lmaging related to underlying immunological and pathological processes in COVlD-19
2022-06-07ElenaIlievaAlexandraBoyapatiLyubomirChervenkovMilenaGulinacJordanBorisovKameliaGenovaTsvetelinaVelikova
Elena Ilieva, Alexandra Boyapati, Lyubomir Chervenkov, Milena Gulinac, Jordan Borisov, Kamelia Genova,Tsvetelina Velikova
Elena llieva, Alexandra Boyapati, Kamelia Genova, Department of Diagnostic Imaging,University Emergency Hospital (UMHATEM) "N. I. Pirogov”, Sofia 1606, Bulgaria
Lyubomir Chervenkov, Department of Diagnostic Imaging, Medical University, Plovdiv,University Hospital "St George", Plovdiv 4000, Bulgaria
Milena Gulinac, Department of General and Clinical Pathology, Medical University, Plovdiv,University Hospital "St George", Plovdiv 4000, Bulgaria
Jordan Borisov, Department of Diagnostic Imaging, MBAL-Dobrich” AD, Dobrich 9300,Bulgaria
Tsvetelina Velikova, Department of Clinical Immunology, University Hospital “Lozenetz”, Sofia 1407, Bulgaria
Tsvetelina Velikova, Medical Faculty, Sofia University “St. Kliment Ohridski”, Sofia 1407,Bulgaria
Abstract The introduction of coronavirus disease-2019 (COVID-19) as a global pandemic has contributed to overall morbidity and mortality. With a focus on understanding the immunology and pathophysiology of the disease, these features can be linked with the respective findings of imaging studies. Thus, the constellation between clinical presentation, histological, laboratory, immunological, and imaging results is crucial for the proper management of patients. The purpose of this article is to examine the role of imaging during the particular stages of severe acute respiratory syndrome coronavirus 2 infection - asymptomatic stage, typical and atypical COVID-19 pneumonia, acute respiratory distress syndrome,multiorgan failure, and thrombosis. The use of imaging methods to assess the severity and duration of changes is crucial in patients with COVID-19.Radiography and computed tomography are among the methods that allow accurate characterization of changes.
Key Words: Coronavirus disease-2019; Ultrasound; Computed tomography; Magnetic resonance imaging;Ground-glass opacity; Acute respiratory distress syndrome; Cytokine storm; COVID-19 reporting and data system; High-resolution computed tomography; Severe acute respiratory syndrome coronavirus 2
lNTRODUCTlON
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent of coronavirus disease-2019 (COVID-19), caused a global pandemic dominated by acute respiratory failure mortality.COVID-19 manifestations are heterogeneous and overlapping. Therefore many challenges in the diagnostics and management of COVID-19 are still present[1]. In addition, typical symptoms without positive proof of infection (PCR/antigen tests) make the diagnosis inconclusive. However, a variety of imaging methods and techniques can be used for this purpose. Furthermore, imaging is also essential for classifying and managing patients during the infection.
Typically, imaging studies are carried out to determine the disease stage and estimate the organ involvement and severity. However, the features, such as ground-glass opacities (GGO), consolidations,interstitial fluid shifts,etc., seen on imaging are not unique to COVID-19. Therefore, a combination of the clinical picture and laboratory assessment is necessary to fully evaluate the patient’s state[2].
In the era of COVID-19, radiology plays a crucial role in the disease work-up and follow-up. Initially considered a purely pulmonary process, COVID-19 turned out to be a multisystemic disease that requires a comprehensive imaging approach, involving all techniques and studying all anatomical areas. As the primary manifestation of COVID-19 is a pneumonia-like respiratory process, the radiology modalities most involved in its diagnosis and follow-up are chest imaging, mainly chest X-ray (CXR)and chest computed tomography (CT). Diagnostic imaging capabilities show that CT is more sensitive than the gold standard RT-PCR for diagnosing COVID-19[2,3]. At the same time, it is cheaper and can be performed faster. The CT examination can give a quick, accurate diagnosis of patients, as the PCR test requires hours, even days, to complete as the number of COVID-19 infection cases grow. However,this comes with a large radiation dose, where the capacity is still lacking in many countries[4]. CXR is ubiquitous worldwide, with a 30-70-fold lower amount of radiation. Despite its low sensitivity and specificity, it is commonly performed as an initial investigation in COVID-19[4,5]. It is the modality of choice in the intensive care unit to detect disease progression and assess the position of the individual resuscitation means.
The imaging findings both on CXR and CT can be invaluable, especially for an atypical or organizing pneumonia with bilateral, multifocal randomly scattered GGO, in subpleural, mainly peripheral distribution with thickened pulmonary interstitium giving a reticular pattern, broncho-vascular prominence,and consolidation with increasing severity in the more seriously ill patients[6]. High-resolution CT(HRCT) with its modern available software techniques is the method of choice for an initial examination, staging, and follow-up of patients with suspected COVID infection. CT has higher sensitivity and specificity than radiography. On CT, the main changes are GGO, which tend to be more in the periphery, crazy paving changes are seen in later stages of the disease, thickening of the interstitium is also seen as well as dilation of the terminal lung vessels[7]. For staging of the changes seen on CT, we use the COVID-19 reporting and data system (CO-RADS) classification. It is a standardized classification proposed by the Dutch Radiological Society. It has 6 levels of suspicion from CO-RADS 1 to CO-RADS 6. In CO-RADS 1 patients, the exam is normal or has non-infectious changes.CO-RADS 2 patients have low levels of suspicion, and the visualized changes are consistent with infections other than COVID. CO-RADS 3 patients are those in which the changes are unclear, and there is an indeterminate level of suspicion. CO-RADS 4 and 5 staged patients have high and very high levels of suspicion for COVID- 19 infection, respectively. CO-RADS 6 patients are those who have typical changes and are PCR positive[8].
Chest ultrasound (US) plays a role in emergency settings as a COVID-19 screening technique.However, it is often of limited use as a highly operator-dependent method for lung disease. In addition,point of care echocardiography might have utility in hemodynamically unstable patients.
As stated above, various extrapulmonary manifestations have been reported, including in the gastrointestinal tract, brain, heart, kidneys, or muscles. The identification of most of these pathologies needs imaging, including abdominal CT, US, and magnetic resonance imaging (MRI). Imaging helps detect, diagnose, and assess the virus-induced injury and associated complications of the organs and systems affected by COVID-19. In suspected pulmonary thromboembolism (PTE) cases, CT pulmonary angiography (CTPA) may help correct the diagnosis. PTE is frequently observed in patients with more severe COVID-19 pneumonia involving mainly the segmental (90.2%) and subsegmental arteries (61.0%)of pulmonary segments affected by a consolidation pattern (67.6%)[9].
Furthermore, CXR, chest CT, and echocardiography can readily evaluate the signs of cardiac failure.However, myocardial injury can be best assessed using cardiac MRI. Potential neurovascular complications such as stroke, hemorrhage, or venous sinus thrombosis can be identified by non-enhanced head CT, and in the setting of a suspected infarct, a non-enhanced MRI of the brain can be performed for definitive assessment[10].
The disease may present as a multisystem hyperinflammatory syndrome in pediatric patients,currently termed pediatric multisystem inflammatory syndrome (PMIS). In children with PMIS, a broad spectrum of abdominal abnormalities can be detected by
both abdominal US and CT, with periportal and pericholecystic edema, gallbladder wall, and bowel wall thickening and dilatation, splenic infarcts, hepatosplenomegaly, right lower quadrant mesenteric lymphadenopathy, and free fluid in the pelvis being among the most commonly encountered abnormalities[10,11].
The COVID-19 pandemic has facilitated research on the implementation of artificial intelligence (AI),machine learning, and its subfield deep learning into imaging, which can be used as an essential adjunct or alternative to the diagnosis and follow-up assessment of progression and therapeutic development of the disease[12]. Deep learning is the most successful machine learning technique, which provides helpful analysis to study a large number of chest images that can critically impact the screening of COVID-19. AI algorithms have been developed to help with the early detection of COVID-19 both on CXR and CT. A deep learning model was also trained to discriminate between COVID and non-COVID pneumonia[13]. In line with this, CT pneumonia analysis (developed by Siemens Healthineers and partners) is another algorithm designed to automatically identify and quantify abnormal patterns in the lungs, enabling simple-to-use analysis of non-contrast chest CT scans for research purposes. The results could be used to analyze the severity and progression of abnormalities in patients exhibiting COVID-19 symptoms.
ASYMPTOMATlC DlSEASE
A person infected with SARS-CoV-2 who has not developed any signs or symptoms of COVID-19 is defined as an asymptomatic case. Immunological features, including any of the innate immunity pathways (i.e., natural killer (NK) cells, interferon, and other cytokine production), play a role at the onset of infection[14]. Stage I, or the asymptomatic incubation with or without detectable virus, is the period when treatment for improving immunity is given, such as the use of antiserums (ready-made antibodies from survivors), and is undoubtedly crucial[14]. However, due to the initial pathological changes in the target organs, sometimes imaging studies are the only way of detecting problems.
In asymptomatic individuals, this stage begins with inhalation of the SARS-CoV-2 virus replicating in the epithelial cells of the nasal cavity. It is well-known that the virus primarily uses the receptors for ACE2[15]; thus the first affected cells are ciliated cells. Therefore, PCR for SARS-CoV-2 RNA in nasal swabs can diagnose the virus at this point. The virus is then distributed in the lungs, digestive tract,reproductive system,etc., while innate immune tolerance is minimal[15].
It has been shown that asymptomatic persons can be infectious and secrete SARS-CoV-2, promoting the dissemination of COVID-19 - a significant concern from an epidemiological point of view.Moreover, while asymptomatic, some patients present with substantial lung changes, for example,when they seek medical attention. This observation requires a stringent search and examination of the interactions of proven infected persons with COVID-19 to diagnose asymptomatic infections[14].
According to the literature and personal experience of our team, the incidence of asymptomatic occurring COVID-19 infection is much higher, about four times more common than symptomatic moderate to severe ongoing cases[16]. The described histological changes in the respiratory system in an asymptomatic case of COVID-19 infection based on autopsy and biopsy are exceptional[17]. The only way to examine histological changes, mainly of the lung, in asymptomatic infection in the early stages of the disease is to take a biopsy for other pathological processes, “accidental” sampling of COVID-19,most often in the case of neoplastic diseases in which surgeries were performed for lung tumors at a time when superimposed infections were not recognized[16-20].
The main morphological changes found in routine histopathological examinations in the lungs are edema, proteinaceous exudate, focal reactive hyperplasia of pneumocytes, some with viral inclusions,and polymorphonuclear inflammatory infiltration composed mainly of lymphocytes and multinucleated giant cells. Hyaline membranes are scattered or not apparent in the early (asymptomatic) stage of COVID-19 infection, unlike in acute respiratory distress syndrome (ARDS). In addition, histologically,protein and fibrin exudates can be found in the lung parenchyma, as well as diffuse thickening of the alveolar walls, consisting of proliferating interstitial fibroblasts and type II pneumocystic hyperplasia,with random cell atypia and multinucleated giant cells, indicating varying degrees of the proliferative phase of diffuse alveolar damage. In some areas, an abundance of alveolar macrophages can be found together with type II pneumocystic hyperplasia[17,18,21].
Most patients with COVID-19 infection are diagnosed with pneumonia. The patients who have no symptoms could be transmitters. However, some asymptomatic patients progress quickly, even to ARDS. In asymptomatic patients, radiography has lower sensitivity and specificity than CT. In patients with slight changes, X-ray imaging can appear normal. This is why CT is the preferred method for diagnosis. Based on the published data, to date, almost all patients with COVID-19 have characteristic changes on CT[8,22].
The X-ray of a 60-year-old female patient is presented in Figure 1. The patient had no symptoms, but a positive PCR test was performed because her husband had COVID pneumonia. Her X-ray showed no abnormalities. This is a typical case of an asymptomatic patient with negative radiography.
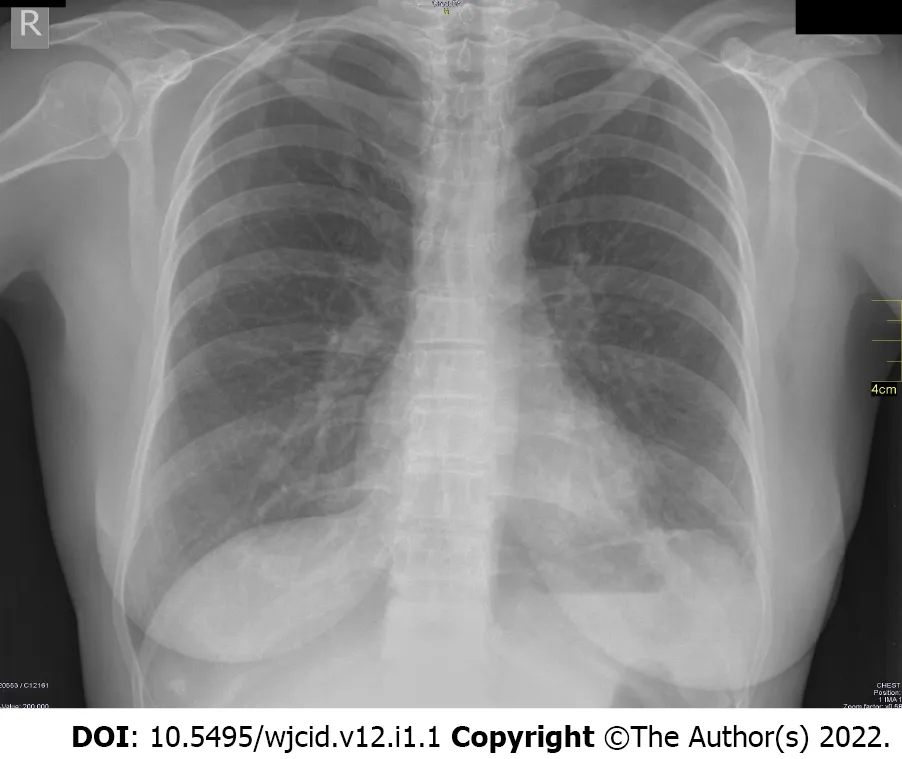
Figure 1 Negative radiography of an asymptomatic patient.
We also present the CT scan of a 45-year-old male, shown in Figure 2. He had no symptoms but a positive PCR test because he had been in contact with a verified COVID-19 patient. Both lungs showed no abnormalities, no infiltrates, no GGO, and no pleural effusions.
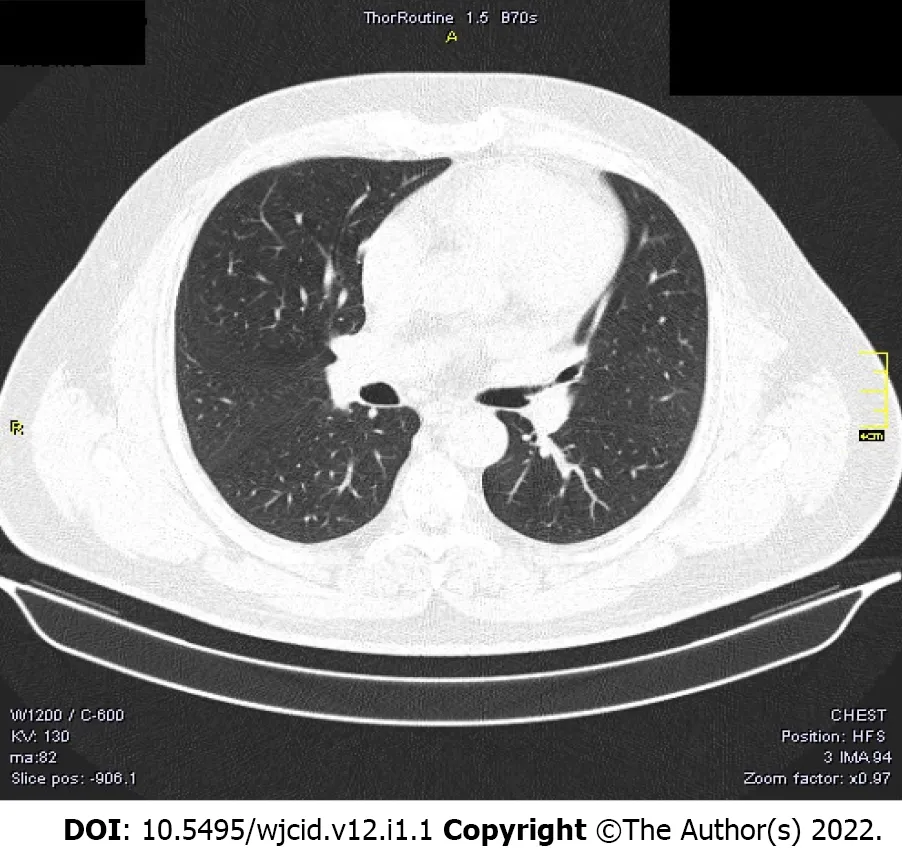
Figure 2 Negative computed tomography of an asymptomatic patient.
Atypical clinical findings in pregnant women with COVID-19 may increase the difficulty in establishing the diagnosis. Recently, data have demonstrated that pregnant women are more vulnerable to severe COVID-19, the development of complications, and death. CT is the favored approach for detecting pulmonary involvement early, especially in asymptomatic cases[22]. In asymptomatic children or those with atypical presentation of COVID-19, CT may be beneficial, especially in sparing time while waiting for PCR results or false-negative results.
In the case of pregnant women or children, it is always preferred to perform CT instead of waiting because any delay in confirming the diagnosis and management of the disease can be fatal and can contribute to the spread of the virus. Furthermore, the CT results in asymptomatic COVID-19 are usually distinct from the normal appearance, where the most common findings are GGO and consolidations in the lungs[22].
MlLD TO MODERATE CASES - TYPlCAL COVlD-19 PNEUMONlA
The onset of pneumonia in COVID-19 includes extreme antigen presentation, followed by enhanced production of C-reactive protein, D-dimer, and liver amino-transferases, accompanied by infiltrates in the lungs, representing involvement of antigen-presenting cells, T-helper cells, B cells, and NK cells[14].
The virus spreads across the airways in the respiratory tract and further to the gastrointestinal tract. It activates robust innate immune reactions. Nasal secretion, sputum or swabs contain SARS-CoV-2 but also early immune response molecules. Cytokine production during this stage may predict the clinical presentation and the subsequent disease course[23]. Significant amounts of interferon type I can confine the infection within the lungs, which is valid for approximately 80% of affected patients. Most of those with typical COVID-19 pneumonia on conservative symptomatic treatment can stay at home. However,about 20% of infected patients will progress to the next severe stage of the disease. Around 2% will develop life-threatening illnesses[23].
However, we have to keep in mind that COVID-19 pneumonia is a heterogeneous disease. It may include tracheobronchitis, vascular injury, and capillary microthrombi, along with inflammation[23].Active injury leads to chronic and permanent lung trauma. The late effects of SARS-CoV-2 on the lungs have not been elucidated, but fibrosis development is suggestive.
Histologically, a hallmark of typical viral pneumonia is the interstitial nature of the inflammatory reaction. In addition, the most common morphologic changes in the lungs correlate with ARDS, which is described in the next section (Severe cases of COVID-19). On the other hand, no apparent morphological changes were observed in cardiac tissue due to the direct action of the coronavirus[17]. However,other findings established on necropsy material taken at autopsy are hypertrophy of cardiomyocytes with microscopic evidence of acute ischemia due to hypertensive heart disease and coronary artery atherosclerosis, but no other substantial damage[5].
Interestingly, we documented a case of sinus cavernosus thrombosis in a 49-year-old man hospitalized with COVID-19 pneumonia. One week after hospitalization, he lost vision in his right eye, and presented with exophthalmos and swelling of the soft tissues around the eye. CT with contrast enhancement was performed immediately after the onset of symptoms. Thrombosis of the right sinus cavernosus is presented in Figure 3A and B.
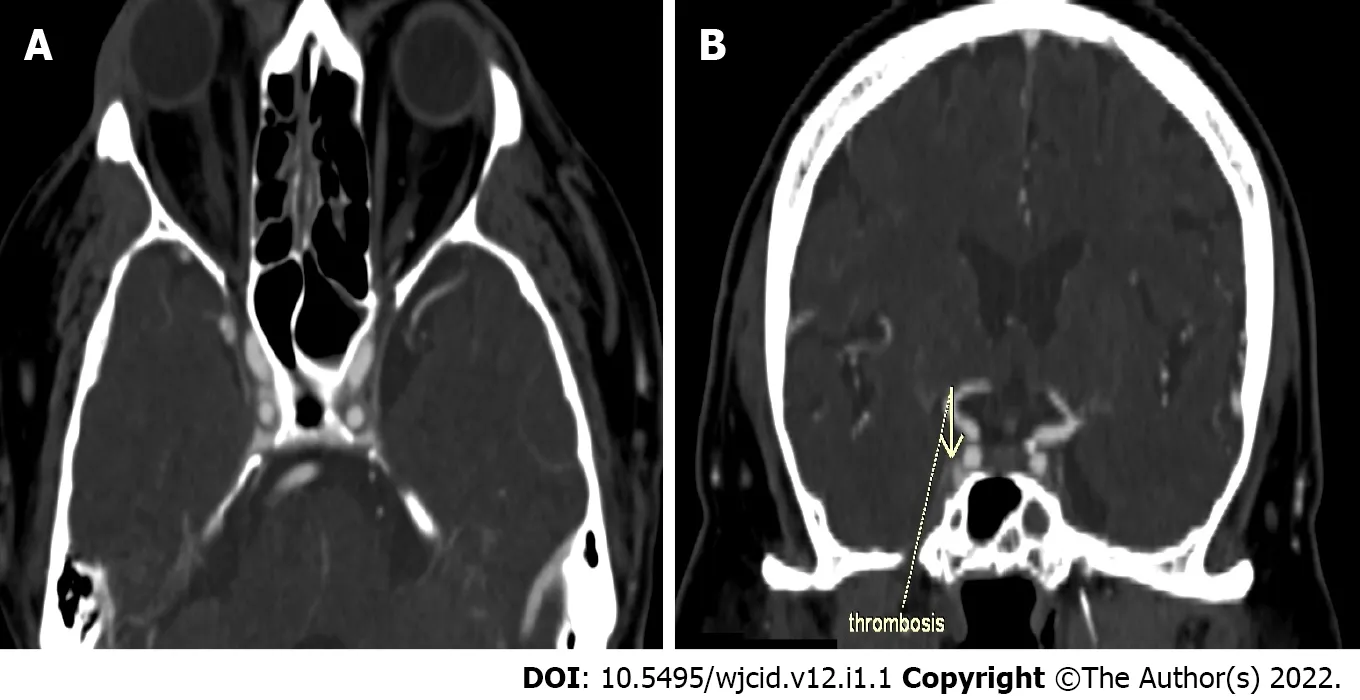
Figure 3 Computed tomography with contrast enhancement. A: Axial slice of thrombosis of the right sinus cavernosus; B: Coronal reconstruction of thrombosis of the right sinus cavernosus.
COVID-19 pneumonia is classified as atypical pneumonia because it overlaps with the radiographic findings seen in interstitial pneumonia, including other coronavirus infections (SARS, MERS)[24,25].Therefore, imaging methods are the first choice for diagnosing COVID-19 pneumonia including CXR and chest CT, with CT being a more sensitive and specific method than CXR, especially in the early stages of the disease[5].
Imaging findings on conventional radiography range from normal findings to diffuse changes in the lung parenchyma. Patients with multiple comorbidities are more likely to have bilateral and diffuse lesions. Findings considered as highly specific for COVID-19 pneumonia on CRX are GGO (Figure 4A)and areas of non-segmental consolidation of parenchyma (Figure 4B) with peripheral and caudal distribution[26]. These findings are most pronounced 10-12 d after the onset of symptoms[27]. Additional findings are confluent ill-defined patchy opacities (Figure 4C), an interstitial lung pattern, decreased lung attenuation, inhomogeneous and linear parenchymal opacities (Figure 4D), and often different features are combined.
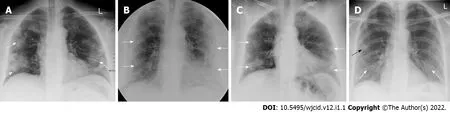
Figure 4 lmaging findings on conventional radiography range from normal findings to diffuse changes in the lung parenchyma. A:Posteroanterior (PA) chest X-ray 7 d after symptom onset. Predominantly peripheral ground glass opacities in both mid and lower zones of the lungs (white arrows);B: PA chest X-ray 11 d after symptom onset. Bilateral dense peripheral and basal air space consolidation (white arrows) more pronounced on the left and loss of lung markings in the mid and lower zones on the left; C: PA chest X-ray 3 d after symptom onset. Bilaterally, approximately proportionally in the middle and lower lung lobes, confluent ill-defined patchy opacities are visualized (white arrows). There is a relative preservation of the central areas of the lungs; D: PA chest X-ray 4 d after symptom onset. Bilaterally, predominantly in the right lower lobe, fine linear opacities (white arrows) are seen. Additionally, small patchy ground glass opacities (black arrow) are visualized in the peripheral region of right middle lobe.
On the one hand, for intensive care unit patients, CRX plays a crucial role in detecting disease progression and assessing the position of the individual resuscitation means - endotracheal tubes,drains, and venous sources as it is performed at the patient’s bedside with a mobile X-ray machine[26].
On the other hand, CT is a vital component in the diagnosis of suspected COVID-19 infection. CT features vary with the patient’s age, immunity status, disease stage, underlying diseases, and drug interventions at the time of scanning[28]. CT findings considered "typical" for COVID-19 pneumonia include GGO, parenchymal consolidations, and crazy-paving pattern (Figure 5)[29]. The changes are usually multifocal, bilateral with a peripheral subpleural distribution predominantly in the posterior segments of the inferior lobes[30,31].
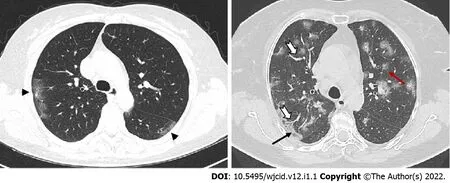
Figure 5 Axial computed tomography images show different patterns of ground glass opacities - round (red arrow), linear (arrowheads)and crazy paving (black arrow) with vascular enlargement (thick white arrow) within ground glass opacities areas.
A GGO is the most frequent and earliest finding defined as increased attenuation on CT, which does not obscure the bronchovascular structures. It can be observed in three typical patterns: rounded, linear and crazy paving[30] (Figure 6). In the area of GGO, widening of vessels and traction bronchiectasis are commonly seen[32,33]. GGO with reticular interstitial thickening, known as crazy-paving, is defined as thickening of the pulmonary interstitium with thickened interlobular septae and visualization of intralobular pulmonary septae[34]. Crazy paving may be seen with areas of GGO or consolidation in the subacute to chronic phase of the disease[35] (Figure 7).
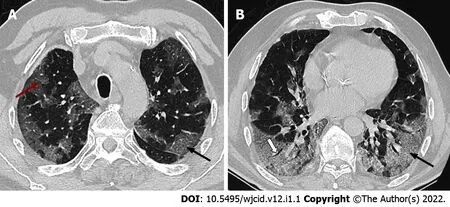
Figure 6 Axial computed tomography scans (7 days after symptom onset) show peripheral bilateral ground-glass opacities. A:Superimposed reticular interstitial thickening (red arrow) within the ground-glass opacities (GGO) giving a ‘crazy-paving’ appearance (black arrow); B: Air bronchogram (thick arrow) within the GGO.

Figure 7 Axial computed tomography images 11 days after symptom onset demonstrate bilateral confluent zones of ground-glass opacities and crazy paving (arrows) with subpleural, predominantly dorsal distribution and with superimposed areas of consolidation(arrowheads), more extensive in the right thoracic half.
By definition, consolidation is a homogenous increase in the lung parenchyma's attenuation with obscuration of the underlying vessels (Figure 8) and bronchi[34]. Therefore, it is considered a sign of disease progression, especially in the intermediate and late stages of the disease[30,34].
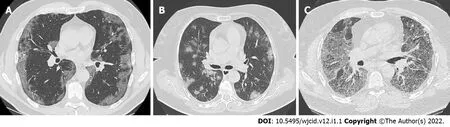
Figure 8 Axial computed tomography images of patients with different time of symptoms onset showing various types of lung abnormalities distribution. A: Subpleural with involvement of peripheral one third of lungs; B: Random with central and subpleural location; C: Diffuse with confluence of changes and continuous involvement.
The distribution of lung abnormalities was recorded as predominantly subpleural (involving mainly the peripheral one-third of the lung), random (without predilection for subpleural or central regions), or diffuse (continuous involvement without respect to lung segments)[33] (Figure 5). Additional CT features of COVID-19 pneumonia include vascular dilation, bronchial wall thickening, and traction bronchiectasis within the areas of GGO, architectural distortion with reticular thickening and subpleural bands formation, halo sign, or reversed halo sign (Figure 9). Changes considered atypical for COVID-19 pneumonia include lobar or segmental consolidations, small nodules (centrilobular lung nodules and tree-in-bud opacities), pulmonary cavitation, lymphadenopathy, and the presence of pleural or pericardial effusions[34].
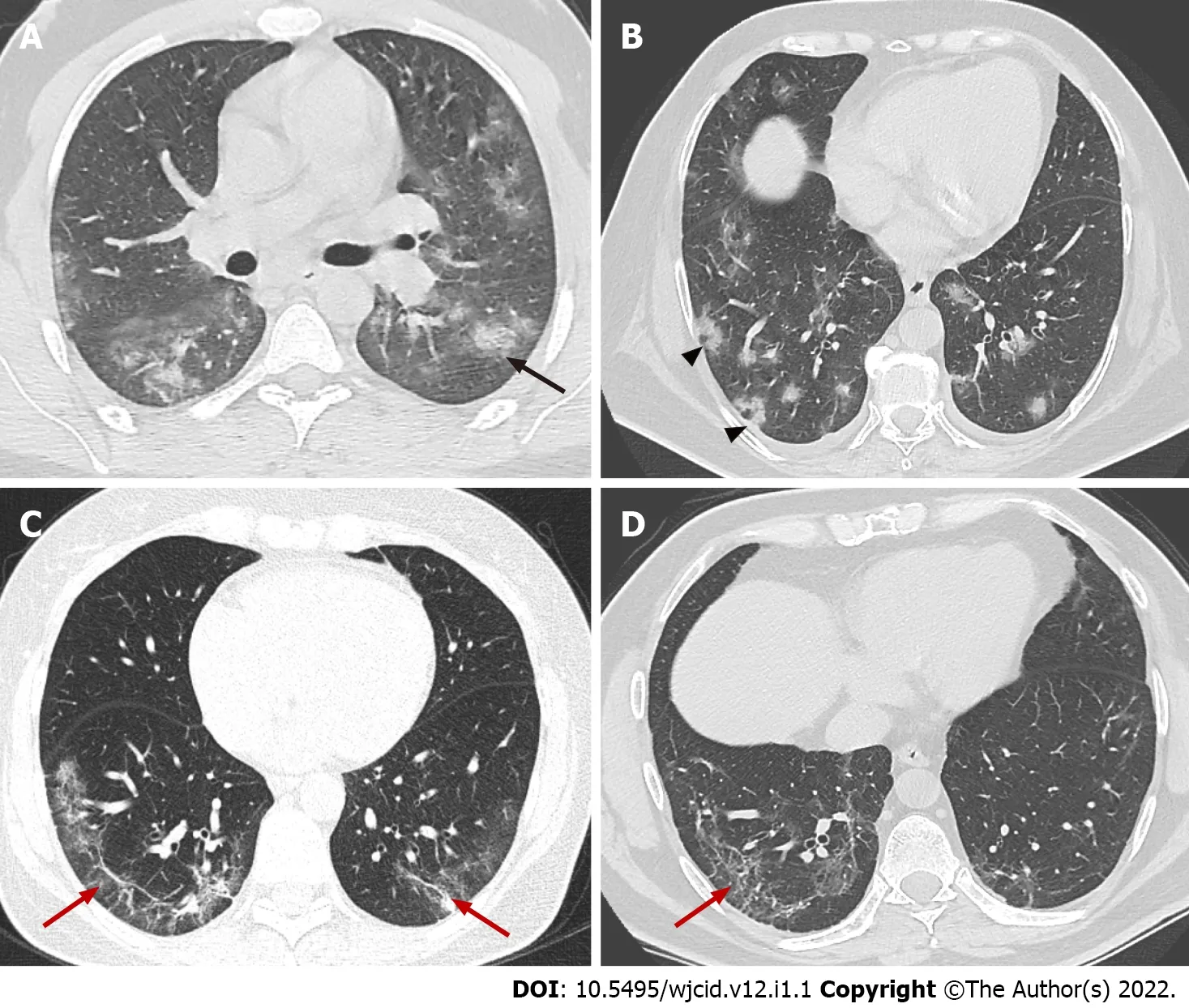
Figure 9 Axial computed tomography images demonstrate features seen in organizing pneumonia. A: Ground-glass opacities (GGO) surrounding small area of consolidation, “halo sign” (black arrow); B: Central GGO surrounded by denser consolidation of crescentic shape, “reverse halo sign” (arrowheads); C and D: Architectural distortion with interstitial thickening and irregular fibrous bands (red arrows).
CT findings also vary depending on the onset of clinical symptoms. Approximately four stages of COVID-19 on chest CT have been described: (1) Early stage (0-5 d after onset of symptoms) marked by either normal or predominantly GGO (Figure 10); (2) Progressive stage (5-8 d after onset of symptoms)marked by enhanced GGO and a crazy-paving look (Figure 11A and B); (3) Peak stage (9-13 d after onset of symptoms) characterized by progressive consolidation (Figure 11C and D); and (4) Late stage (≥14 d after the onset of symptoms) denoted by a gradual decrease in consolidation and GGO, although signs of fibrosis (including parenchymal bands, architectural distortion, and traction bronchiectasis)may occur[31,36-39] (Figure 12). It should also be remembered that the temporal development and degree of lung defects are heterogeneous among different individuals, depending on the disease severity[31,36]. Similar findings can be observed in other viral types of pneumonia, pneumonia caused by Mycoplasma or Chlamydia, vasculitis, and connective tissue disease. Therefore, the clinicallaboratory correlation with the radiological finding is essential for the diagnosis of COVID-19 pneumonia.
CT scanning can be a useful tool in evaluating the individual disease burden[40]. The quantitative severity can be assessed using a visual method or software that determines the percentage of affected lung volumes using deep learning algorithms[41,42]. Furthermore, the severity of lung involvement on CT correlates with the severity of the disease. It can be measured by scoring the percentages of each of the five lobes that are involved and can range from 0 (no involvement) to 25 (maximum involvement)when all five lobes show more than 75% involvement[41] (Figure 13).
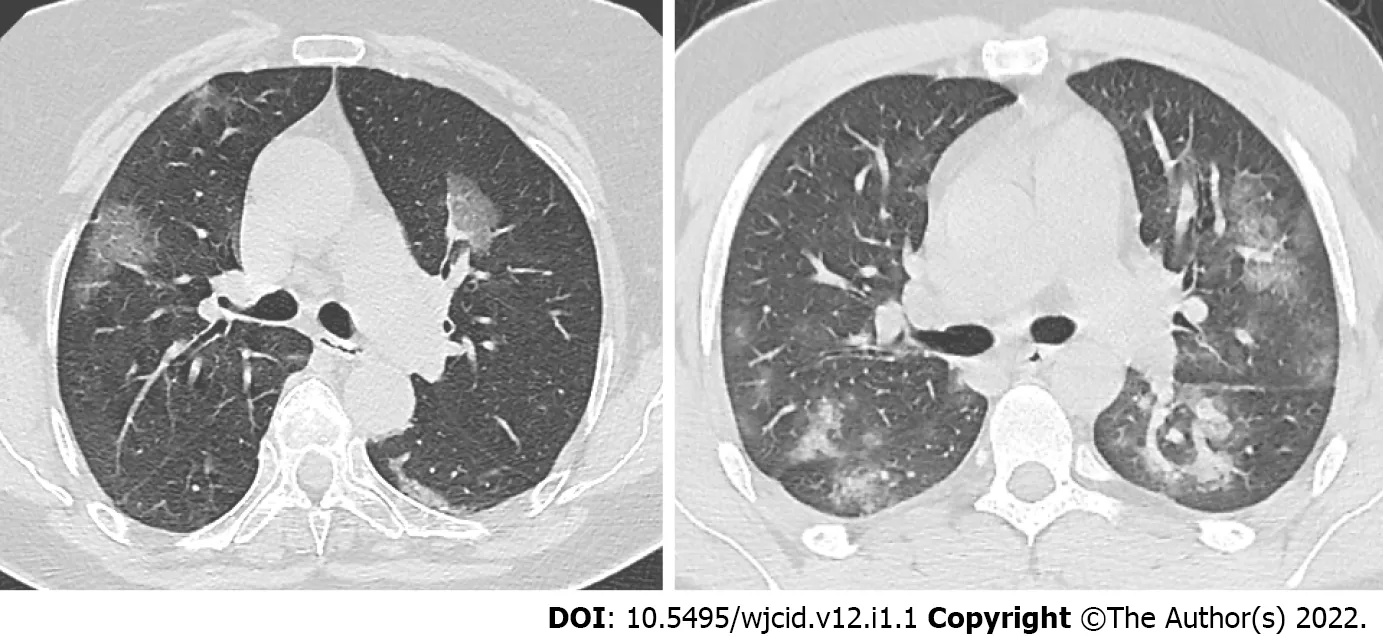
Figure 10 Axial computed tomography images in two different patients 5 days after onset of symptoms show various degree of groundglass opacities abnormalities (early stage of COVlD-19).
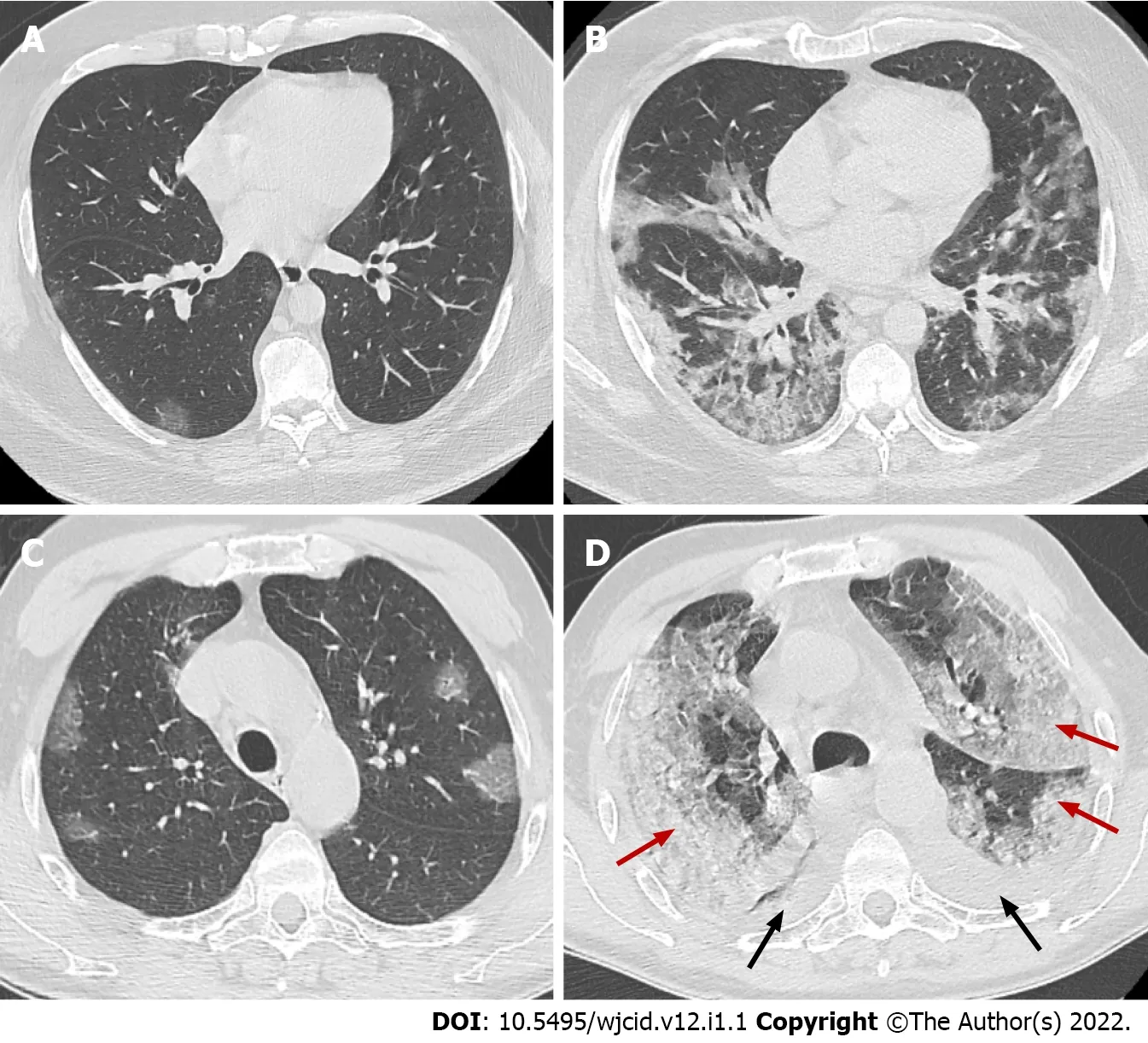
Figure 11 Axial computed tomography images. Axial computed tomography images on day 2 (A) and day 10 (B) from onset of symptoms of patient with clinical worsening demonstrate increased ground-glass opacities with confluence, crazy-paving appearance and typical peripheral subpleural location, early (A) and progressive (B) stage. Axial images from CT on day 5 (C) and day 15 (D) from onset of symptoms of patient with pronounced clinical worsening demonstrate increased ground-glass opacities with confluence, crazy-paving appearance and extensive areas of consolidation (red arrows) with predominant posterior and lateral distribution. In addition, bilateral pleural effusions (black arrows) are noted, early (C) and peak (D) stage.
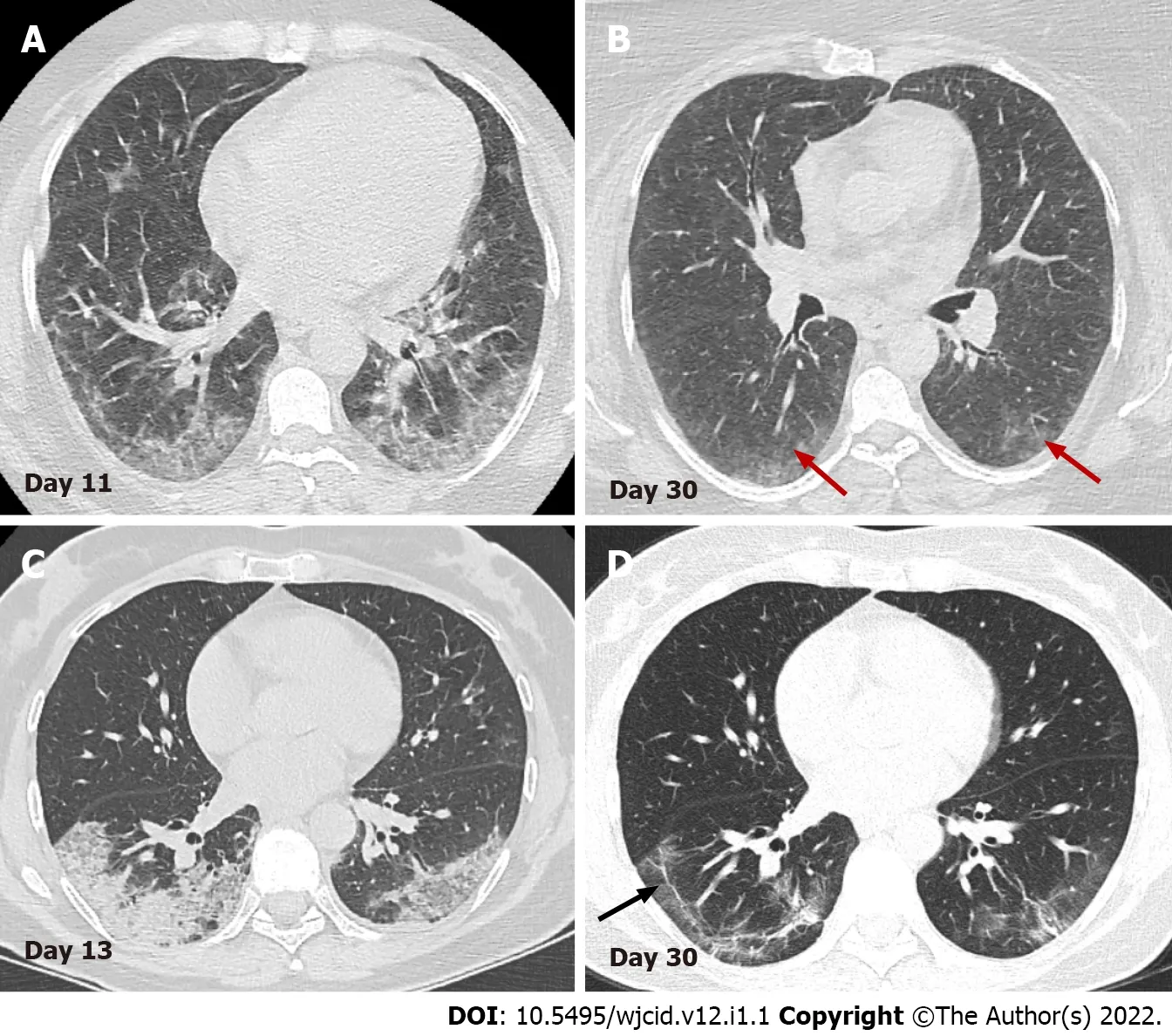
Figure 12 Serial axial computed tomography images. Serial axial computed tomography images in two different patients show gradual decrease of GGO and consolidations in the late stage of disease (B) and (D). In patient 1 (A) and (B) advanced resorption of the abnormalities is seen with very low density GGO at the sight of previous lung changes (red arrows). In patient 2 (C) and (D) architectural distortion with fibrous bands parallel to the pleura (black arrow) and traction bronchiectasis are visualized (arrowheads).
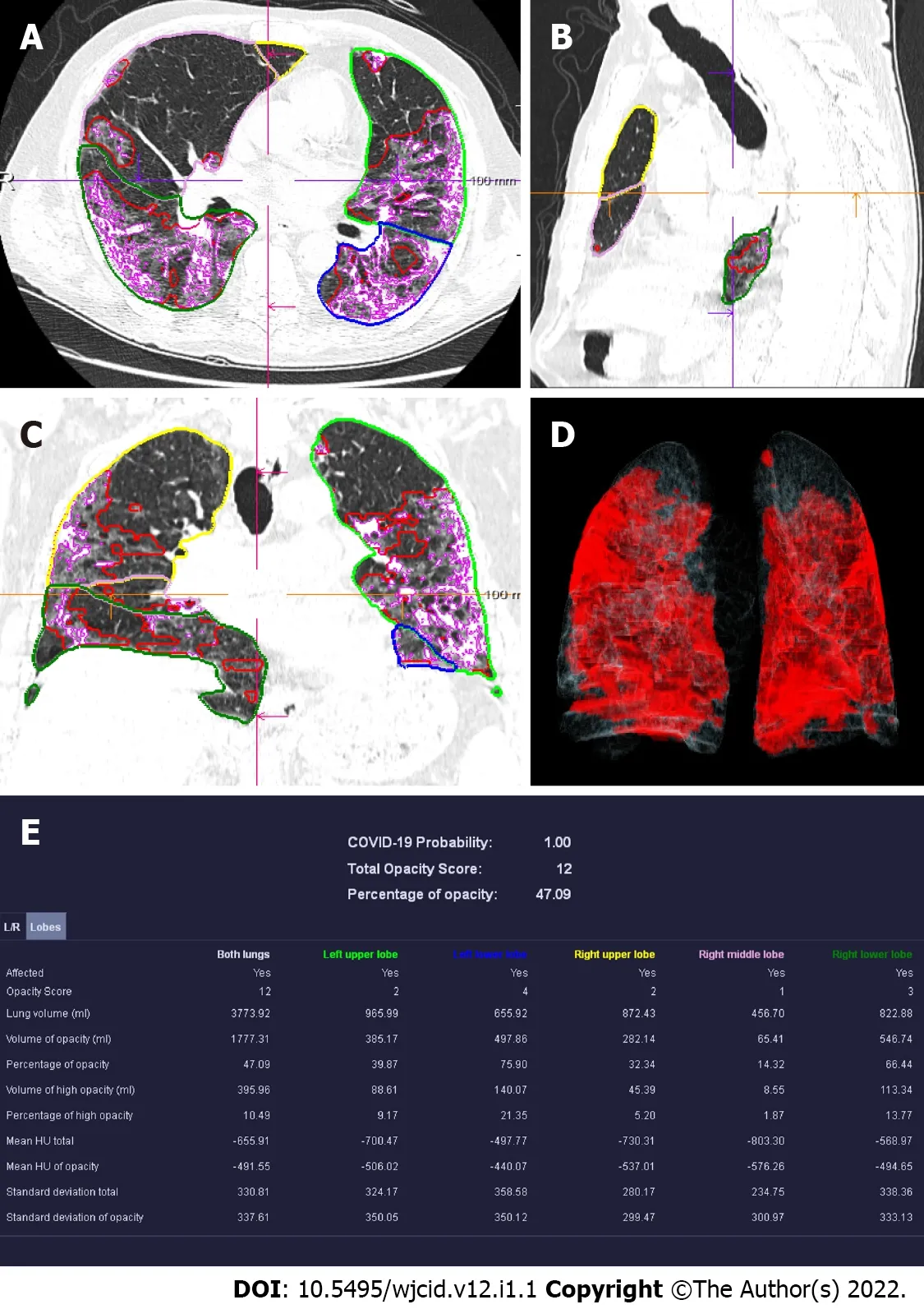
Figure 13 Computed tomography pneumonia analysis (automated lung opacity analysis). A-C: Multi-planar reconstruction views overlaid with delineations of the opacities and the lungs; D: Volume rendering image showing a quick overview of the spatial distribution of the opacities; E: Table with measurements demonstrating relative (“percentage of opacities”) and absolute volume of opacities, mean and standard deviation of HU values between lung parenchyma and the detected opacities, separately segmented quantitative results per left and right lung and per lung lobe.
Lung ultrasound is increasingly used as a complementary method in diagnosing COVID-19 pneumonia. It allows assessment of both the lung parenchyma and the pleural space with the ability of bedside and real-time assessment of pulmonary function and changes. In addition, US can easily distinguish normally aerated from pathologically altered lung parenchyma. The most common findings observed in interstitial pneumonia are irregularly thickened pleura, B-lines, and subpleural consolidations of the parenchyma[43,44].
B-lines are vertical hyperechoic artifacts arising from the pleura that resemble a comet tail(Figure 14A) and move with the lung sliding. The increase in subpleural lung density (in the absence of consolidated tissue) may lead to the coalescence of many vertical artifacts in more extended echogenic patterns and a single homogeneous subpleural echogenic area can be seen. This phenomenon is known as “white lung”[44] (Figure 14B). In addition to B-lines, small, oval subpleural consolidations are visible in the parenchyma (Figure 14C). With disease progression to ARDS, the number of B-lines and parenchymal consolidations increases with the formation of sizeable gravitational consolidation[44],and the characteristic lung sliding is no longer visible. In the recovery phase of the disease, the consolidations and B-lines gradually disappear. Instead, A-line artifacts found in normally aerated parenchyma reappear and lung sliding is improved.
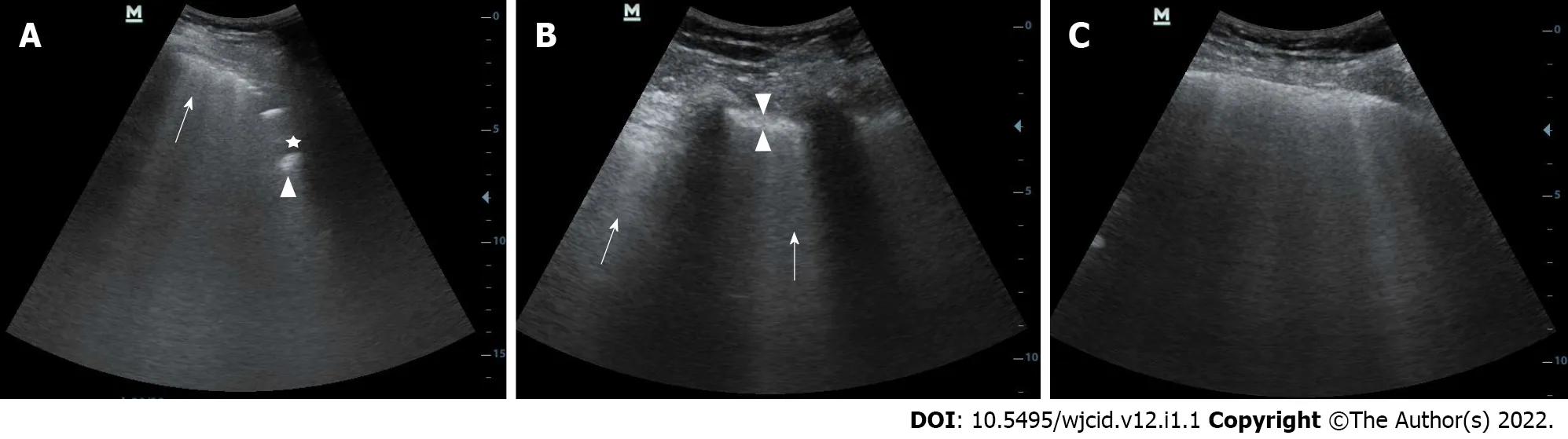
Figure 14 Lung ultrasound image. A: Lung ultrasound image obtained with convex probe shows multiple B-lines (arrow) and subpleural consolidations seen as hypoechoic regions (asterix) that appear tissue-like with an irregular deep border (shredded fractal line) abutting the more aerated lung, which has echogenic artefacts (arrowhead); B: Lung ultrasound image obtained with convex probe. Multiple linear vertical artefacts - B-lines (arrows), and thickened pleural line (between arrowheads) are visualized; C: Lung ultrasound image obtained with convex probe. Multiple confluent B-lines are seen (white lung).
SEVERE CASES OF COVlD-19
ARDS/cytokine storm
In some cases, COVID-19 pneumonia proceeds to the typical complications such as ARDS and cytokine storm. ARDS remains the most frequent immunopathological complication in SARS-CoV-2, SARS-CoV,and MERS-CoV viruses. The cytokine storm is one of the critical pathways for ARDS[45]. Both ARDS and cytokine storm represent an unregulated autoimmune inflammatory response arising from the release of significant quantities of pro-inflammatory cytokines (such as IFNg, IFNa, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-5-007, TGFβ,etc.) and chemokines (C-C Motif Chemokine Ligand 2 (CCL2), CCL3, CCL5,C-X-C motif ligand 8 (CXCL8), CXCL9, CXCL10,etc.) from immune or viral-infected cells[46]. Therefore,it is essential that these factors be detected and evaluated early. Thus, the appropriate therapy can be administered.
Histological examination of lung specimens in patients with severe infection is characterized by severe and extensive diffuse alveolar damage, fibromyxoid exudates, interstitial and intra-alveolar edema. However, in one of the cases described in the literature, the respiratory bronchial mucosa was intact, without evidence of squamous metaplasia. This is a significant difference compared with the pathology observed in the first epidemic of SARS[48]. In such patients, it is often found denuded with necrosis of type I pneumocytes and the formation of hyaline membrane and the proliferation of type II pneumocytes, which indicate ARDS in the acute stage without evidence of interstitial organization[17,21]. In addition, there is diffuse thickening of alveolar walls due to congestion, and patchy to mild mononuclear inflammatory infiltrate comprised of lymphocytes, macrophages, and some plasma cells.Perivascular lymphocyte aggregates have also been identified[47]. No eosinophils or neutrophils were identified, but if these are found, they usually suggest secondary bacterial infection[21,47]. Occasionally,in proliferated alveolar epithelial cells, viral inclusions in intranuclear and/or intracytoplasmic and multinucleated syncytial cells with atypical enlarged pneumocytes can be seen characterized by large eosinophilic nuclei with prominent nucleoli and granular cytoplasm (probably representing a viral effect similar to that reported in the first SARS epidemic)[17,47-49]. Common in ARDS, pulmonary thrombi and microangiopathy may be noted within a few small pulmonary artery branches and pleural adhesions[21,47]. This thrombotic process may involve activation of megakaryocytes, probably those that are naturally found in the lung, with platelet aggregation and platelet formation, in addition to fibrin deposition. The formation of small vascular thrombi in the lungs is often accompanied by focal alveolar hemorrhage[47,49].
With regard to imaging, COVID-19 related ARDS is diagnosed when a patient with confirmed COVID-19 infection meets the Berlin 2012 ARDS diagnostic criteria: acute hypoxemic respiratory failure;presentation within one week of worsening respiratory symptoms; bilateral airspace disease on CRX,CT, or US that is not fully explained by effusions, lung collapse, or nodules; and cardiac failure is not the primary cause of acute hypoxemic respiratory failure[50,51]. The risk factors for patients with COVID-19 to develop ARDS in the course of the disease are older age, concomitant conditions - most often hypertension and diabetes, and specific clinical symptoms on initial presentation such as marked dyspnea and fever ≥ 39°C[52]. However, clinical manifestations may be relatively mild regarding the severity of imaging findings in COVID-19[53].
Imaging studies have an essential role in the initial evaluation of the pattern and extent of lung involvement and in the follow-up of hospitalized patients. Chest radiographic findings of ARDS are non-specific and resemble those of typical pulmonary edema or pulmonary hemorrhage with diffuse,patchy or homogeneous, bilateral, and coalescent opacities[54] (Figures 15 and 16). Radiography in the first 24 h of deterioration may be unchanged[55]. CT imaging features depend on the phase of the disease[56]. In the early exudative stage (first week), CT scans usually display a non-homogeneous distribution and ventrodorsal gradient of density. More dense consolidations are observed in dependent regions, extensive GGO, and comparatively regular or hyper-inflated parenchyma (in the case of mechanical ventilation) in non-dependent areas[54] (Figure 17).
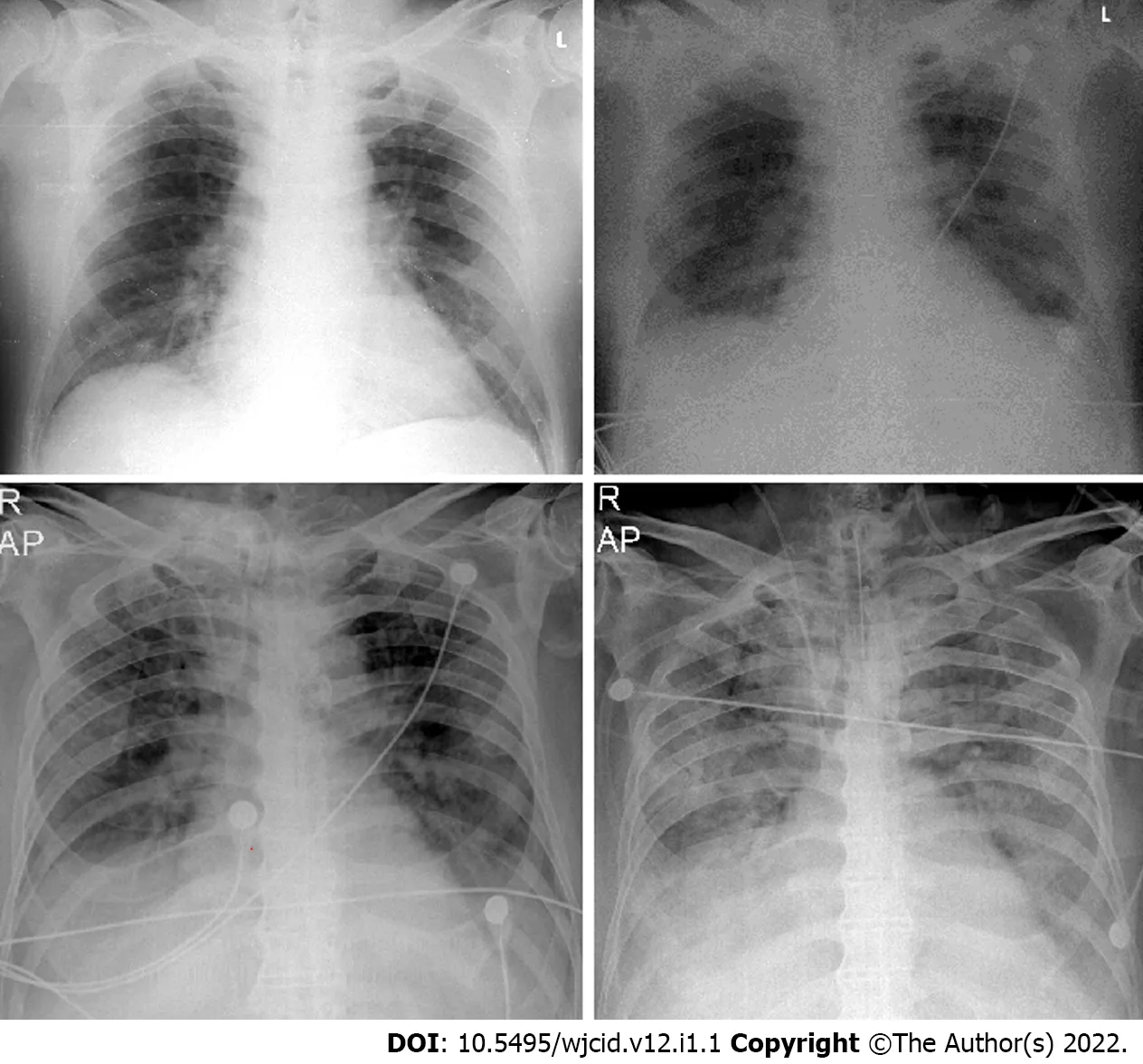
Figure 15 Serial chest X-ray imaging of coronavirus disease-2019 infected 65-year-old man with rapid respiratory deterioration after symptom onset showing progression from lower lung predominant interstitial and airspace opacities on day 1 and day 3 to diffuse and worsening involvement with extensive airspace disease on days 4 and 6.
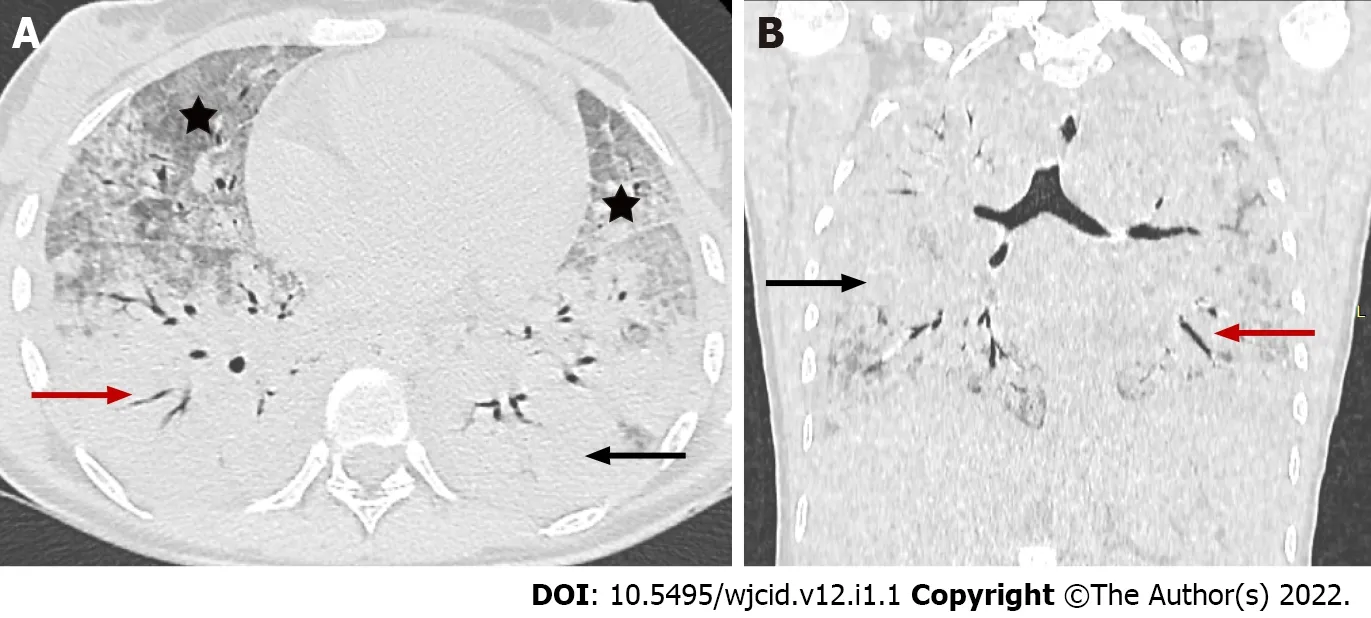
Figure 16 Coronavirus disease-2019 related acute respiratory distress syndrome, early stage. Axial (A) and coronal (B) computed tomography images demonstrate widespread ground glass opacities (asterix) and large areas of consolidation (black arrow) with air bronchogram (red arrow) showing anteroposterior density gradient in both lungs.
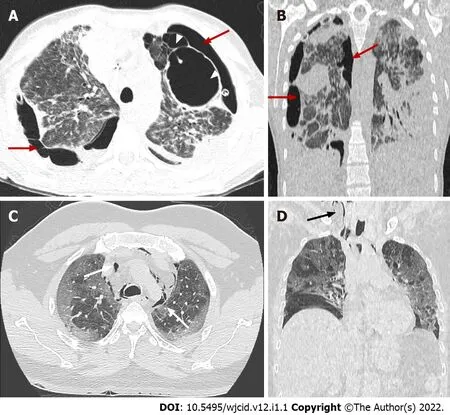
Figure 17 Coronavirus disease-2019 related acute respiratory distress syndrome, late stage. Axial (A) and coronal computed tomography (CT) (B)images demonstrate typical complications in mechanically ventilated patients with subpleural bullae formation (arrowheads) and bilateral pneumothorax (red arrows).Axial (C) and coronal (D) CT images in different patient showing spontaneous pneumomediastinum (white arrows), extending into the neck as subcutaneous emphysema (black arrow).
In the late fibrotic phase (over two weeks), CT appearance can be variable. Complete resolution may occur in some cases, but the coarse reticular pattern and ground-glass opacification in the anterior (nondependent) part of the lungs are considered more typical CT features. Pulmonary cysts of varying sizes and bullae have also been reported, which probably developed due to prolonged ventilation[56].Subsequent imaging studies can show the development of pneumomediastinum, pneumothorax (often hypertensive in mechanically ventilated patients), and subcutaneous emphysema[54] (Figure 18).
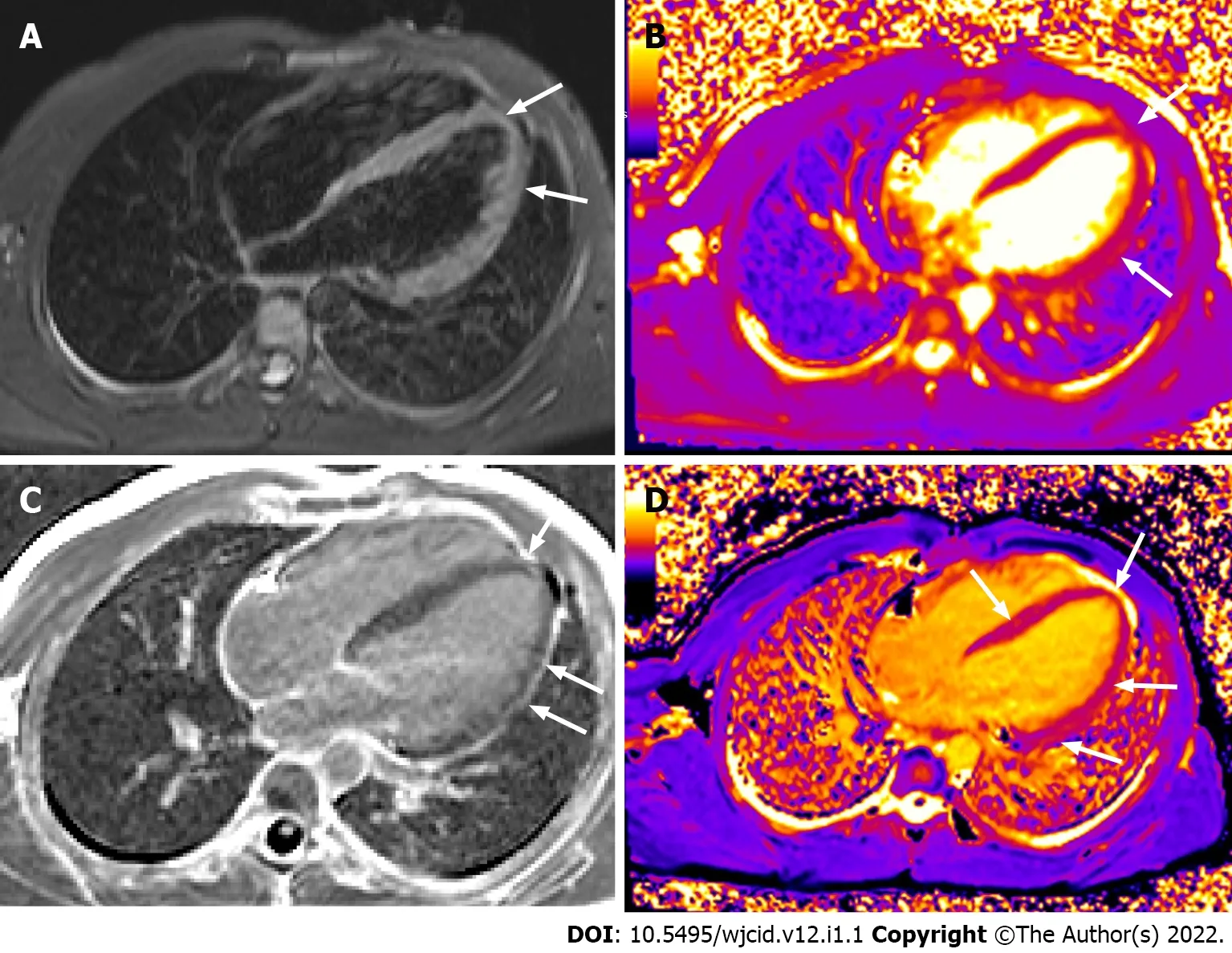
Figure 18 Cardiac magnetic resonance imaging. Horizontal long axis TIRM image (A) displaying high signal in the myocardium at the apex and along the free wall of the left ventricle (LV). Horizontal long axis T2 (B) and T1 (D) mapping depicting prolonged T2 and T1 relaxation times at the same area and in the middle septum (D). Horizontal long axis image post gadolinium administration (C) showing late enhancement at the apex and along the free wall of LV. There is also pericardial enhancement along the free wall of LV.
Multiorgan failure
The cytokine storm causes ARDS and multiorgan failure, leading to death in severe cases of coronavirus infections[45]. Although COVID-19 is known to cause pulmonary disease, including pneumonia and ARDS, various extrapulmonary manifestations of COVID-19 have been reported, affecting the gastrointestinal tract, brain, heart, kidneys, or muscles. Therefore, imaging helps to estimate the presence of complications and the extend of COVID-19.
Histologically, there is no noticeable viral cytopathic effect on the heart in the case of moderate infection on light microscopy[21,47]. However, liver biopsy specimens in patients with COVID-19 showed moderate fatty degeneration of hepatocytes and mild lobular and portal activity with slight interstitial mononuclear inflammatory infiltrates, indicating that the injury that could have been caused by either COVID-19 infection or drug-induced liver injury.
The pathogenesis of kidney injury due to COVID-19 is not well-described. However, it seems to be multifactorial, involving mechanisms related to systemic hypoxia, coagulation disorders, inflammatory changes, or even cell destruction due to viruses. According to the literature, renal impairment was related to multiple organ failure[57]. Furthermore, post-mortem examination of the kidney from a patient who died of COVID-19 infection demonstrated that viral antigens accumulated in the renal tubules, inducing acute kidney injury[57].
Histological examination confirmed that a diffuse proximal tubular lesion, loss of brush border, nonisometric vacuolar degeneration, and a small area of necrosis were observed. In addition, interstitial inflammation and hemorrhage were found in some of the published cases, probably due to secondary bacterial infection[58]. However, most of these findings are caused by comorbidities. The renal changes described above may be directly due to COVID-19 infection. These pathological observations may only provide a basis for further study and investigation of COVID-19[58].
The main focus of COVID-19 is respiratory system complications, which are also a leading factor determining the severity of the disease course. More and more is known about the pathophysiology of the disease and the mechanism of lung damage. However, the extrapulmonary manifestations of the disease remain unclear, which in some cases is decisive for the disease course[59]. Cardiovascular complications are receiving increasing attention, and according to various studies range from 30 to 78%[60-62]. Cardiovascular disease (CVD) is known to be one of the main risk factors for severe disease. A study of 44672 patients with COVID-19 infection in China showed that a history of concomitant CVD was associated with nearly five-fold higher mortality (10.5%vs2.3%) than patients without a history of CVD[63]. In addition, increasing evidence suggests that the virus may directly affect the cardiovascular system and lead to complications such as myocarditis, acute coronary syndrome, arrhythmias, and venous thromboembolism[64].
Cardiomagnetic resonance imaging (CMR) represents the gold standard in assessing the structure and function of the heart and provides information on the tissue characteristics of the myocardium.That, together with its non-invasive nature and lack of ionizing radiation, makes it essential for both early diagnosis and monitoring of cardiac complications due to COVID-19.
The pathogenesis of cardiac damage is currently unclear. However, presumed mechanisms include direct viral invasion, cytokine-mediated injury, a mismatch between needs and oxygen supply, and ischemic impairment resulting from microvascular thrombosis[65,66].
The CMR findings in the acute phase of the disease reported so far are based on small groups of patients. The presence of myocardial edema is a characteristic feature observed in almost all reported cases[67-70]. In a review of 31 publications with 51 patients, only one patient did not have edema at baseline, and two patients developed reversible edema within two weeks[68].
The extent of edema varies - from diffuse to limited to separate segments of the myocardium - most often in the LV inferior wall regions, the mid inferoseptal regions, and the apical region[68]. Another characteristic CMR sign is late gadolinium enhancement (LGE) of the myocardium due to necrosis/fibrosis following nonischemic myocardial injury localized at the sub-epicardial and/or intramural regions in the non-coronary territory. The presence and extent of zones of LGE correlate with the prognosis[68]. An additional feature in the acute phase of the disease is diffuse hypokinesis of the left and/or right ventricle and, much less frequently, segmental hypokinesis[68].
Similar CMR findings - edema, necrosis/fibrosis, and impaired systolic function are also found in patients following COVID-19 infection[62,67,69-71]. Signs of persistent inflammation with prolonged T2 relaxation time due to edema, prolonged T1 relaxation time and LGE with a different distribution, and pericardial enhancement are seen. In some cases, reduced ejection fraction[62] is found.
Coagulopathy and thrombotic accidents
The cytokine storm is one of the leading causes of disseminated intravascular coagulation (DIC)affecting the entire organism. Pro-inflammatory cytokines, such as TNFa and IL-1, are able to inhibit endogenic anticoagulation. Inflammation injures the endothelium and results in activation of tissue plasminogen activator, which may explain the rise in D-dimer and fibrin degradation products[72,73].In summary, COVID-19 is associated with a hypercoagulable disease and an elevated risk of thromboembolic complications.
The histological findings in organs other than lungs do not indicate significant changes directly related to COVID-19. However, the most commonly observed histological changes were due to thrombotic microangiopathy involving the lungs, spleen, and kidney. The most common complications which have been described in the literature include acute limb ischemia, aortic and mesenteric thrombosis, myocardial and brain infarction, and DIC[74].
A retrospective study on CTPA reported that pulmonary embolism in COVID-19 patients appears to be primarily distributed in the segmental arteries of the right lung[75]. Furthermore, only the determination of D-dimer and IL-6 at admission to CT scan appears to differentiate patients with pulmonary embolism from patients with a negative CT pulmonary angiogram. However, inter-individual heterogeneity calls for the establishment of cut-off values in COVID-19 patients in future research[76].
The pre-pandemic understanding of the predictive value of perfusion scintigraphy in assessing chronic thromboembolic disease and chronic thromboembolic pulmonary hypertension is wellestablished. Dhawanet al[75] proposed perfusion imaging as a triage tool for post-COVID-19 recovery.They suggested the potential of perfusion imaging to examine thein situthrombotic small vessel signature of COVID-19. It is widely accepted thatin situthrombotic microbial pulmonary hypertension is present. Thus, lung perfusion imaging provides a primary triage instrument within the broader panel of investigations to enhance understanding of the natural history of thromboembolic phenomena in COVID-19 and distinguish hemodynamic sequelae from deconditioning dysfunctional breathing-related functional limitations. The authors also suggest that such imaging should be incorporated in routine post-COVID-19 follow-up pathways[75].
CONCLUSlON
Early observed changes in critical organs such as lungs, kidneys, blood vessels,etc., might benefit the timely and appropriate treatment of COVID-19 to alleviate the complications and avoid fatal outcomes.Along with typical clinical and imaging results, atypical results are also beneficial. Early detection of pathological changes at different stages of life-threatening COVID-19 can improve patient management,treatment, and outcome.
FOOTNOTES
Author contributions:Boyapati A, Chervenkov L, Gulinac M, and Genova K performed the literature review;Chervenkov L, Ilieva E, Gulinac M, and Borisov Y collected and prepared the images from their institutes’ database;Genova K, Ilieva E, and Chervenkov L provided the text and explanations of the images; Ilieva E and Velikova T wrote the draft; all the authors wrote additional sections in the paper according to their specialty; Velikova T revised the final draft; all authors revised and approved the final version of the article.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Bulgaria
ORClD number:Elena Ilieva 0000-0003-2016-8591; Alexandra Boyapati 0000-0003-1003-4127; Lyubomir Chervenkov 0000-0002-8380-5992; Milena Gulinac 0000-0001-7970-9378; Jordan Borisov 0000-0001-5842-6096; Kamelia Genova 0000-0002-3168-819X; Tsvetelina Velikova 0000-0002-0593-1272.
S-Editor:Liu JH
L-Editor:Webster JR
P-Editor:Liu JH
