Novel approaches in search for biomarkers of cholangiocarcinoma
2022-06-14LaviniaPatriciaMocanMariaIlieCarmenStancaMelincoviciMihaelaSprchezRareCrciunIulianaNenuAdelinaHorhatCristianTefasZenoSprchezCristinaAdelaIugaTudorMocanCarmenMihaelaMihu
Lavinia-Patricia Mocan, Maria Ilieș, Carmen Stanca Melincovici, Mihaela Spârchez, Rareș Crăciun, Iuliana Nenu, Adelina Horhat, Cristian Tefas, Zeno Spârchez, Cristina Adela Iuga, Tudor Mocan, Carmen Mihaela Mihu
Abstract Cholangiocarcinoma (CCA) arises from the ductular epithelium of the biliary tree,either within the liver (intrahepatic CCA) or more commonly from the extrahepatic bile ducts (extrahepatic CCA). This disease has a poor prognosis and a growing worldwide prevalence. The poor outcomes of CCA are partially explained by the fact that a final diagnosis is challenging, especially the differential diagnosis between hepatocellular carcinoma and intrahepatic CCA, or distal CCA and pancreatic head adenocarcinoma. Most patients present with an advanced disease, unresectable disease, and there is a lack in non-surgical therapeutic modalities. Not least, there is an acute lack of prognostic biomarkers which further complicates disease management. Therefore, there is a dire need to find alternative diagnostic and follow-up pathways that can lead to an accurate result, either singlehandedly or combined with other methods. In the "-omics" era, this goal can be attained by various means, as it has been successfully demonstrated in other primary tumors.Numerous variants can reach a biomarker status ranging from circulating nucleic acids to proteins,metabolites, extracellular vesicles, and ultimately circulating tumor cells. However, given the relatively heterogeneous data, extracting clinical meaning from the inconsequential noise might become a tall task. The current review aims to navigate the nascent waters of the non-invasive approach to CCA and provide an evidence-based input to aid clinical decisions and provide grounds for future research.
Key Words: Cholangiocarcinoma; Biomarker; Proteomics; Metabolomics; Extracellular vesicles; Circulating nucleic acids
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor arising from the biliary epithelial cells. The latest World Health Organization Classification of Tumors-Digestive System Tumors acknowledges the heterogeneous nature of CCA, emphasizing the importance of tumor localization. In this matter, there are two main types of CCAs: Intrahepatic CCA (iCCA) and extrahepatic CCA (eCCA) [including both:Perihilar CCA (pCCA) and distal (dCCA)], featuring different aspects in etiology, molecular alterations,pathogenesis, behavior, potential diagnostic or prognostic biomarkers and hence a different clinical management[1].
iCCAs represent approximately 10%-15% of liver tumors and the second primary liver malignancy,after hepatocellular carcinoma (HCC)[2], while eCCAs account for 0.5-2 cases/10.000 person-years[3].Although considered a relatively rare type of cancer, the incidence of CCA is rising in most geographic areas[4]. Both HCC and iCCA, although they are considered different diseases, do share some common risk factors including hepatitis B or C, non-biliary hepatic cirrhosis, alcoholic and non-alcoholic steatohepatitis, or metabolic syndrome. On the other hand, eCCA typically occurs in conditions associated with chronic biliary inflammation, such as primary sclerosing cholangitis, lithiasis, cysts, or liver fluke infections. In most cases, the exact etiology remains difficult to pinpoint[5].
To this point, CCA is notoriously difficult to diagnose. Diagnosing these tumors requires the correlation of clinical, imaging, and, when available, histopathologic data. In terms of treatment,surgical resection with curative intent remains the best option. However, most patients with CCA(approximately 70%) are diagnosed at late stages due to lack of specific symptoms[6]. Mortality rates are high, and thus the prognosis is poor[7], especially in the case of large tumors, satellite nodules, vascular or lymphatic invasion, positive resection margins, or advanced pathological tumor-node-metastasis stages (TNM)[8,9]. For surgically resectable tumors, the 5-year survival rate reaches 20%-30%, but the percentage drops to a bitter 0% for the rest of the cases[10]. After surgery, the recurrence rate is relatively high, reaching from 49% to 70%[11] and relapse occurs early, typically within 2 or 3 years after surgery[8].
These circumstances emphasize the necessity of novel, clinical-suited tools that would serve for early diagnosis, as prognostic indicators or in treatment guidance, such as biomarkers.
Biomarkers were defined by the Food and Drug Administration-National Institute of Health Biomarker Working Group back in 2016 as “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention,including therapeutic interventions. Molecular, histologic, radiographic, or physiologic characteristics are types of biomarkers.”. While α-fetoprotein (AFP) is the most convenient and non-invasive serum biomarker for detecting HCC, elevated AFP was observed only in approximately 20% of a subgroup of CCA, namely iCCA patients[12].
The charbohydrate antigen 19-9 (CA19-9) is currently used worldwide in clinical practice as a nonspecific serum marker for orientation in diagnosing CCA, but it bears a low sensitivity in European patients[13]. In terms of prognosis there are some validated tools that are useful in the clinical practice.These markers are not specific for CCA but rather apply to all human malignancies. Tumor size and differentiation, vascular involvement, lymph node status, margin status and presence of occult metastasis were all shown to be good predictors for overall survival (OS) for both iCCA and eCCA[14].
One option could be the study of tumor tissue in search of novel biomarkers. This strategy appeared to be fruitful, as several tumor tissue-based biomarkers were already identified. Mutations in TP53 and KRAS proto-oncogene are associated with an impaired outcome-lower OS and higher tumor recurrence than other mutations in resected CCA while several other genetic signatures with prognostic potential include epidermal growth factor receptor (EGFR), mucin 1 (MUC1), MUC4, and fascin (FSCN)expression[15]. Moreover, alteration in targetable pathways [e.g., fibroblast growth factor receptor 2 gene (FGFR2) involved in MAP kinase signaling, isocitrate dehydrogenase 1 and 2 (IDH1, IDH2)] were also depicted in CCA patients[16] and currently, several clinical trials are actively recruiting patients.Nevertheless, several microRNAs (miRs) expressions in tissue or deregulated immune responses[expression levels of cytotoxic T-lymphocyte antigen 4 (CTLA-4), forkhead box P3 (FOXP3), and programmed death-ligand 1 (PD-L1)] might have predictive capabilities in CCA patients[17,18]. Many other diagnostic and prognostic tissue-derived biomarkers have already been previously described[19].
Unfortunately, biopsy collection for tissue analysis is not an ideal biospecimen for biomarker assessment and translation to clinical practice. Although it offers absolute insights into tumor biology,the collection procedure presents several caveats and poses the risk of serious clinical complications[20]. As an alternative to tissue biopsy, a much more reliable biospecimen, already implemented in the clinical practice with several advantages over tissue, is the liquid biopsy (blood). Serum, plasma, or urine, collected non-invasively using well-established low-cost techniques are considered "ideal fluids"in biomarker research. Moreover, liquid biopsy encloses molecules from the whole body, and a single sample can offer a wide range of information and is enough for multiple measurements.
The current review aims to explore the nascent waters of the non-invasive biomarkers reported for CCA by taking an in-depth look at the fields of circulating nucleic acids, proteomic and metabolomicderived biomarkers, extracellular vesicles, and circulating tumor cells (Figure 1) and provide an evidence-based input which could provide grounds for future research to pave the way for prospective validation and translation into the clinical practice of novel biomarkers. In this review the term CCA will make reference to all types of CCA, while the terms iCCA, hCCA, pCCA, and dCCA will stand for intrahepatic, hilar, perihilar and distal CCA, respectively.
CIRCULATING NUCLEIC ACIDS
Circulating nucleic acids represent snippets of genetic material, either DNA (cell-free DNA-cfDNA) or RNA (usually miR), reaching various fluid compartments (serum, urine, bile) through active cellular export or following cell death. The road from bench to bedside for circulating nucleic acids has taken a relatively long time and has not quite reached the point of clinical applicability in cancer diagnosis.However, more than four decades have passed between the initial proof-of-concept[21] and present-day genome-wide cfDNA mutational integration[22]. The world of cfDNA and miRs seems to be emerging more promising than ever, as the highly effervescent field has started to deliver on the early expectations. Moreover, CCA might provide a unique setting for the method to flourish: A conventional diagnostic challenge, sometimes a hard to biopsy tumor, all while having a relatively underdeveloped therapeutic arsenal.
CfDNA–the mutational fingerprint
The analysis of circulating cfDNA can provide a quick, complete, and non-invasive mutational profile of any tumor, by amplifying each mutation encountered throughout the tumor burden. The method reflects the entirety of mutations, thus not being the subject of selection bias in the case of heterogeneous cancers. More specifically, tissue samples can provide the mutational palette only for the available specimen. Therefore, the genetic fingerprint of a tumor might be incomplete, as metastases or distant regions of a tumor might have additional alterations. Consequently, cfDNA provides (at least in principle) a better understanding of the disease, with a substantial impact in disease management, from diagnostics to guide therapeutic choices.
This concept has been recently validated for CCA, using plasma samples of patients with fully characterized mutation status[23]. According to the study design, 31 mutations in theKRAS,NRAS,BRAF, andPIK3CAgenes were screened using multiplex polymerase chain reaction (PCR) and further quantified. The results were then compared with the mutational profile of the primary tumor, resulting in a perfect match. These results were partially reinforced by the work of a German team that performed the deep sequencing of 15 genes involved in CCA (n= 32), revealing a 74% overall blood-tissue sample concordance and 92% for intrahepatic tumors. Moreover, the patients were followed throughout chemotherapy, during which 63% of the patients had their mutational fingerprint altered[24]. This finding might have particular implications regarding treatment selection, especially in the case of loss of response. There is evidence of resistance to BGJ398, a pan-FGFR inhibitor, due tode novopoint mutations in the FGFR-2 kinase domain, revealed by cfDNA analysis[25].
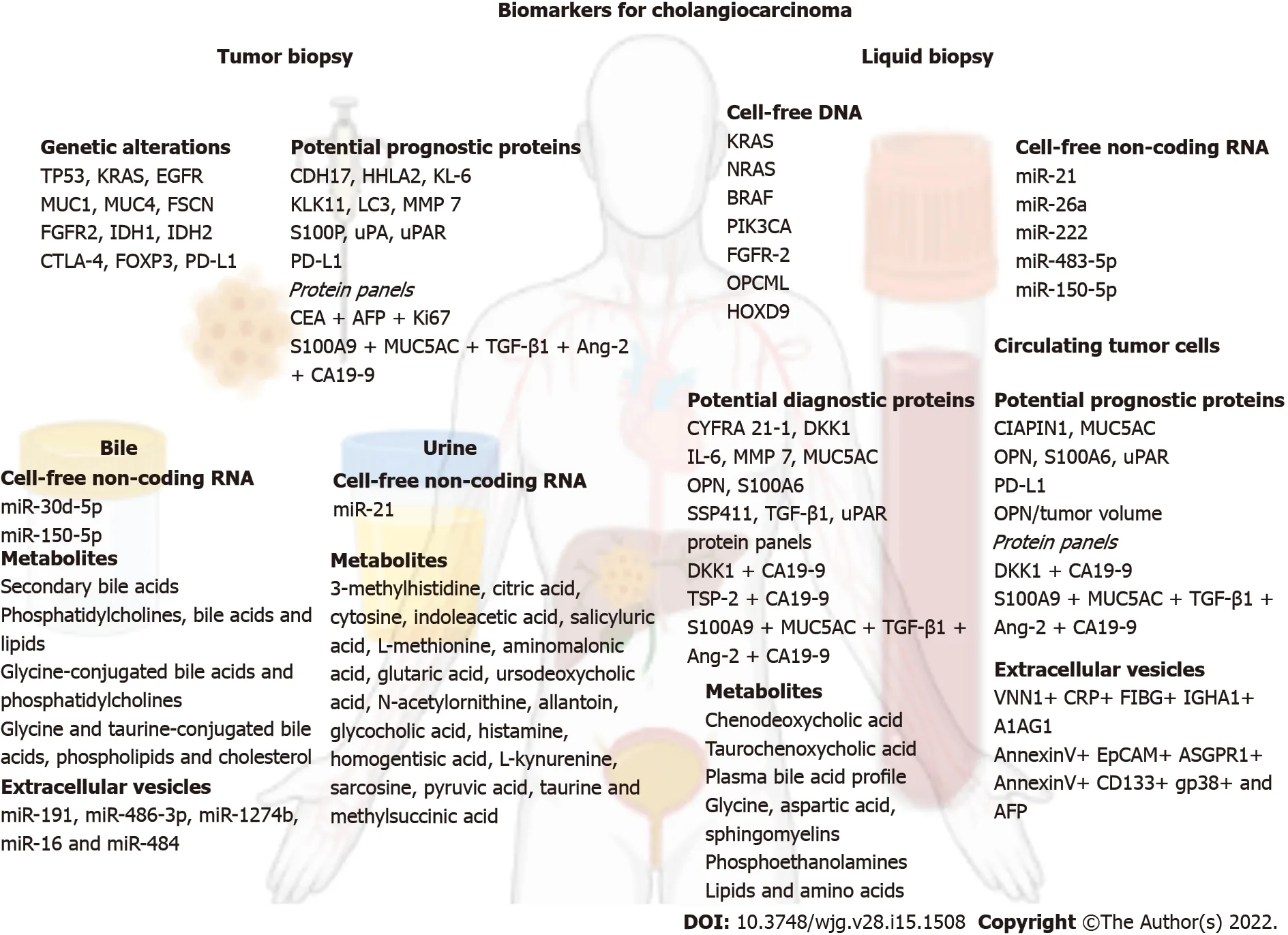
Figure 1 An overview on the biomarkers for cholangiocarcinoma. Created with biorender.com. A1AG1: Alpha-1 acid glycoprotein; AFP: Alpha fetoprotein; Ang-2: Angiopoietin-2; ASGPR1: Asialoglycoprotein receptor 1; CA19-9: Charbohydrate antigen 19-9; CDH17: Cadherin-17; CEA: Carcinoembryonic antigen; CIAPIN1: Cytokine-induced apoptosis inhibitor 1; CRP: C-reactive protein; CTLA-4: Cytotoxic T-lymphocyte antigen 4; CYFRA 21-1: Cytokeratin 19 fragment; DKK1: Dickkopf-1; EGFR: Epidermal growth factor receptor; EpCAM: Epithelial cell adhesion molecule; FGFR2: Fibroblast growth factor receptor 2; FIBG:Fibrinogen gamma chain; FOXP3: Forkhead box P3; FSCN: Fascin; HHLA2: Human endogenous retrovirus-H long terminal repeat-associating protein 2; IDH1:Isocitrate dehydrogenase 1; IDH2: Isocitrate dehydrogenase 2; IGHA1: Immunoglobulin heavy constant alpha 1; IL-6: Interleukin 6; Ki67: Proliferation marker protein Ki67; KL-6: Krebs von den Lungen 6; KLK11: Kallikrein related peptidase 11; LC3: Microtubule-associated protein 1A/1B-light chain 3; MMP-7: Metalloproteinase 7;MUC1: Mucin 1; MUC4: Mucin 4; MUC5AC: Mucin 5AC; OPN: Osteopontin; PD-L1: Programmed death-ligand 1; S100A6: S100 calcium-binding protein A6; S100A9:S100 calcium-binding protein A9; S100P: Tissue protein S100P; SSP411: Spermatogenesis-associated protein 20; TGF-β1: Transforming growth factor-β1; TSP-2:Thrombospondin-2; uPA: Urokinase-type plasminogen activator; uPAR: Urokinase-type plasminogen activator receptor; VNN1: Pantetheinase.
Another promising subfield of cfDNA in CCA is the study of cell-free epigenetics. A recently published report, which analyzed 40 samples of each patient group, hints towards distinct methylation profiles between benign biliary tract disease (BTD) and CCA. The methylation pattern of opioid-binding protein/cell adhesion molecule (OPCML) and homeobox D9 (HOXD9) had a promising discriminative potential, with an area under the receiver operating characteristic (AUROC) of 0.85 for diagnosing CCA[26].
However, we believe that, to this point, there is a dire need for more data to support these initial findings. Barriers regarding study design: Method synchronization, number of patients included, data heterogeneity, cost, and lack of validation prevent their use in clinical settings, while also preventing the funding of large-scale translational endeavors.
Cell-free non-coding RNA
Research in the past decade has revealed an increasing role of miRs as cancer biomarkers for multiple primary tumors, including CCA[27-31]. There are several qualities that, at least in theory, favor miRs as useful biomarkers: Relative specificity, long-term stability, presence in multiple fluids, as well as relative ease of detection and amplification through ever more accessible PCR techniques[19]. To this point,numerous studies[32-36] have investigated the role of miRs in CCA, some showing substantial promise[37-41]. These studies are briefly analyzed in Table 1. The viability of miRs as biomarkers in CCA wastested in two meta-analyses, each including approximately 500 patients and testing the diagnostic capabilities of the cell-free non-coding RNA method, without focusing on specific miRs. Overall, the results were promising, with an AUROC ranging between 0.88 and 0.90 for CCA detection[42,43].
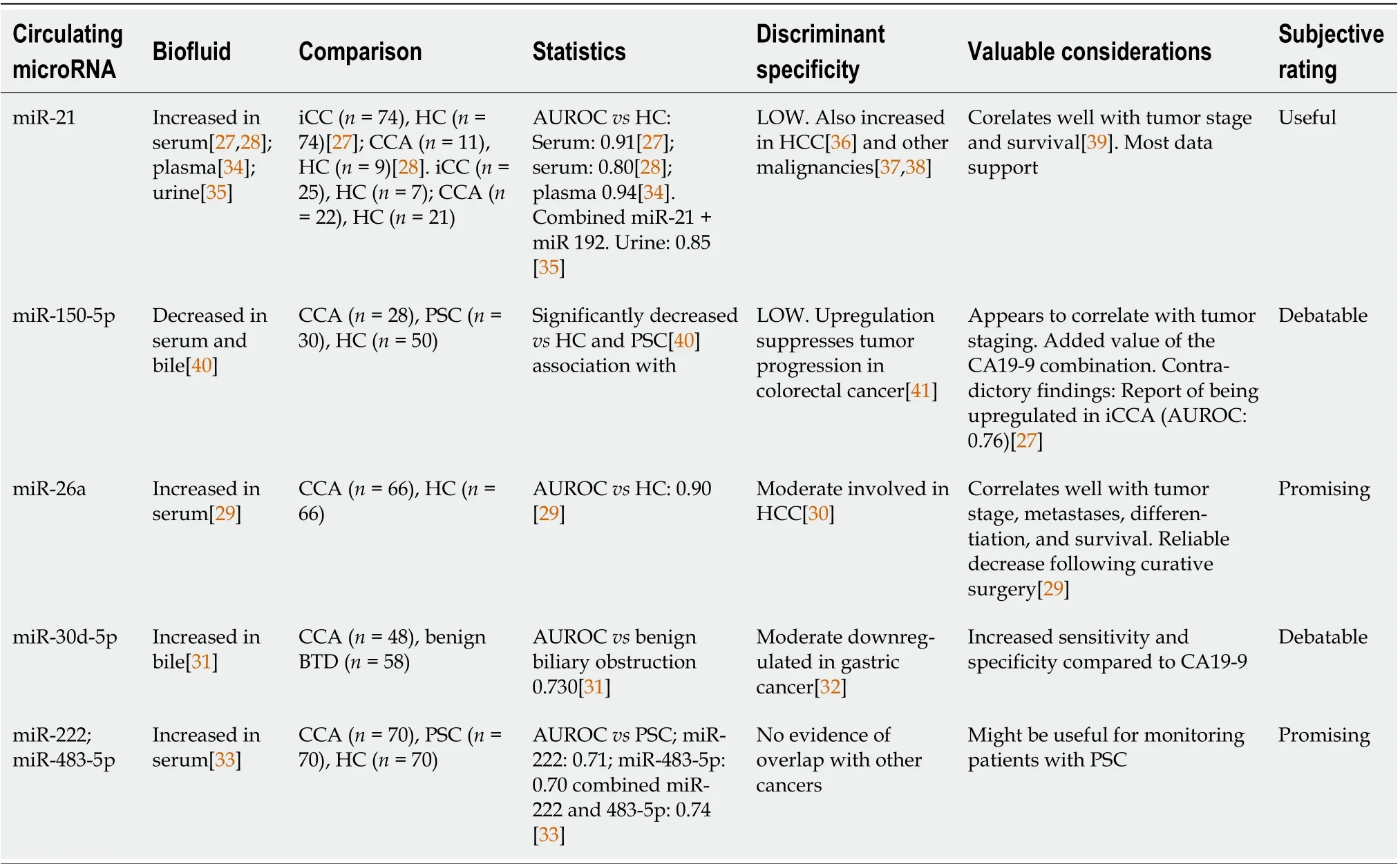
Table 1 The role of microRNAs as diagnostic and prognostic biomarkers in cholangiocarcinoma
However, there are some nuances in the study of miRs, which are worth addressing since the clinical future of the method might be at stake. Of critical relevance is the problem of specificity. Most biomarkers fare relatively well against healthy controls (HC), which is helpful for initial validation, yet far from desirable in a clinical scenario where the setting is less straightforward. This issue has been partially addressed in some study designs by comparisons with other benign BTD conditions, probably the most relevant being primary sclerosing cholangitis (PSC), which shares a common natural history pathway with CCA. However, in such conditions, the specificity and AUROCs tend to drop at least by 0.10-0.20 (as shown in Table 1). Consequently, their diagnostic biomarker value remains only slightly above the threshold for utility in the clinical scenario where the discriminative capabilities were most sought after. Moreover, there is the issue of overlapping with other cancers, which might further complicate the matter. In response, some designs have tried to implement a panel of up to eight miRs to generate distinct profiles depending on CCA subtypes (n= 14) and tumor progression[44].
The use of circulating nucleic acids in CCA diagnosis and prognosis is undoubtedly promising.Nevertheless, the field is still nascent, and most of the data come from studies with heterogeneous designs, most of which are proof-of-concept. Therefore, a potential research direction might be to stimulate reproducibility instead of novelty to provide the grounds for a quicker clinical application.
PROTEINS
Protein-based biomarkers in the clinical practice
Proteomics is a rapidly growing field of biomedical research in the postgenomic era, given the everexpanding role of personalized medicine. Proteome-based biomarker studies target proteins that could serve as agents to fit a patient's molecular profile in the clinical practice for diagnostic, prognostic, and predictive molecules, their levels being measured from serum samples usually by ELISA.
There are three protein-based biomarkers currently used in the clinical practice towards assisting CCA diagnosis and prognosis: CA19-9 and CA125, and carcinoembryonic antigen (CEA)[45,46].
CA19-9 is a circulating high molecular weight glycoprotein produced by the biliary duct and pancreatic cells and secreted by the gastric and colonic epithelia. Up to 7% of the general population is not producing CA19-9 because of blood cell Lewis antigen deficiency. For CCA, CA19-9 is by far the most frequently used biomarker. Concerning CCA diagnosis, CA19-9 showed a somewhat limited diagnostic accuracy, with following performances: Sensitivity: 72% and specificity: 84%[13]. Hence its promise resides in assessing CCA prognosis. As recently reviewed by Langet al[45] CA19-9 appears to be an independent prognostic biomarker associated with treatment outcome, as elevated CA19-9 serum levels pre- and postoperatively after systemic therapy show impaired OS. Nevertheless, several factors hamper CA19-9 use as a unique CCA prognostic biomarker[47], thus making its clinical use tumorassociated rather than tumor-specific.
Also known as MUC16, CA125 is the largest membrane-associated mucin, which is also, a glycoprotein. Being a well-known biomarker, CA125 is primary used for the ovarian cancer clinical management[48]. CA125 showed incipient potential diagnostic and prognostic value towards clinical management of CCA[45].However, CA125 proves its predictive power only in combination with other biomarkers, such as CA19-9, CAE and AFP.
Being produced by the gastrointestinal tissue during fetal development, CEA is a cell surface glycoprotein and functions as an intracellular adhesion molecule. In clinical practice, CEA is extensively used in colorectal cancer monitoring[49]. CEA proved its potential value as a diagnostic biomarker, with rages of sensitivity reported between 40% and 79%, and specificity between 48% and 90%. CEA was also reported as a prognostic indicator for CCA, with expanded predictive capabilities in several biomarker combinations, such as with CA19-9[45].
The three glycoproteins are the most used biomarkers in the clinical management of CCA, and their role is to assist rather than provide a definite diagnostic or prognostic statement. Various other proteinbased biomarker candidates reported as single molecules, combined with CA19-9 or as biomarker panels, have been spotlighted in several CCA studies. Towards identifying the potential biomarkers,several approaches have been used. With respect to the study design, CCA patient samples have been compared to (1) Only HC; (2) Only to benign BTD; (3) To benign BTD and HC; and (4) To other disease related conditions and HC. Other studies were interested only in searching biomarkers for iCCA and only one approach was headed towards subtypes of CCA, such as perihilar iCCA, hCCA, and eCCA.While the biospecimens are limited to serum and tissue, and the methods to ELISA or immunohistochemistry, the number of samples included appears to be very heterogenous, ranging from around 20 to up to over 200. The proteins associated with favorable diagnostic and with poor prognostic, potential protein-based biomarkers, are desciphered in Tables 2 and 3.
Proteins associated with favorable diagnostic potential in CCA patients
Multiple proteins appeared to have a role in CCA diagnosis, typically showing increased serum levels.These findings were reported in studies using serum as biospecimen and ELISA assays for their absolute quantification (Table 2). Such examples are osteopontin (OPN)[50] and S100 calcium-binding protein A6 (S100A6)[51] which efficiently discriminated between CCA and HC, and dickkopf-1 (DKK1)between iCCA and HC[52]. Studies reporting serum cytokine interleukin 6 (IL-6)[53], spermatogenesisassociated protein 20 (SSP411)[54] and MUC5AC[55] also included groups of metastatic liver cancer,HCC, and benign BTD disease. Metalloproteinase 7 (MMP-7) was assessed only in groups of CCAvsbenign BTD[56,57].
After several reports, cytokeratin 19 fragment (CYFRA 21-1) was included in a comprehensive metaanalysis[58] and the pooled diagnostic indices showed a sensitivity of 81% and a specificity of 86% for iCCA diagnosis. More recently, the urokinase-type plasminogen activator receptor (uPAR)[59], reported as a single protein-based biomarker, proved to be a reliable tool for differentiating CCA from HC with sensitivity of 95% and specificity close to 90%, while transforming growth factor-β1 (TGF-β1) appears to help distinguishing CCA from other pro-inflammatory conditions and HC.
Moreover, the combination of MMP-7[57], DKK1[60], thrombospondin-2 (TSP-2)[61] and uPAR[59]assessed together with CA19-9 showed higher values of sensitivity and specificity than the markers measured individually to diagnose CCA patients. Not least, a biomarker panel consisting of five proteins investigated in a decision tree algorithms based study, namely S100A9, MUC5AC, TGF-β1,angiopoietin-2 (Ang-2), and CA19-9, showed to have the greatest diagnostic potential among all mentioned proteins towards CCAvsHC (sensitivity: 95%, specificity: 90%) and towards CCAvsnon-CCA (sensitivity: 70%, specificity: 83%) differentiation[62].
Proteins associated with poor outcome in CCA patients
Concerning prognosis, several protein-based potential biomarkers have shown increased levels in CCA,most frequently by employing immunohistochemistry in tissue samples. The serum has also emerged as a biospecimen towards prognostic biomarkers exploration (Table 3). As such, high levels of tissue Krebs von den Lungen 6 (KL-6 mucin)[63], cadherin-17 (CDH17)[64], kallikrein-11[65], uPAR[59] and high levels of serum cytokine-induced apoptosis inhibitor 1 (CIAPIN1)[66], MUC5AC[55], OPN[50], S100A6[67] and uPAR[59,68] were found adverse prognostic factors for CCA patient’s survival. Subjected to meta-analysis, high levels of tissue PD-L1[17] and tissue protein S100P[69] were also proposed as potential prognostic markers of CCA. Out of a protein multimarker panel consisting of serum S100A9,MUC5AC, TGF-β1, Ang-2, and CA19-9, serum levels of TGF-β1 and Ang-2 provided predictive potential for both metastasis and TNM stage prognosis in CCA patients[62].

Table 2 Proteins associated with favorable cholangiocarcinoma diagnostic potential
For patients with tumors of combined HCC and CCA (cHCC-CC), microtubule-associated protein 1A/1B-light chain 3 (LC3) increased tissue expression was found to predict postresection OS (5-year OS,61.2%) and disease-free survival (74.6%)[70].
iCCA prognostic biomarkers were also of particular interest in some studies. High tissue levels of urokinase-type plasminogen activator (uPa)[71], MMP-7[72,73] and human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2), reported from a recent meta-analysis[74], were associated with adverse outcomes in iCCA patients. High serum DKK1 in combination with CA19-9 was independently associated with shorter survival[60]. Recently, Qianget al[75] found that the biomarker panel consisting of CEA, AFP, and proliferation marker protein Ki67 are significant prognostic indicators in iCC patients.
Proteins that showed decreased levels in association with CCA were PD-L1 and OPN. The lack of serum PD-L1 level normalization after surgery seems to identify patients at high risk for recurrence and adverse outcomes[76]. By applying an innovative approach, decreased serum OPNpertumor volume was associated with invasive behavior and early recurrence of iCCA[77].
There is vast evidence of protein-based biomarkers reported in CCA diagnosis and prognosis, but only CA19-9 and CEA are currently employed in routine clinical practice. The data above reveals exciting results for new potential protein-based biomarkers used as single molecules (e.g., uPAR) or biomarker panels (e.g., S100A9, MUC5AC, TGF-β1, Ang-2, and CA19-9) for CCA.
Extrapolating from proteomic-derived biomarker studies in other diseases, it appears that using multiple-molecule panels instead of individual proteins provides better predictive results and shows more promise for a translation to clinical practice. However, future validation studies on large patient cohorts are needed towards establishing the real applicability and the subsequent translation of these biomarkers into the clinical practice.
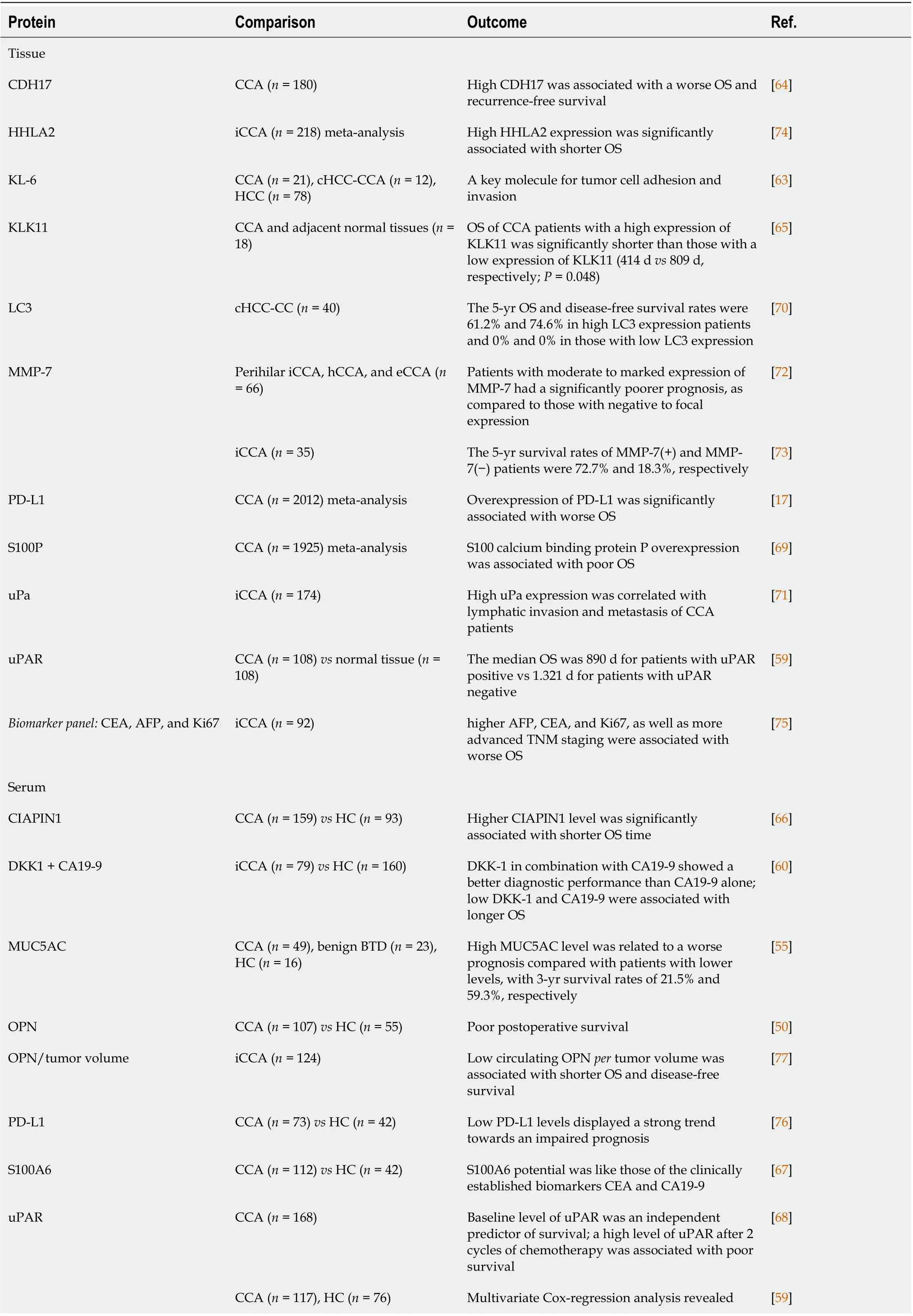
Table 3 Proteins associated with poor outcome in cholangiocarcinoma patients
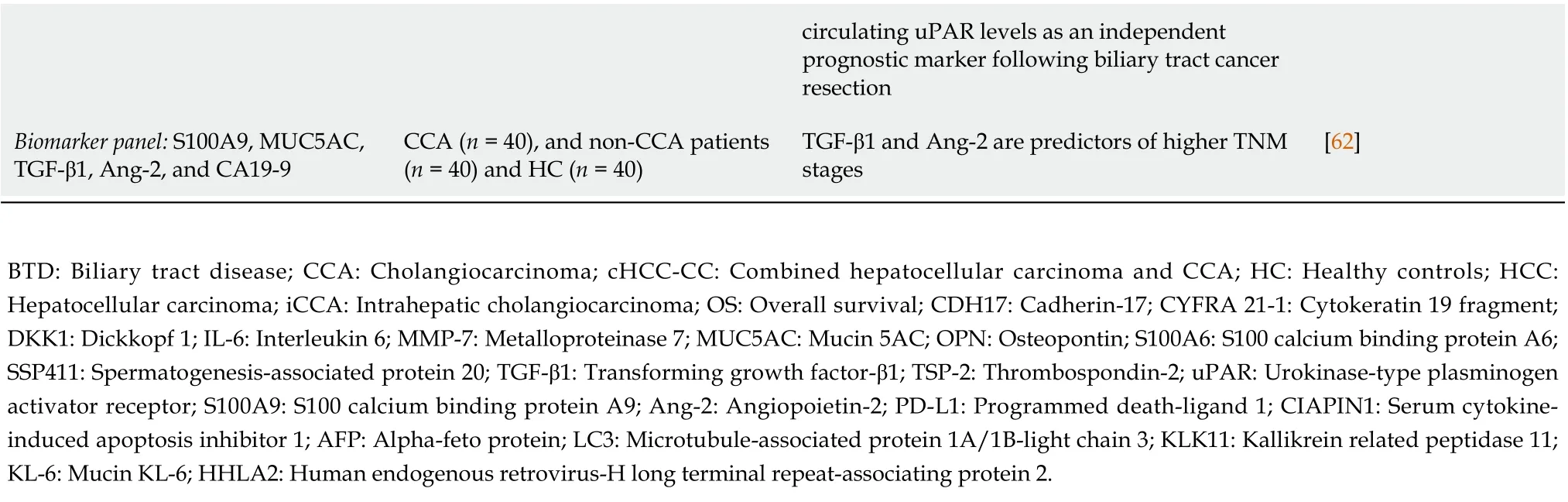
circulating uPAR levels as an independent prognostic marker following biliary tract cancer resection Biomarker panel: S100A9, MUC5AC,TGF-β1, Ang-2, and CA19-9 CCA (n = 40), and non-CCA patients(n = 40) and HC (n = 40)TGF-β1 and Ang-2 are predictors of higher TNM stages[62]BTD: Biliary tract disease; CCA: Cholangiocarcinoma; cHCC-CC: Combined hepatocellular carcinoma and CCA; HC: Healthy controls; HCC:Hepatocellular carcinoma; iCCA: Intrahepatic cholangiocarcinoma; OS: Overall survival; CDH17: Cadherin-17; CYFRA 21-1: Cytokeratin 19 fragment;DKK1: Dickkopf 1; IL-6: Interleukin 6; MMP-7: Metalloproteinase 7; MUC5AC: Mucin 5AC; OPN: Osteopontin; S100A6: S100 calcium binding protein A6;SSP411: Spermatogenesis-associated protein 20; TGF-β1: Transforming growth factor-β1; TSP-2: Thrombospondin-2; uPAR: Urokinase-type plasminogen activator receptor; S100A9: S100 calcium binding protein A9; Ang-2: Angiopoietin-2; PD-L1: Programmed death-ligand 1; CIAPIN1: Serum cytokineinduced apoptosis inhibitor 1; AFP: Alpha-feto protein; LC3: Microtubule-associated protein 1A/1B-light chain 3; KLK11: Kallikrein related peptidase 11;KL-6: Mucin KL-6; HHLA2: Human endogenous retrovirus-H long terminal repeat-associating protein 2.
METABOLITES
Metabolomics, another branch of omics-derived technologies, analyzes low molecular weight metabolites (< 1500 Da) in various biological fluids. One of the hallmarks of cancer is energy metabolism reprogramming. In order to promote cancer survival and subsequently cancer growth, there are several shifts in normal metabolic pathways (e.g., a higher glucose uptake rate and an increase in lactate production)[78,79]. A different or "wiser" use of metabolic pathways in cancer cells leads to the release of several metabolites in various body fluids, providing an opportunity for diagnosis and monitoring.Metabolic profiling is, therefore, a promising approach for the identification of potential biomarkers in several cancers, including CCA[80]. To date, several studies have investigated the potential of metabolomics in CCA diagnosis or prognosis in various body fluids.
We believe that investigating the molecular composition of the bile could provide more crucial information than other fluids due to at least two reasons. Firstly, it could unravel mechanistic information regarding the pathological alteration of the biliary epithelium. Secondly, it could identify biomarkers from nearby tumor cells, markers that might or might not be present in other body fluids.Several metabolite profiling studies of human bile have been performed over the past few years. One such study reported a reduction in the proportion of secondary bile acids in patients with CCA compared to those with biliary tract stones and healthy individuals[81]. Another study showed that changes in phosphatidylcholines, bile acids, and lipids could discriminate CCA from PSC and benign BTD (sensitivity: 88.9%; specificity: 78.1%)[82]. When comparing inoperable eCCA to non-malignant,non-cholestatic biliary diseases (including PSC), CCA was associated with increased levels of glycineconjugated bile acids and phosphatidylcholines. Moreover, constructed models could discriminate CCA patients from those with non-malignant biliary diseases with an 80% sensitivity and 95% specificity:95%[83].
Unfortunately, the impact of cholestasis on the metabolic profile was not investigated, and it is difficult to reach a solid conclusion. In contrast, the analysis of metabolites in patients with CCA, HCC,and non-malignant liver diseases showed a decrease in glycine and taurine-conjugated bile acids,phospholipids, and cholesterol in patients with CCA compared to control groups but only to a certain extent when compared to HCC[84].
In theory, if one biomarker is detected and validated in bile, it might provide sufficient grounds further to test it in more accessible and less invasive fluids (e.g., serum, urine, plasma). Nevertheless,metabolomics studies can also be performed directly on serum, plasma, or urine. Using serum, one study from the United Kingdom failed to show any differences between profiles from patients with benign biliary strictures and CCA[85]. In contrast, one study from China showed that two bile acids,chenodeoxycholic acid (CDCA) and taurochenoxycholic acid (TCDCA) (from plasma), had higher sensitivity and specificity than CA19-9 for CCAvsbenign bile duct disease and CCAvsHC[86].Furthermore, a study from Europe (Italy), using an artificial intelligence approach, found a plasma bile acid profile that could discriminate between CCA and benign BTD with an accuracy of 86.4%[87].However, all the beforementioned studies could not offer more answers to some of the most critical clinical dilemmas when caring for patients with liver cancer.
In the liver cancer community, there are at least two primary clinical necessities. The first clinical dilemma is probably the most common scenario: One patient with advanced liver disease and focal liver lesions: Is it cancer? If the answer is yes, is it HCC or iCCA? In this setting, one study (on serum) has shown that the development of an algorithm combining glycine, aspartic acid, sphingomyelin (SM)(42:3), and SM (43:2) permitted accurate discrimination between HCC and iCCA with a sensitivity of 75% and specificity of 90%. In the same study, another algorithm discriminated PSC from iCCA with a sensitivity of 100% and specificity of 70%. Of note, these results were further validated in an independent cohort[88]. A similar finding was also reported in one study from China. A panel of four metabolites {PE (19:0/0:0), PE [18:2 (9Z, 12Z)/0:0], PC (14:0/0:0) and PC (18:0/0:0)} attained a diagnostic accuracy (HCCvsiCCA) of 99.7%[89].
The second clinical dilemma is: One patient with distal bile duct obstruction: Is it cancer? dCCA or pancreatic ductal adenocarcinoma (PDAC)? A combination of serum levels of nine metabolites [acylcarnitine AC (16:0), ceramide Cer (d18:1/24:0), phosphatidylcholines PC (20:0/0:0) and PC (O-16:0/20:3),lysophosphatidylcholines PC (20:0/0:0) and PC (0:0/20:0), lysophosphatidylethanolamine PE (P-18:2/0:0), and sphingomyelins SM (d18:2/22:0) and SM (d18:2/23:0) and CA 19-9] could discriminate between dCCA and PDAC with a sensitivity of 55.9% and specificity of 89.5%[90]. Metabolic profiling of urine in patients with CCA was also applied, showing some metabolic differences in the urine of CCA compared to controls. As such, a urine metabolomic panel consisting of 3-methylhistidine, citric acid,cytosine, indoleacetic acid, salicyluric acid, L-methionine, aminomalonic acid, glutaric acid, ursodeoxycholic acid, N-acetylornithine, allantoin, glycocholic acid, histamine, homogentisic acid, L-kynurenine,sarcosine, pyruvic acid, taurine and methylsuccinic acid were identified as potential biomarkers for primary extrahepatic CCA[91]. Nevertheless, in terms of prognosis, only a few studies have shown the potential of metabolites to predict recurrence or OS[91,92].
The road ahead is still long for metabolomics in CCA. Metabolome studies in CCA have just begun,and some promising metabolites have already been identified. However, a shift from bench to bedside is not expected to appear in the next few years. First, identifying a specific metabolite with diagnostic or prognostic properties is a challenging goal due to the presence of many confounding factors (e.g., age,gender, diet, underlying liver disease, concomitant disease, drugs, and others). Secondly, the results from untargeted metabolomics might be different from targeted metabolomics, according to at least one recent metabolomics study, investigating plasma fetal bile acids towards assessing liver cirrhosis severity[93]. Not least, the reproducibility of many of these studies is a genuine concern (due to multiple analytical platforms, different sample preparation protocols), and standardized procedures are urgently needed.
EXTRACELLULAR VESICLES
In terms of minimally invasive biomarkers, EVs are the "new kids on the market". They hold great promise in the diagnosis and prognosis of cancer. EVs are encountered in all body fluids including blood[94], urine[95] and bile[96]. According to their size and biogenesis there are two classes: (1) Large EVs [also called microvesicles (MVs)] roughly ranging from 100 to 1000 nm in size, which directly bud from the plasma membrane of their parental cell; and (2) Small EVs (also called exosomes) are considerably smaller (below 100 nm) and originate from accumulated intraluminal vesicles within the endomembranous system, forming so-called multivesicular bodies[97].
The function of EVs depends on the type and content (e.g., lipids, proteins, nucleic acids) of their parent cells. They orchestrate many of the processes described by Hanahanet al[98] as "Hallmarks of Cancer"[98] eitherviaparacrine signaling or horizontal transfer of bioactive agents[99]. In the initial steps of cancer genesis, EVs (released by cancer cells) appear to be responsible for the differentiation of mesenchymal stem cells into fibroblasts, contributing to stroma generation ant thus preparing their tumor niche[100]. Furthermore, EVs could transport miR species from human CCA cells to cancerassociated fibroblasts, a communication between cancer cells and the cancer microenvironment responsible for tumor growth and, later on, CCA cells-derived EVs can transfer oncogenes to normal cholangiocytes, increasing their migration and invasive potential [viaincreasing the expression of betacatenin (CTNNB1) and decreasing the expression of E-cadherin (CDH1)], hence preparing the final processes of carcinogenesis: Tumor invasion and metastasis[101].
Some studies have already revealed the great potential of EVs content or surface markers in terms of diagnosis. Proteomic profiling of serum EVs has identified a panel of five proteins that could assist CCA diagnosis. The study design included CCA (n= 43), PSC (n= 30), HCC (n= 29) patients and HC (n= 32).As such, pantetheinase (VNN1), C-reactive protein, fibrinogen gamma chain (FIBG), immunoglobulin heavy constant alpha 1 (IGHA1) and alpha-1 acid glycoprotein (A1AG1) showed to have an increased concentration in serum EVs of CCA compared to all PSC, HCC and HC. Moreover, a panel of three EVs proteins, namely ficolin-2 (FCN2), inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4), and FIBG showed to be able to discriminate between early-stage CCA and PSC patients with an AUC > 0.88[102].
A major challenge nowadays is the differential diagnosis between HCC and iCCA or between dCCA and PDAC. More often, the final diagnosis (HCCvscCCA) in clinical practice is based on liver biopsy.In terms of EVs surface antigens, one study enrolling 172 patients with liver cancer (HCC or CCA), 54 with cirrhosis and no liver neoplasia, and 202 control subjects, found a combination of tumor-associated microparticles (AnnexinV+ epithelial cell adhesion molecule (EpCAM+) and asialoglycoprotein receptor 1 (ASGPR1+)) could diagnose CCA from healthy individuals and other cancer entities with up to 90%sensitivity. However, it was unable to differentiate between CCA and HCC[103]. Interestingly, later on,the same group in another study including a large set of patients, including 77 CCA, 67 HCC, identified a combination between EVs surface antigens (AnnexinV+ CD44v6, cut-off = 34 numberper103AnnexinV+ EVs) together with AFP (cut-off = 30 ng/mL) that could discriminate between HCC and CCA (iCCA and eCCA) with both sensitivity and specificity of 100%[104]. Indeed, it is a novel potential diagnostic biomarker that could help clinicians diagnose CCA non-invasively and accurately. Further large multicenter studies are urgently necessary. In search of novel biomarkers for the differential diagnoses between dCCA and PDAC, one study including 50 patients (n= 20 pancreatic cancer,n=dCCA,n= 15 chronic pancreatitis,n= 10 common bile duct obstruction due to biliary stones patients)reported that the concentration of EVsper sein bile and serum could discriminate malignant from nonmalignant pancreaticobiliary diseases with 100% sensitivity in bile and 47% in serum[105]. EVs cargo profile could also have diagnosis potential. In particular, a panel of 5 miRs (miR-191, miR-486-3p, miR-1274b, miR-16 and miR-484) isolated from bile EVs showed good diagnostic values for CCA diagnosis compared to non -malignant biliary diseases (sensitivity: 67%; specificity: 96%)[106].
CIRCULATING TUMOR CELLS
Circulating tumor cells (CTCs) have been evaluated as a diagnostic marker in pancreatic, colorectal,breast or prostate cancer, and are associated with poor survival rates. However, only a handful of studies have assessed their potential in CCAs. CTCs are cancer-derived cells released from a primary solid tumor or local lymphoid reservoirs into the bloodstream, harboring tumor-initiation properties,and possibly enabling distant metastasis. Even after primary tumor resection, the permanence of viable CTCs in the portal venous blood seems to be a consequence of T-cell suppression by myeloid-derived suppressor cells and CTC-induced apoptosis[107]. Subsequently, CTCs proliferate and cluster, possibly under the influence of cell adhesion molecules such as plakoglobin, leading to tumor growth and immune resistance[107,108].
Identification of CTCs in peripheral blood relies on their overexpression of EpCAM. It has been performed using immunocytochemistry, reverse transcriptase-PCR, flow cytometry, or an enzymelinked immunosorbent spot assay. The most used assay is CellSearch™, which uses ferrofluid nanoparticles with antibodies that target EpCAM, which is expressed in various of human epithelial tissues, carcinomas, and stem cells, and is involved in cell signaling, migration, proliferation, and differentiation[109-111]. In a study of 26 CCA patients, targeting CTCs using antibodies against EpCAM,DAPI, cytokeratin 8, 18, and/or 19, Al Ustwaniet al[112] showed that 25% of patients with CCA had a significant amount of CTCs (≥ 2/7.5 mL of blood). Similar results were reported in another study, where out of 95 CCA patients, 24% had a count of two cells or higherper7.5 mL blood, while 22% had a count of one cell, and the remainder of 54% no detectable cells[113]. Since CTCs seem to be relatively rare in peripheral blood, their potential as a diagnostic marker might be more evident in patients with metastatic disease and less in early tumors.
CTCs are also seemingly associated with more aggressive tumors, as patients with no CTCs in their blood sample had the best survival rate. In contrast, the presence of two or more CTCs was strongly associated with worse OS (median 18.1 movs8.7 mo)[113]. However, their presence does not seem to predict treatment outcome, as evidenced in the ABC-03 trial[113,114].
The high degree of variability in detection rates might be explained by suboptimal EpCAM levels for detection, loss of epithelial surface antigens, or epithelial-mesenchymal transition[112,115,116]. To overcome these shortcomings, a novel glycosaminoglycan-SCH45-probe on a microfluidic platform has been employed to isolate CCA CTCs by combining multiple-capture approaches in a shorter period and using lower blood volumes compared to the traditional method. Using EpCAM as a conventional protein biomarker, the authors showed, by analyzing peripheral blood of 65 metastatic CCA patients,that CTCs could be detected in all advanced or metastatic CCA, suggesting that CTCs may maximize the predictive performance of liquid biopsies if the proper diagnostic tool is used[117]. Reduzziet al[118] assessed an alternative to improving detection rates in a prospective study of 21 patients with advanced-stage biliary tract cancer. Using non-conventional CTCs lacking epithelial and leukocyte markers, but presenting aberrant genomes, the detection rate increased from 19% to 83%.
CONCLUSION
A non-invasive approach towards diagnosis and prognosis is the path forward in CCA, a type of cancer that sometimes appears to be hiding in plain sight. The previously discussed methods aim to provide the necessary leap forward towards a personalized approach and might allow for a refined characterization of the disease. However, most available reports are deeply heterogeneous, study protocols are not harmonized, and the number of included patients is inconsistent. These caveats appear to be the primary reasons for the gap between the wide range of cancer biomarkers that appear to be effective in individual studies and the relatively low number of biomarkers ready to be translated into the clinic.Consequently, the most challenging task in the short term might be not to find new molecules and pathways but rather to validate or infirm the role of current methods to shorten the bench to bedside gap.
FOOTNOTES
Author contributions:Ilieș M, Mocan T, Mihu CM made the conception and design; Mocan LP, Crăciun R, Nenu I,Horhat A, Spârchez M, Melincovici CS, Ilieș M, Mocan T made the acquisition, analysis, and interpretation of data;Spârchez Z, Ilieș M, Iuga CA, Mocan T and Mihu CM performed critical revisions of the manuscript; all authors contributed their expert opinion to the concept of the paper, critically revised the article, and approved the final article version to be published.
Supported byThe Romanian National Ministry of Research, Innovation and Digitalization, CNCS-UEFISCDI:Postdoctoral Research Project PN-III-P1-1.1-PD-2019-0852/PD113 within PNCDI III, awarded to Maria Ilieș.
Conflict-of-interest statement:All authors have no conflict of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Romania
ORCID number:Lavinia-Patricia Mocan 0000-0003-4957-4390; Maria Ilieș 0000-0002-0659-8926; Carmen Stanca Melincovici 0000-0002-2757-2565; Mihaela Spârchez 0000-0001-8620-9160; Rareș Crăciun 0000-0002-5872-8630; Iuliana Nenu 0000-0002-1690-6689; Adelina Horhat 0000-0002-8701-8750; Cristian Tefas 0000-0002-8263-7923; Zeno Spârchez 0000-0002-3813-1677; Cristina Adela Iuga 0000-0002-0345-0993; Tudor Mocan 0000-0001-7785-6403; Carmen Mihaela Mihu 0000-0002-9419-4824.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Gastroenterology的其它文章
- Liquid biopsy in colorectal cancer: No longer young, but not yet old
- Establishing a rabbit model of perianal fistulizing Crohn’s disease
- Reevaluation of the expanded indications in undifferentiated early gastric cancer for endoscopic submucosal dissection
- Validation model of fibrosis-8 index score to predict significant fibrosis among patients with nonalcoholic fatty liver disease
- Prognostic factors of recurrent intrahepatic cholangiocarcinoma after hepatectomy: A retrospective study
- Development and validation of a prediction model for moderately severe and severe acute pancreatitis in pregnancy
