance was above 0.5% in T, J, and D samples. Each circle represents oneOTU, and the size of each circle represents its relative abundance. The position of each circle was determined by the contribution of the indicated compartments to the total relative abundance. Blue, green, purple, and red circles denote that OTUs were significantly enriched in the Tianbanzi microhabitat (P < 0.05), chili pei microhabitat (P < 0.05), both Tianbanzi and chili pei microhabitats (P < 0.05), and paste microhabitat (P < 0.05), respectively. (C) Relationships among bacterialcommunities. The size of each node is proportional to the number of connections, and the nodes are colored by phylum (Firmicutes, green; Proteobacteria, pink;Bacteroidetes, yellow). The thickness of each connection (edge) between two nodes is proportional to the value of Spearman’s correlation coefficient (ρ). The color of the edges corresponds to a positive (red) or negative (blue) relationship.
Paste fermentation begins by mixingTianbanziand chilipei, and the microorganisms will be a mixture of both samples. The microbiota might also be reconstructed due to changes in fermentation conditions.A linear model analysis was employed to determine bacterial OTUs that were considerably enriched inTianbanziand chilipeicompared with those in paste fermentation to identify bacteria responsible for diversification between the two paste-associated microhabitats. Three distinct bacteria sub-communities thriving in the entire fermentation process were identified by this approach (Fig. 5B). In one subcommunity, a small number of OTUs were specifically enriched inTianbanzisamples (3 blue circles in Fig. 5B and Table S3). Two of them belong to family Enterococcaceae and one to Lactobacillaceae.The second sub-community was defined by bacteria that were substantially enriched in chilipeisamples and was characterized by numerous J-enriched OTUs mainly comprising Enterococcaceae,Bacillaceae, Lactobacillaceae, and Staphylococcaceae (11 green circles in Fig. 5B and Table S3). The third sub-community was defined by bacteria enriched inTianbanziand chilipeisamples from the paste and represented the largest fraction of the CBBPC bacterial communities, which mostly belong to phylum of Firmicutes, Proteobacteria, Cyanobacteria, and Bacteroidetes. These samples mainly comprised families belonging to Enterococcaceae,Enterobacteriaceae, Lactobacillaceae, Bacillaceae, Streptococcaceae,Pseudomonadaceae, Leuconostocaceae, and Staphylococcaceae(57 purple circles in Fig. 5B and Table S3). In particular, the representative bacteria enriched in T-J revealed a similar abundance trend. Moreover, bacteria that were considerably enriched in paste samples mainly comprised members of Enterobacteriaceae and Xanthomonadaceae (17 red circles in Fig. 5B and Table S3). Among the 17 bacteria, 4 were succeeded fromTianbanzi, 10 were from chilipei, and 3 were particularly enriched in the fermentation process of paste samples. These results reveal that paste-associated microecosystem comprised several bacteria fromTianbanziand chilipeiand specific bacteria from the environment, and then they enriched in the paste fermentation stage.
The correlations of microbes were explored based on Spearman’s rank correlations (|ρ| > 0.8 andP< 0.05) to further illustrate the interactions among microbes during the entire fermentation process.Twenty-three abundant genera were identified, among which 8 genera (green nodes) belonged to Firmicutes and were mostly predominant, 14 genera (pink nodes) belonged to Proteobacteria,and one (yellow nodes) belonged to Bacteroidetes (Fig. 5C). The generated network showed that the 23 genera were divided into three groups. The first group includedAlcaligenes,Rhizobium,Sphingomonas,Brevibacterium,Methylobacterium,Pseudomonas,andStenotrophomonas. The second group includedProvidencia,Morganella, andKerstersia. And the rest genera belonged to the third group. The third group presented a negative correlation with the first and second groups, whereas the bacteria within the third group showed strong positive correlation with each other. In particular,Tetragenococcus,Lactococcus,Enterobacter, andLactobacillusshowed close correlation with the other bacteria. These results suggest that the three groups of bacteria express different succession orders due to their different characteristics. Recent research has demonstrated that co-occurring taxa exert considerable influence on microbial structure and function regardless of their abundance across space and time [43]. Network analysis facilitates the exploration of many simultaneously interconnected correlations in a complex biological system [44].
3.4 Factors that promote the succession of bacterial communities throughout the entire fermentation process
Traditional CBBPC fermentation process was empirically conducted. Total acidity, pH, reducing sugar, and amino acid nitrogen, which are important factors that influence the growth of microorganisms, are not controlled during the fermentation process.The bacterial community construction changes along with the fermentation process, and a high correlation is found between those factors and the structure of microbial communities [24]. However,the factors that can promote the succession of bacterial communities during each different fermentation stage remain unclear. Therefore,the relationships between bacterial community succession and fermentation conditions were analyzed [45-48]. Multiple endogenous factors (pH, total acidity, NaCl, reducing sugar, and amino acid nitrogen) were selected to reveal their connection with the succession of bacterial communities by using the Mantel test and to demonstrate the major factors associated with bacterial succession at the different stages of CBBPC production. Significant correlation of changes in the bacterial community structure with pH was shown (P< 0.001,Table 1). Moreover, the two axes of CCA explained 74.2%, 68.6%,and 75.8% of the total variance in bacterial community differentiation duringTianbanzi, chilipei, and paste fermentation, respectively(Fig. 6). These results indicate that pH, total acidity, and NaCl are important endogenous factors that promote the succession of bacteria throughout the CBBPC fermentation process.
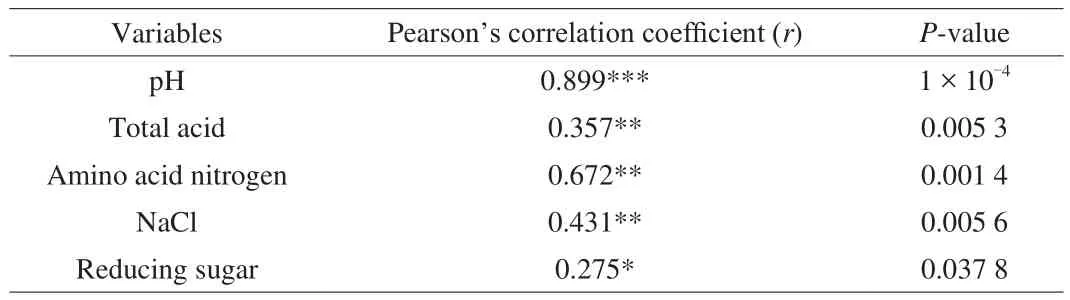
Table 1Pearson’s correlation and relative contributions of fermentation parameters to bacterial community structure analyzed by the Mantel test.

Fig. 6 CCA of bacterial community and fermentation conditions. (A) Tianbanzi fermentation, (B) chili pei fermentation, and (C) paste fermentation. Samples and responding bacterial communities are indicated on the graph. Correlations with fermentation conditions are indicated by arrows.
ForTianbanzifermentation, pH showed a strong positive correlation withTetragenococcus(P< 0.01) andPseudomonas(P< 0.05) (Table 2) at the initial fermentation stage (T1, T2, and T3)and a negative correlation at the later stages (T4 and T5) (Fig. 6A).AcinetobacterandEnterococcuswere negatively correlated with pH. The use ofTetragenococcusas a starter to improve the flavor of several fermented foods, including fish sauce, nampla [49], and Pixiandouban [7], has been reported. The pH values observed inTianbanziare possibly due to the changes in organic acids [41,50]. Total acidity was negatively correlated withTetragenococcus(P< 0.01)andPseudomonas(P< 0.01), but was significantly correlated withAcinetobacter(P< 0.05) andEnterococcus(Table 2, Fig. 6A).This result is consistent with that of pH. In the fermentation of doubanjiang-meju, a Chinese traditional fermented broad bean paste,Tetragenococcusis significantly correlated with pH, andPseudomonasandTetragenococcusare correlated with the production or consumption of organic acids [41]. Thus, pH and total acidity are two key factors that promote the succession ofPseudomonasandTetragenococcusinTianbanzifermentation. Amino acid nitrogen, reducing sugar, and NaCl showed negative correlations withTetragenococcus(P< 0.05),Staphylococcus(P< 0.05), andPseudomonas(P< 0.05) at the initial fermentation stage, but were positively correlated withLactobacillus(P< 0.01),Enterococcus(P< 0.05), andAcinetobacter(P< 0.05) at the later fermentation stages (Table 2, Fig. 6A). Amino acid nitrogen produced through proteolysis of proteins are consumed by microorganisms [51].Pseudomonasis one of the major components in kimchi [52]and doubanjiang [53]and is significantly correlated with nitrogenous compound metabolites [41,53]. Free sugars might be derived from polysaccharides through hydrolysis and can be consumed by lactic acid bacteria (LAB) to produce organic acids, including lactic acid and acetic acid [54]. The increased content of organic acids resulted in the decrease in pH.Staphylococcushas been reported with high tolerance against NaCl, and is able to grow in 15% NaCl [55-57]. This agrees with the result of CCA, indicating that in CBBPC fermentationStaphylococcusmay tolerate osmotic stress.Staphylococcushas been frequently found in meju, a Korean traditional soybean fermentation starter [58], Chinese traditional low-salt fermented whole fish [59],and the fermentation of Chinese strong- flavor liquor [60]. It has been reported that some strains ofStaphylococcuscan extend the shelf life of fermented foods by producing antimicrobial compounds [61], or improve the flavor due to lipolysis and proteolytic activity [62]. TheTianbanzisamples from T3 (60 days) were not only significantly correlated with a number of important genera but also associated with most of the metabolites (Fig. 6A). The most kinds and highest concentration of volatile flavor compounds were also detected in T3 samples. These results demonstrate that 60-day fermentation ofTianbanziis the best period for flavor formation.
For chilipeifermentation,Tetragenococcus(P< 0.01) andStaphylococcus(P< 0.05) were significantly correlated with pH(Table 2 and Fig. 6B) at the initial fermentation stage (J1 and J2).The two bacteria genera were negatively correlated with total acidity.Lactobacillus(P< 0.01),Pseudomonas(P< 0.05),Bacillus(P< 0.05), andWeissellawere negatively correlated with pH.Tetragenococcus,Lactobacillus, andWeissellaare all LAB. Their different correlations with total acidity are probably due to their different acid tolerance levels, resulting in the succession of bacteria along with the fermentation process. Previous studies found thatBacillusis correlated with organic acid in soy preprocessing [3,63,64].Chang et al. [65]reported thatBacillusproduces amylase and proteases duringdoenjangfermentation, which would provide the substrates for the formation of organic acids.Lactobacillus(P< 0.01),Bacillus(P< 0.05) andProteuswere positively correlated with amino acid nitrogen and NaCl.Tetragenococcus(P< 0.05) andStaphylococcuswere negatively correlated with NaCl. These results indicate that in chilipeifermentation stage,Lactobacillus,Bacillus, andProteuspossess higher NaCl tolerance thanTetragenococcusandStaphylococcus. Most of the flavor compounds (esters, phenols, and ketones) were positively correlated with the middle fermentation stage (210 days, J3). The addition ofLactobacillusduring vegetable fermentation can reduce the fermentation time [66,67].
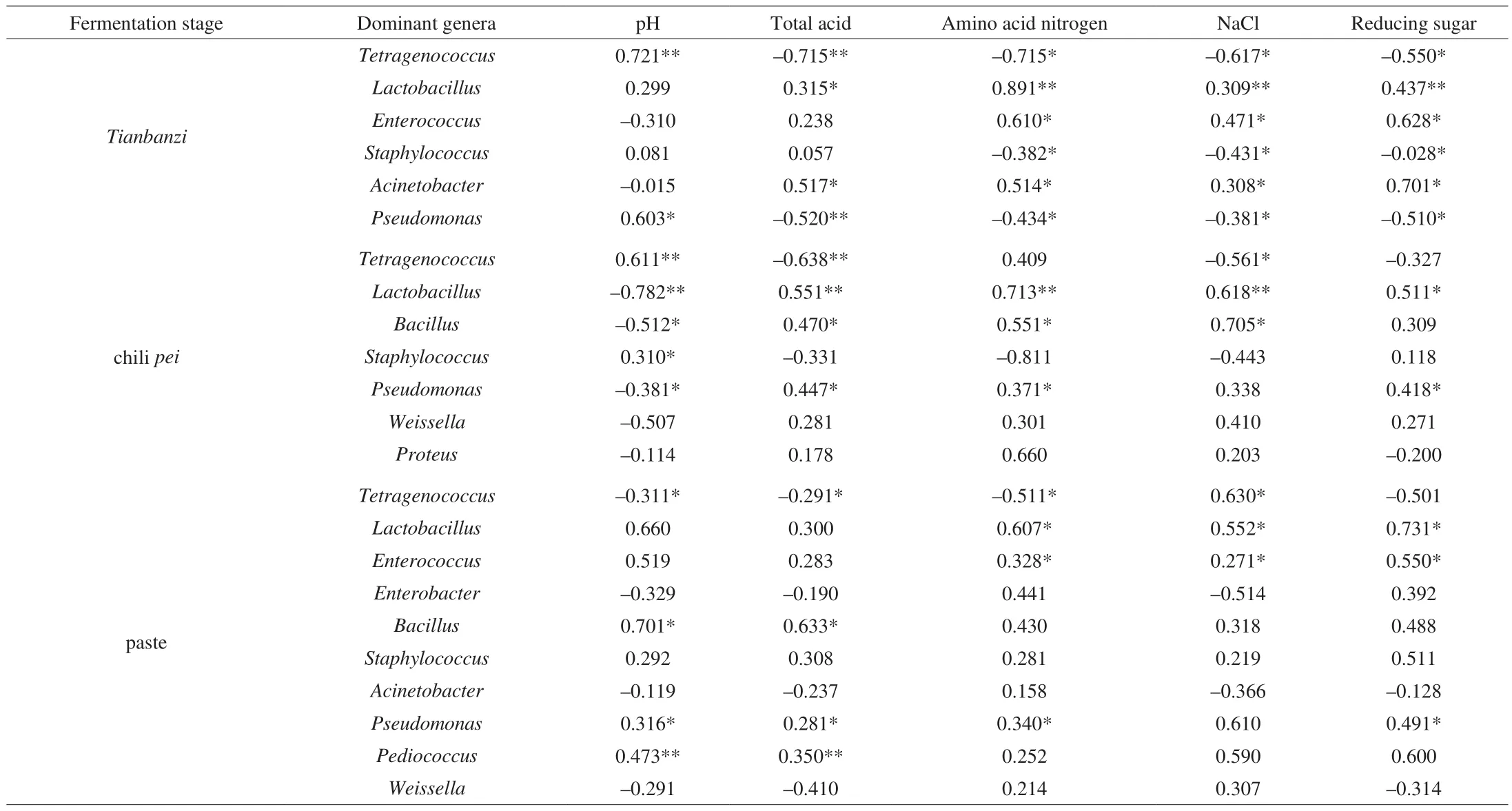
Table 2Pearson’s correlation between the relative abundance of dominant genera (relative abundance > 1%) and fermentation conditions variables.
For paste fermentation, total acidity and pH were positively correlated toPediococcus(P< 0.01),Pseudomonas(P< 0.05),andBacillus(P< 0.05), but were negatively correlated withTetragenococcus(P< 0.05) (Table 2, Fig. 6C).Pediococcushas also been reported to be related to the decrease in pH during fermentation [68].The total acidity declined at the paste fermentation stage (Fig. 2B).Therefore,Pediococcuscan be responsible for the sourness of paste.The correlation map showed thatEnterococcusandEnterobacterfeatured a close relationship to D1 and D2, respectively (Fig. 6C).Enterococcusis used as a starting culture for the fermentation of various foods, including cheese, milk, and sausages [69,70].Enterococcusmay also play a role in softening the texture and lowering the powdery and viscosity mouthfeel indoenjangfermentation [3]. D3 sample (180 days) was not only significantly correlated with numerous important genera but was also associated with most metabolites (Fig. 6C). This finding also proves that a 6-month duration is the optimal period for paste fermentation because many bacteria contribute to the formation of most flavor compounds during this fermentation period.
Considering the entire fermentation process, a high relationship exists between microorganisms and the fermentation process.Three (Tetragenococcus,Lactobacillus, andPseudomonas), four(Tetragenococcus,Lactobacillus,Bacillus, andPseudomonas), and five genera (Tetragenococcus,Lactobacillus,Bacillus,Pseudomonas,andPediococcus) were the core functional bacteria ofTianbanzi,chilipei, and paste fermentation, respectively. These genera were highly associated with different fermentation stages. However, the quality of fermented food by multi-microorganism is affected by a rich consortium of microbes rather than individual ones. This study presents the succession of bacterial communities throughout the three stages of CBBPC fermentation. In particular, the reconstruction of bacterial community after mixingTianbanziand chilipeiwas analyzed considering the changes in fermentation conditions. The characteristics offlavor compounds formed were provided to correlate the microbial assortments involved in each fermentation stage.
4. Conclusion
In this work, the entire fermentation process of CBBPC was continuously monitored up to 390 days. There were 89 genera shared in three individual stages, and 1, 1, and 3 genera exclusive toTianbanzi, chilipei, and paste stages, respectively. A high relationship exists between microorganisms and the fermentation process. The physicochemical properties are responsible for the succession of bacterial community, among which total acidity, pH, and NaCl are important endogenous factors. According to the dynamic of organic acid, reducing sugar, amino acid, and volatile compounds, 60-, 210-,and 180-day are the best fermentation periods forTianbanzi, chilipei,and paste, respectively, to balance time cost and product quality.
Conflict of interest
The authors have no conflicts of interest.
Acknowledgement
This work was supported by the National Key R&D Program of China (2016YFD0400505), China Postdoctoral Science Foundation (2018M640241), Tianjin Postdoctoral Foundation(TJQYBSH2018010), Tianjin Municipal Education Commission(TD13-5013, 2018ZD08), and Key Laboratory of Industrial Fermentation Microbiology, Education Ministry of China(2018KF005).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.03.011.
猜你喜欢
杂志排行
食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study
