Validation model of fibrosis-8 index score to predict significant fibrosis among patients with nonalcoholic fatty liver disease
2022-06-14ThanineePrasoppokakornWahKheongChanVincentWaiSunWongPanyaveePitisuttithumSanjivMahadevaNikRaihanNikMustaphaGraceLaiHungWongHowardHoWaiLeungPimsiriSripongpunSombatTreeprasertsuk
Thaninee Prasoppokakorn, Wah-Kheong Chan, Vincent Wai-Sun Wong, Panyavee Pitisuttithum, Sanjiv Mahadeva, Nik Raihan Nik Mustapha, Grace Lai-Hung Wong, Howard Ho-Wai Leung, Pimsiri Sripongpun,Sombat Treeprasertsuk
Abstract BACKGROUND Identifying hepatic fibrosis is crucial for nonalcoholic fatty liver disease (NAFLD)management. The fibrosis-8 (FIB-8) score, recently developed by incorporating four additional variables into the fibrosis-4 (FIB-4) score, showed better performance in predicting significant fibrosis in NAFLD.AIM To validate the FIB-8 score in a biopsy-proven NAFLD cohort and compare the diagnostic performance of the FIB-8 and FIB-4 scores and NAFLD fibrosis score (NFS) for predicting significant fibrosis.METHODS We collected the data of biopsy-proven NAFLD patients from three Asian centers in three countries. All the patients with available variables for the FIB-4 score (age, platelet count, and aspartate and alanine aminotransferase levels) and FIB-8 score (the FIB-4 variables plus 4 additional parameters: The body mass index (BMI), albumin to globulin ratio, gamma-glutamyl transferase level, and presence of diabetes mellitus) were included. The fibrosis stage was scored using nonalcoholic steatohepatitis CRN criteria, and significant fibrosis was defined as at least fibrosis stage 2.RESULTS A total of 511 patients with biopsy-proven NAFLD and complete data were included for validation. Of these 511 patients, 271 (53.0%) were female, with a median age of 51 (interquartile range: 41, 58) years. The median BMI was 29 (26.3, 32.6) kg/m2, and 268 (52.4%) had diabetes.Among the 511 NAFLD patients, 157 (30.7%) had significant fibrosis (≥ F2). The areas under the receiver operating characteristic curves of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis were 0.774, 0.743, and 0.680, respectively. The FIB-8 score demonstrated significantly better performance for predicting significant fibrosis than the NFS (P = 0.001) and was also clinically superior to FIB-4, although statistical significance was not reached (P = 0.073).The low cutoff point of the FIB-8 score for predicting significant fibrosis of 0.88 showed 92.36%sensitivity, and the high cutoff point of the FIB-8 score for predicting significant fibrosis of 1.77 showed 67.51% specificity.CONCLUSION We demonstrated that the FIB-8 score had significantly better performance for predicting significant fibrosis in NAFLD patients than the NFS, as well as clinically superior performance vs the FIB-4 score in an Asian population. A novel simple fibrosis score comprising commonly accessible basic laboratories may be beneficial to use for an initial assessment in primary care units, excluding patients with significant liver fibrosis and aiding in patient selection for further hepatologist referral.
Key Words: Nonalcoholic fatty liver disease; Fibrosis-8 score; Fibrosis-4 score; Nonalcoholic fatty liver disease fibrosis score
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a global health issue and has become the most common liver disease in Western countries, accounting for an estimated 25% of the adult population[1] and affecting an estimated 25%-30% of the adult population in the Asia Pacific region[2]. A meta-analysis in Asia during 1999 to 2019, described the overall pooled incidence rate was 50.9per1000 person-years[3].According to our previous study, the prevalence of significant fibrosis (defined as ≥ F2 fibrosis) is 18.4%in asymptomatic NAFLD patients[4]. Nonalcoholic steatohepatitis (NASH) has emerged as the most common cause of cryptogenic cirrhosis and hepatocellular carcinoma worldwide. The presence of hepatic fibrosis is the major determinant of future risk of mortality and liver-related morbidity[5], and detecting significant fibrosis is crucial for NAFLD because no well-accepted and proven therapy is available for this disease to date[6]. However, patients with F2 or higher are at a higher risk of long-term liver-related death than patients with F0-1. Those with significant fibrosis should be intensively followed up or considered to participate in the therapeutic trial for NAFLD.
Liver biopsy remains the gold standard for evaluating hepatic fibrosis. However, because of several drawbacks, including invasiveness, the risk of bleeding complications, intrinsic sampling and pathologist reader variability[7], and cost, noninvasive tests are more practical. Thus, the 2018 American Association for the Study of Liver Diseases (AASLD) practice guidance recommends the use of the fibrosis-4 (FIB-4) score, the NAFLD fibrosis score (NFS), vibration-controlled transient elastography, and magnetic resonance elastography[8] to identify those at low or high risk for advanced fibrosis [bridging fibrosis (F3) or cirrhosis (F4)]. Noninvasive tests using only clinical and routine laboratory parameters are inexpensive and particularly important in primary care or resource-limited settings where the pretest probability of advanced fibrosis is low because these scores have good negative predictive values (NPVs) to exclude advanced fibrosis[9]. Therefore, using simple fibrosis scores as an initial assessment in primary care is reasonable. The FIB-4 score comprises four parameters, age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelets, while the NFS score comprises six parameters in addition to those comprising the FIB-4 score, such as the body mass index (BMI),presence of diabetes, and serum albumin level[10].
According to Sripongpunet al[11], their AASLD 2019 abstract reported a new model for a fibrosis-8 score (FIB-8) score developed by incorporating the following four additional variables: BMI,albumin/globulin (A/G) ratio, gamma-glutamyl transferase (GGT) level, and diabetes. The subjects were enrolled in the PIVENS and FLINT trials, of which 522 participants all had histologically confirmed NASH[12,13]. The optimal low and high cutoffs for the FIB-8 score to exclude and include F ≥2 were < 0.88 and ≥ 1.77, respectively, with a sensitivity of 95.3% and a specificity of 79.2%. The areas under the receiver operating characteristic curves (AUROCs) of the FIB-8 score were 0.79 and 0.78 in the training and validation datasets, respectively. The FIB-8 score provided significantly better AUROCs than the FIB-4 score (P< 0.001) and NFS (P= 0.005) in the validation dataset for predicting significant and advanced fibrosis in NAFLD patients. Following the study, the field test and validation of the FIB-8 score in a real-world cohort of NAFLD patients revealed that the AUROCs of the FIB-8 score were 0.84 with imputed data (n= 130) and 0.91 when only patients with complete data without imputation were included (n= 31). The FIB-8 score again outperformed the FIB-4 score and NFS, with AUROCs of 0.86vs0.80 and 0.77, respectively, for diagnosing advanced fibrosis (F3)[14].
To our best knowledge, no validation of the FIB-8 score has been reported in a larger cohort.Therefore, this study was to validate the FIB-8 score in a biopsy-proven NAFLD cohort and compare the diagnostic performance of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis.
MATERIALS AND METHODS
Study population and data collection
We collected the data of biopsy-proven NAFLD patients from the following three Asian centers in three countries: (1) Chulalongkorn University, Thailand; (2) The Chinese University of Hong Kong, Hong Kong; and (3) University of Malaya, Malaysia. The data from Thailand were collected from April 2008 to May 2019, those from Hong Kong were collected from July 2006 to November 2017, and those from Malaysia were collected from November 2012 to October 2015.
NAFLD was diagnosed based on ultrasonographic findings of fatty liver as well as transient elastography and the exclusion of viral hepatitis B and C infection, significant alcohol intake, and current usage of medications causing hepatic steatosis. Only patients with biopsy-proven NAFLD were included. Patients with other causes of chronic liver disease, incomplete histological data, and without significant hepatic steatosis were excluded. The laboratory data for the FIB-4 score (age, platelet count,and aspartate and ALT levels), FIB-8 score [the FIB-4 variables plus 4 additional parameters: The BMI,albumin to globulin ratio, gamma-glutamyl transferase level, and presence of diabetes mellitus (DM)],and the NFS were collected. The time interval between the enrolled laboratories and the date of liver biopsy was within 1 year. The fibrosis stage was scored using the NASH Clinical Research Network(CRN) criteria, and significant fibrosis was defined as at least fibrosis stage 2 (F ≥ 2).
Noninvasive methods
We validated the noninvasive methods from the FIB-8 score, FIB-4 score, and NFS and the test variables for predicting significant fibrosis (Table 1)[11,15,16].
Outcomes
We aimed to validate the FIB-8 score in a biopsy-proven NAFLD cohort and compare the diagnostic performance of the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis (≥ F2) in an Asian cohort.
Ethical permission
The study was reviewed and approved by the Institutional Review Board, Faculty of Medicine,Chulalongkorn University, Bangkok, Thailand (IRB number 238/59). This is a retrospective study, and signed informed consent was waived by the Ethics Committee. The analysis used anonymous clinical data after each patient agreed to treatment by written consent.
Statistical analysis
Categorical and continuous variables were compared between patients with and without significant fibrosis using Chi-squared and Student’s t-test or the Wilcoxon rank-sum test (according to the distribution of the data), respectively. Most of the numerical values did not follow a normal distribution and were expressed as medians and interquartile ranges. The diagnostic performance of each scoring system was then evaluated using receiver operating characteristic curves, and comparisons between the correlated AUROCs were performed using DeLong’s test[17]. The sensitivities (Sens) and specificities(Spec) of each scoring system were analyzed using the given low and high cutoffs for predicting F2, as reported previously-i.e., 0.88 and 1.77 for the FIB-8 score, 0.81 and 1.81 for the FIB-4 score, and -2.45 and 0.03 for the NFS, respectively[11,18]. All statistical analyses were performed using the SPSS statistical analysis package (version 18.0.0; SPSS Inc., Chicago, Illinois, United States), Stata (version 15;StataCorp), and R program version 4.1.1. APvalue < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
A total of 1013 patients with biopsy-proven NAFLD were included in the database. Of those, 511 patients had complete data on variables, including the NFS and FIB-4 and FIB-8 scores, and were eligible for the current study (Figure 1). Of the 511 patients, 271 (53.0%) were female, with a median age of 51 [interquartile range (IQR): 41, 58] years. The median BMI was 29 (26.3, 32.6) kg/m2, and 268(52.4%) had diabetes. Among the 511 NAFLD patients, 157 (30.7%), 88 (17.2%), and 16 (3.1%) patients had significant fibrosis (≥ F2), advanced fibrosis (≥ F3), and cirrhosis (F4), respectively. The baseline characteristics comparing NAFLD F0-1 and significant fibrosis (F ≥ 2) are shown in Table 2. The significant factors associated with significant fibrosis were an older age [55 (48, 61)vs49.5 (39, 57) years;P< 0.001], the presence of diabetes (71.3%vs44.0%;P< 0.001), higher levels of AST [53.5 (36, 75)vs35(26, 52) U/L;P< 0.001], ALT [75 (50, 111)vs59.5 (40, 98) U/L;P< 0.001] and GGT [81 (48, 151)vs56.5(35, 92) U/L;P< 0.001], a lower platelet count [230 (189, 277)vs266 (226.8, 302) × 109/cu.mm;P< 0.001],lower levels of total cholesterol [182 (159, 209)vs193 (170, 220) mg/dL;P= 0.004] and LDL-cholesterol[107 (85, 132)vs116 (96, 143) mg/dL;P= 0.003], and a higher median Controlled Attenuation Parameter(CAP) [324 (294, 347)vs299 (211, 339) dB/m] (Table 2).
Performance of the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis (≥ F2)
The AUROCs of the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis were 0.774(95%CI: 0.729-0.820), 0.743 (95%CI: 0.695-0.791), and 0.680 (95%CI: 0.630-0.730), respectively (Figure 2).The FIB-8 score showed a significantly better performance for predicting significant fibrosis (≥ F2) than the NFS (P= 0.001) and was numerically higher than the FIB-4 score, but the difference was not statistically significant (P= 0.073). The sensitivities and specificities of the cutoffs specified to exclude and include significant fibrosis for each score are reported in Table 3.
Diagnostic accuracy of the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis (≥ F2)by age group
The cohort was stratified by age into three groups: Age < 35 (n= 66), 35-65 (n= 412), and > 65 years (n=33). The AUROCs of the FIB-8 score, FIB-4 score, and NFS in patients aged 35-65 years for predicting significant fibrosis were 0.79, 0.76, and 0.68, respectively. This patient group comprised most of the cohort and had similar diagnostic performance results as the entire cohort. However, the FIB-8 score,FIB-4 score, and NFS were poor in patients aged < 35 years (AUROC: 0.55, 0.59, and 0.70, respectively)and > 65 years (AUROC: 0.66, 0.71, and 0.54, respectively). The number of patients in each age groupand center is shown in Supplementary Table 1. A detailed summary of the AUROC, sensitivity,specificity, positive predictive value, and NPV for the FIB-8 score, FIB-4 score, and the NFS is shown in Supplementary Table 2.
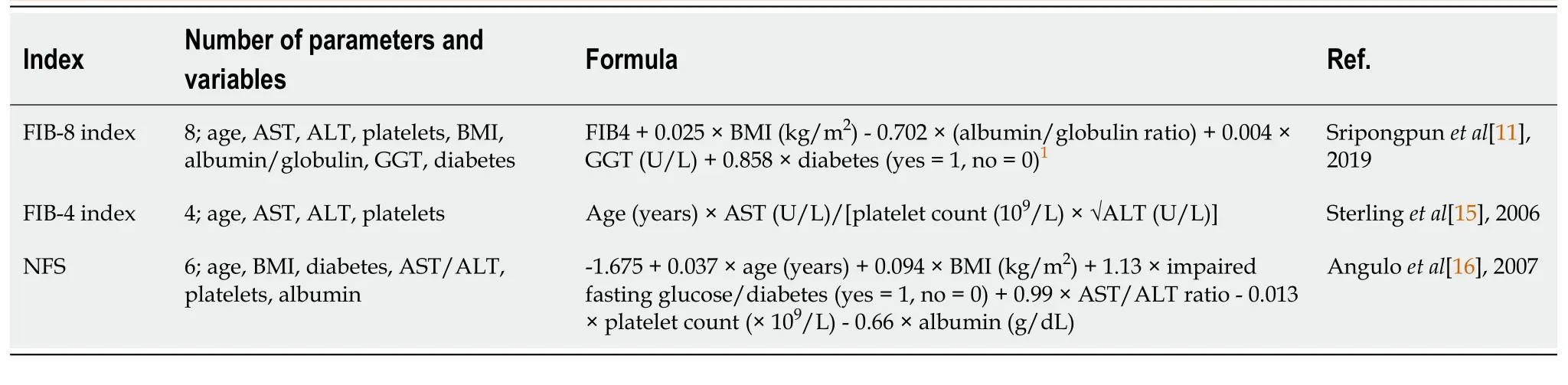
Table 1 Details of the three noninvasive methods used in this study
DISCUSSION
Based on the results of the present study, we validated the diagnostic performance of the FIB-8 score,FIB-4 score, and NFS score in 511 biopsy-proven NAFLD patients for predicting significant fibrosis. The main issue affecting the diagnostic ability of new methods for detecting liver fibrosis in NAFLD patients is the prevalence of fibrosis among the particular population. Our results demonstrated that the overall prevalence rates of significant fibrosis (≥ F2), advanced fibrosis (≥ F3), and cirrhosis (F4) were 157(30.7%), 88 (17.2%), and 16 (3.1%), respectively. The mean incidence rates of significant fibrosis from previous publications were 52.5% and 35.4% in the PIVENS plus FLINT trials and a Stanford University trial, respectively[11,14] (Table 4). The remarkable aspects were as follows: (1) Our study had a lower incidence of fibrosis than the first cohort; (2) Among the noninvasive methods, the FIB-8 score and NFS included the BMI in their models, and our cohort had a lower mean BMI than previous reports (30.4 kg/m2vs34.0 and 31.5 kg/m2), which might have resulted in lower percentages of sensitivity and specificity in our cohort than those previously reported; and (3) GGT is a uniquely incorporated variable in the new FIB-8 scoring system. Some reported studies have demonstrated that a higher GGT level is a risk factor for advanced fibrosis in NAFLD[19,20]. Additionally, considering NAFLD patients with type 2 DM, a serum GGT level over 82 U/L was independently associated with advanced fibrosis using noninvasive methods in multivariate analysis (P= 0.004)[21]. In our study, the baseline characteristics correlatively showed that a higher level of median GGT was a significant factor associated with significant fibrosis [81 (IQR: 48, 151)vs56.5 (35, 92);P< 0.001]. We postulated that GGT may be an additional variable predicting significant fibrosis in NAFLD patients. The diagnostic performance of the FIB-8 score exhibited higher accuracy for diagnosing significant fibrosis (≥ F2) than the NFS but was not superior to the FIB-4 score in previous studies or our study; the AUROCs for the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis were 0.774, 0.743, and 0.680, respectively (FIB-8vsNFS,P= 0.001; FIB-8vsFIB-4,P= 0.073). The sensitivities of the low cutoff of FIB-8 score to exclude significant fibrosis was 92.36%. Consequently, the high sensitivity and NPV for excluded significant fibrosis may be beneficial in primary care units and to select patients for further hepatologist referral.However, the limited specificity of the high cutoff of FIB-8 score to include significant fibrosis may require further step assessment instance transient elastography.
Furthermore, our results demonstrated that the FIB-4 score offered better diagnostic performance than the NFS score (P< 0.001). According to meta-analysis results from Castera[10], the FIB-4 score and NFS showed the best diagnostic performance for detecting advanced fibrosis compared with other blood-based models. However, this meta-analysis included studies that used different cut-off thresholds. Furthermore, a recent meta-analysis from Castellanaet al[22] reported a head-to-head comparison of the FIB-4 score and NFS from 18 studies that used consistent cutoffs. The FIB-4 score offered higher performance for including and NFS for excluding advanced fibrosis. However, our studies used different cutoffs and aimed to predict significant fibrosis, not advanced fibrosis.Consequently, our cohort was not suitable to compare the FIB-4 score and NFS.
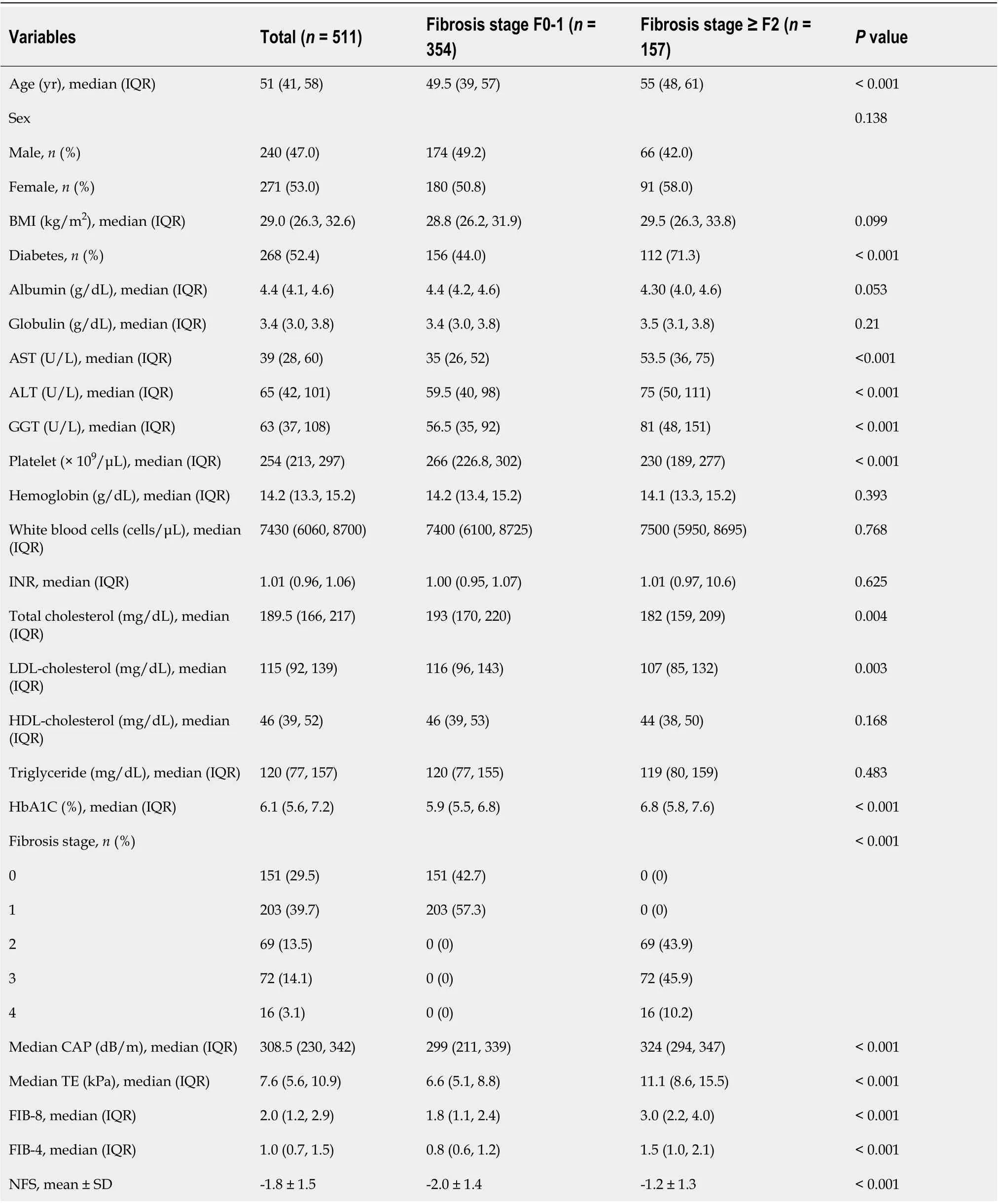
Table 2 Characteristics of patients with F0-1 fibrosis compared to those with F ≥ 2 fibrosis stage (n = 511)
Additionally, our results demonstrated the performance of the FIB-8 score, FIB-4 score, and NFS in patients aged > 65 years (AUROC: 0.66, 0.71, and 0.54, respectively). The performance was poor in patients aged < 35 years (AUROC: 0.55, 0.59, and 0.70, respectively). Thus, these scores have insufficient accuracy for use in NAFLD patients in extreme age groups. Similarly, McPhersonet al[23] demonstrated age as a confounding factor for the accurate noninvasive scoring system predicting advanced fibrosis[23]. The FIB-8 score has low accuracy for predicting significant fibrosis in NAFLD patients, similar tothe FIB-4 score and NFS in patients aged < 35 and > 65 years.

Table 3 Performance of fibrosis-8, fibrosis-4, and nonalcoholic fatty liver disease fibrosis score for predicting significant fibrosis (F ≥ 2)in the Asian population (n = 511)

Table 4 Comparison of study population using the fibrosis-8 score for predicting significant fibrosis (F ≥ 2)
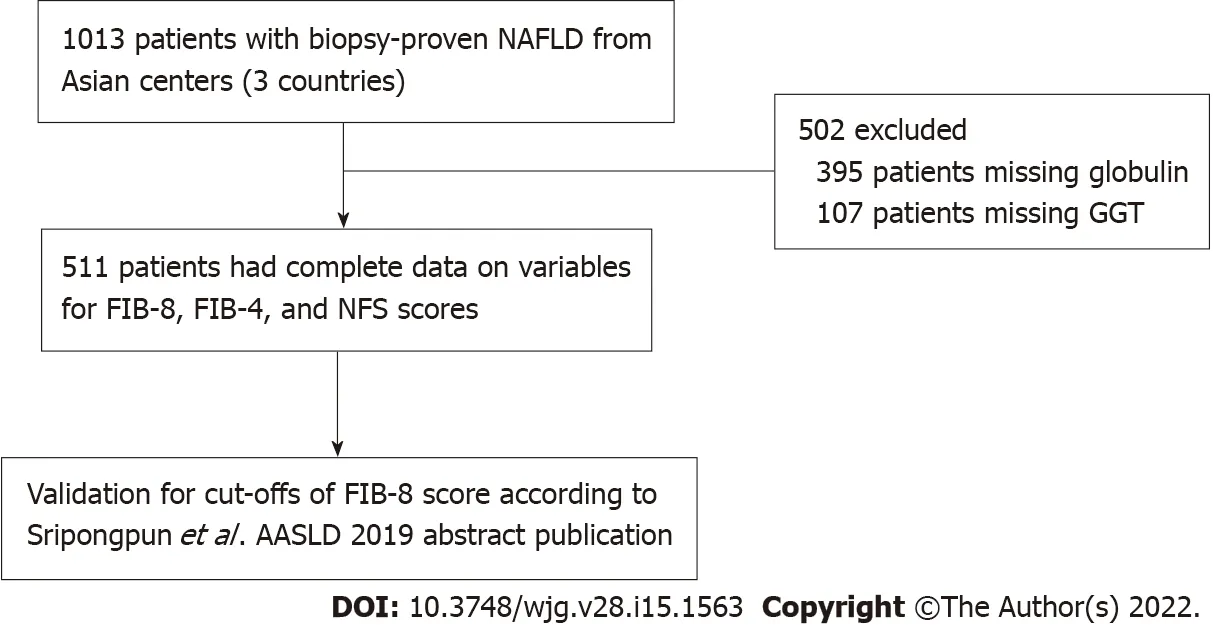
Figure 1 Flow diagram of the study population. NAFLD: Nonalcoholic fatty liver disease; FIB-8: Fibrosis-8 score; FIB-4: Fibrosis-4 score; NFS: NAFLD fibrosis score; AASLD: American Association for the Study of Liver Diseases; GGT: Gamma-glutamyl transferase.
Our study had limitations. First, we had limited complete data for half of our database because of the lack of either globulin or GGT. In usual clinical practice, clinicians do not routinely check both laboratory parameters, and no added value exists for observing or monitoring these values in patients.The second limitation of our study was the lower incidence of fibrosis in our cohortvsother cohorts.The differences in fibrosis may have diagnostic value for novel fibrosis scores for validation. Validations in larger cohorts are needed.
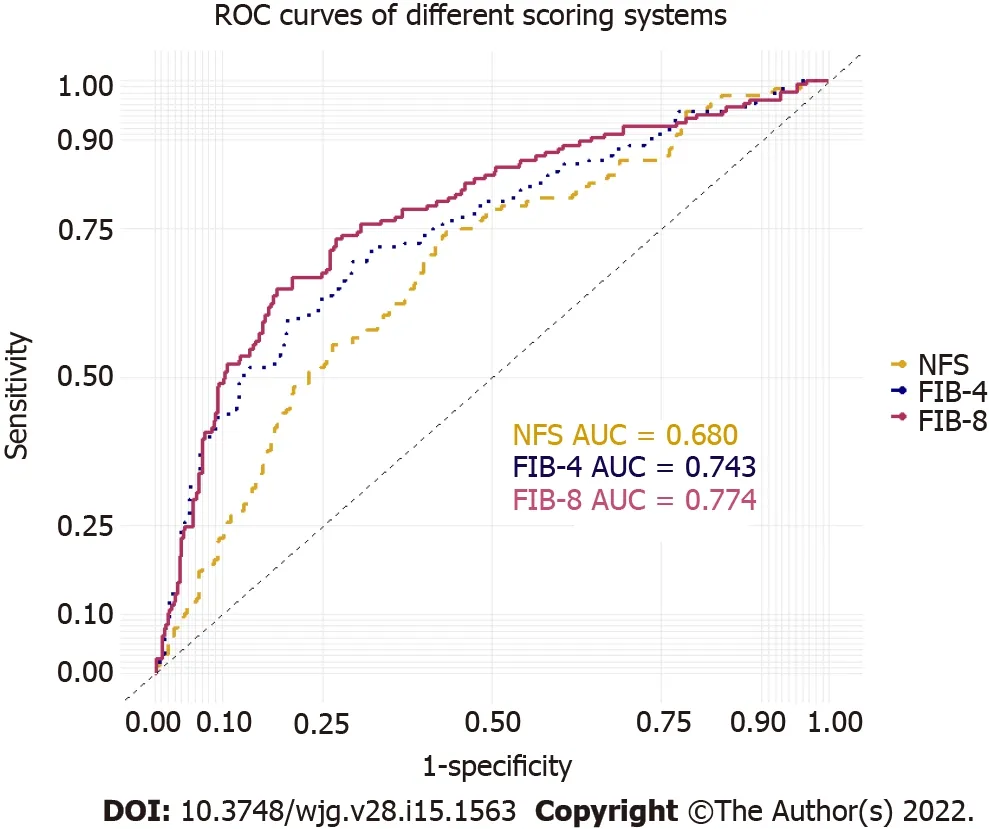
Figure 2 Receiver operating characteristic curves of the fibrosis-8 score, fibrosis-4 score, and nonalcoholic fatty liver disease fibrosis score for predicting significant fibrosis (F ≥ 2) in the Asian population (n = 511). NAFLD: Nonalcoholic fatty liver disease; FIB-8: Fibrosis-8 score;FIB-4: Fibrosis-4 score; NFS: NAFLD fibrosis score; AUROC: Areas under the receiver operating characteristic curves.
To our best knowledge, our study is the first to report a new validation model of the FIB-8 score for predicting significant fibrosis among patients with NAFLD in an Asian population. The FIB-8 score yielded higher accuracy in diagnosing significant fibrosis than the NFS. Additionally, the FIB-8 score was non-inferior but insignificantly superior to the FIB-4 score. A novel simple fibrosis score comprising commonly accessible basic laboratories may be additionally used to add previous fibrosis scores for an initial assessment in primary care units and to select patients for further hepatologist referral.
CONCLUSION
The new, simple fibrosis FIB-8 score had significantly better performance for predicting significant fibrosis in NAFLD patients than the NFS and was non-inferior but insignificantly superior to the FIB-4 score in the Asian population. A simple fibrosis score comprising commonly accessible basic laboratories may be used for an initial assessment in primary care units and to select patients for further hepatologist referral.
ARTICLE HIGHLIGHTS
Research background
In the nonalcoholic fatty liver disease (NAFLD) population, noninvasive fibrosis scores, such as the fibrosis-4 (FIB-4) score and NAFLD fibrosis score (NFS), are generally applied in clinical practice guidelines. The novel fibrosis-8 (FIB-8) score yielded higher accuracy in diagnosing significant fibrosis in a previously reported cohort. A larger cohort may provide more reliability and benefit in clinical practice.
Research motivation
A noninvasive fibrosis score in NAFLD patients using only routine laboratory parameters is particularly important in initial assessment in the primary care unit or resource-limited conditions. We proposed the novel FIB-8 score, which incorporates the additional variables body mass index (BMI), the A/G ratio,gamma-glutamyl transferase (GGT), and diabetes into the FIB-4 score. The additional variables, particularly GGT, may provide better diagnostic accuracy for predicting significant fibrosis in NAFLD patients.
Research objectives
We aimed to validate the FIB-8 score among patients with a biopsy-proven NAFLD cohort and to compare the diagnostic performance of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis.
Research methods
This was a retrospective study involving 1013 biopsy-proven NAFLD patients from 3 Asian centers in 3 countries in an Asian population. All the patients with available baseline biochemical tests for the FIB-8 score calculation and all related variables for predicting liver fibrosis were included.
Research results
A total of 1013 patients were included in the final analysis. Of those, 511 patients had complete data on the variables, including the NFS and FIB-4 and FIB-8 scores. One hundred fifty-seven (30.7%) patients had significant fibrosis (≥ F2). The areas under the receiver operating characteristic curves of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis were 0.774, 0.743, and 0.680, respectively.The FIB-8 score had significantly better performance for predicting significant fibrosis than the NFS (P=0.001) but was not superior to the FIB-4 score (P= 0.073). The low cutoff point of the FIB-8 score for predicting significant fibrosis of 0.88 showed 92.36% sensitivity, and the high cutoff point of the FIB-8 score for predicting significant fibrosis of 1.77 had 67.51% specificity.
Research conclusions
The FIB-8 score, which incorporates the additional variables of the BMI, A/G ratio, GGT level, and diabetes into the FIB-4 score, yielded better performance for predicting significant fibrosis in NAFLD patients than the NFS but was not superior to the FIB-4 score in the Asian population. A simple fibrosis score comprising commonly accessible basic laboratories may be used for an initial assessment in primary care units.
Research perspectives
Future prospective studies are needed to compare the diagnostic accuracy of various noninvasive scores for predicting significant fibrosis and staging fibrosis.
ACKNOWLEDGEMENTS
We would like to thank the supporting team of the Gut and Obesity in Asia workgroup for the database.Additionally, we would like to thank the research coordinator and statistician, Kanokwan Sornsiri, and Chonlada Phathong, from the Division of Gastroenterology, King Chulalongkorn Memorial Hospital.
FOOTNOTES
Author contributions:Treeprasertsuk S designed the study; Pitisuttithum P, Chan WK, Wong VWS, and Treeprasertsuk S contributed to data acquisition; Mahadeva S and Wong GLH recruited and managed the patients;Mustapha NRN and Leung HHW performed the histological assessment; Prasoppokakorn T, Pitisuttithum P,Sripongpun P, and Treeprasertsuk S analyzed and interpreted the data; Prasoppokakorn T drafted the manuscript;Chan WK, Sripongpun P, Wong VWS, and Treeprasertsuk S revised the manuscript critically for important intellectual content; all the authors read and approved the final manuscript.
Supported byThe Fatty Liver Research Fund, Faculty of Medicine Foundation, Chulalongkorn University.
Institutional review board statement:The study was reviewed and approved by the Institutional Review Board,Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, No. 238/59.
Informed consent statement:This is a retrospective study, and an exemption of a signed informed consent application was approved by the Ethics Committee. The analysis used anonymous clinical data after each patient agreed to treatment by written consent.
Conflict-of-interest statement:There are no conflicts of interest to report.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement-a checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-a checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Thailand
ORCID number:Thaninee Prasoppokakorn 0000-0002-1012-9874; Wah-Kheong Chan 0000-0002-9105-5837; Vincent Wai-Sun Wong 0000-0003-2215-9410; Panyavee Pitisuttithum 0000-0002-3530-3621; Sanjiv Mahadeva 0000-0003-0021-8596; Nik Raihan Nik Mustapha 0000-0002-4326-882X; Grace Lai-Hung Wong 0000-0002-2863-9389; Howard Ho-Wai Leung 0000-0001-9930-1783; Pimsiri Sripongpun 0000-0003-0007-8214; Sombat Treeprasertsuk 0000-0001-6459-8329.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Gastroenterology的其它文章
- Liquid biopsy in colorectal cancer: No longer young, but not yet old
- Novel approaches in search for biomarkers of cholangiocarcinoma
- Establishing a rabbit model of perianal fistulizing Crohn’s disease
- Reevaluation of the expanded indications in undifferentiated early gastric cancer for endoscopic submucosal dissection
- Prognostic factors of recurrent intrahepatic cholangiocarcinoma after hepatectomy: A retrospective study
- Development and validation of a prediction model for moderately severe and severe acute pancreatitis in pregnancy
