Preoperative lymphocyte to C-reactive protein ratio as a new prognostic indicator in patients with resectable gallbladder cancer
2022-06-02WenYnYoXingSongWuShiLeiLiuZiYouWuPingDongWeiGong
Wen-Yn Yo , b , # , Xing-Song Wu , b , # , Shi-Lei Liu , b , Zi-You Wu , b , Ping Dong , b ,Wei Gong , b , *
a Department of General Surgery, Xinhua Hospital, Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 20 0 092, China
b Shanghai Key Laboratory of Biliary Tract Disease Research, Shanghai 20 0 092, China
Keywords:Gallbladder neoplasms Inflammation Lymphocyte to C-reactive protein ratio Overall survival
ABSTRACT
Introduction
Gallbladder cancer (GBC) is the most common malignancy of the biliary tract [1-7] . The incidence of GBC is approximately 3.82 per 100 000 persons in China [1] . The prognosis of GBC is extremely poor because of its late diagnosis and highly invasive nature and the lack of effective therapies [ 2 , 3 , 6 ]. The reported 5-year survival of GBC is generally lower than 10% [ 3 , 4 ]. The only possible way to cure this disease is resection [ 3 , 8 ]. The assessment of reliable biomarkers for predicting postoperative outcomes in clinical trials could greatly improve the prognosis of GBC patients. With these useful markers, we can identify patients with a poor prognosis preoperatively, evaluate the feasibility of surgery and select optimal treatment regimens for high risk patients.
Growing evidence has shown that systemic inflammation is closely related to oncogenesis and tumor development in multiple types of malignancies [9-11] . Based on these findings, several inflammatory factors, such as C-reactive protein (CRP), neutrophils, lymphocytes, platelets and albumin [12-16] , were found to be closely related to the prognosis of different types of cancer. Further studies [ 2 , 17-22 ] showed that the combination ratios of these inflammatory factors [e.g., CRP to albumin ratio (CAR), lymphocyte to C-reactive protein ratio (LCR), neutrophil to lymphocyte ratio(NLR), and platelet to lymphocyte ratio (PLR)] yielded more robust results than the single factors. The preoperative LCR is a new prognostic marker in colorectal cancer patients [19] , but the correlation between preoperative LCR and GBC is unknown. This study aimed to investigate the prognostic value of the preoperative LCR in patients with resectable GBC.
Patients and methods
Patients
In total, 104 patients who underwent curative resections for the treatment of GBC between January 20 0 0 and December 2016 at Xinhua Hospital, Affiliated to Shanghai Jiao Tong University School of Medicine were enrolled. All patients met the following criteria: (i) GBC diagnosis was confirmed by pathology and (ii) no neoadjuvant chemotherapy or radiotherapy was administered before surgery. The exclusion criteria were as follows: (i) the presence of other tumors; (ii) no laboratory data within one week before surgery; (iii) infection before surgery, including cholecystitis and cholangitis; (iv) other hematological disease; and (v) the use of palliative resection. This study was approved by the Ethics Committee of the Institutional Review Board of Xinhua Hospital, Affiliated to Shanghai Jiao Tong University School of Medicine (XHECD-2019-122). Written informed consent was obtained from all participants.
Data collection
Preoperative laboratory measurements were obtained within one week prior to the surgical resection of the gallbladder, including the levels of CRP, white blood cells, neutrophils, lymphocytes, platelets, albumin, hemoglobin, carbohydrate antigen 19-9(CA19-9, cutoff value 39 U/mL), carcinoembryonic antigen (CEA,cutoff value 5.2 ng/mL), and carbohydrate antigen 125 (CA125, cutoff value 35 U/mL). The LCR was calculated as follows: lymphocyte count (number/μL)/CRP (mg/L). Margin status was defined as follows: R0 resection: complete resection with histologically assessed clear margins; R1 resection: there was a microscopic residual tumor involving at least one margin; R2 resection: there was macroscopic residual disease [8] . The tumor stage was confirmed by the 8th edition of the American Joint Committee on Cancer Manual [23] .
Clinical treatment and patient follow-up
For T1a tumors, patients were treated with simple cholecystectomy. For T1b-T4 tumors, patients were treated with radical cholecystectomy, which consisted ofen-blocresection of adjacent liver parenchyma and regional lymph nodes. Combined common bile duct or other organ resections were performed if these surrounding structures were infiltrated. For incidental GBC, T1b-T4 stage tumors needed radical resection after the first operation. For these patients, the laboratory measurements mentioned above were collected before the first operation.
The patients were followed up via telephone interviews every 3 months after surgery. The first day of the follow-up started on the day of surgery. Patients who survived until the last follow-up (June 2020) were considered censored data.
Statistical analysis
Categorical variables are presented as frequency and percentage. Non-normally distributed variables are presented as median(interquartile range, IQR). Overall survival (OS) was defined as the time from the day of operation to death or last follow-up. A timedependent receiver operating characteristic (ROC) curve for OS was constructed to evaluate the predictive value of different markers,and the area under the ROC curve (AUC) was used to compare the accuracy. The optimal cutoff value was determined by the formula“surv_cutpoint {survminer}” in R, which is an outcome-oriented method providing a value of a cutpoint that corresponds to themost significant relation with outcome, using the maximally selected rank statistics from the ‘maxstat’ R package. Differences between non-normally distributed variables were determined by the Mann-Whitney test or Kruskal-Wallis test. The Kaplan-Meier method and the log-rank test were used to generate and assess survival curves. Univariate and multivariate Cox proportional hazard models were used to define factors associated with overall survival. AllPvalues were two-sided, and differences withPvalues less than 0.05 were considered statistically significant. Statistical analyses were performed by SPSS version 22.0 (IBM Corp., Armonk, NY, USA), while the time-dependent ROC curve analysis and optimal cutoff value analysis were performed by R version 3.6.1(www.r-project.org).
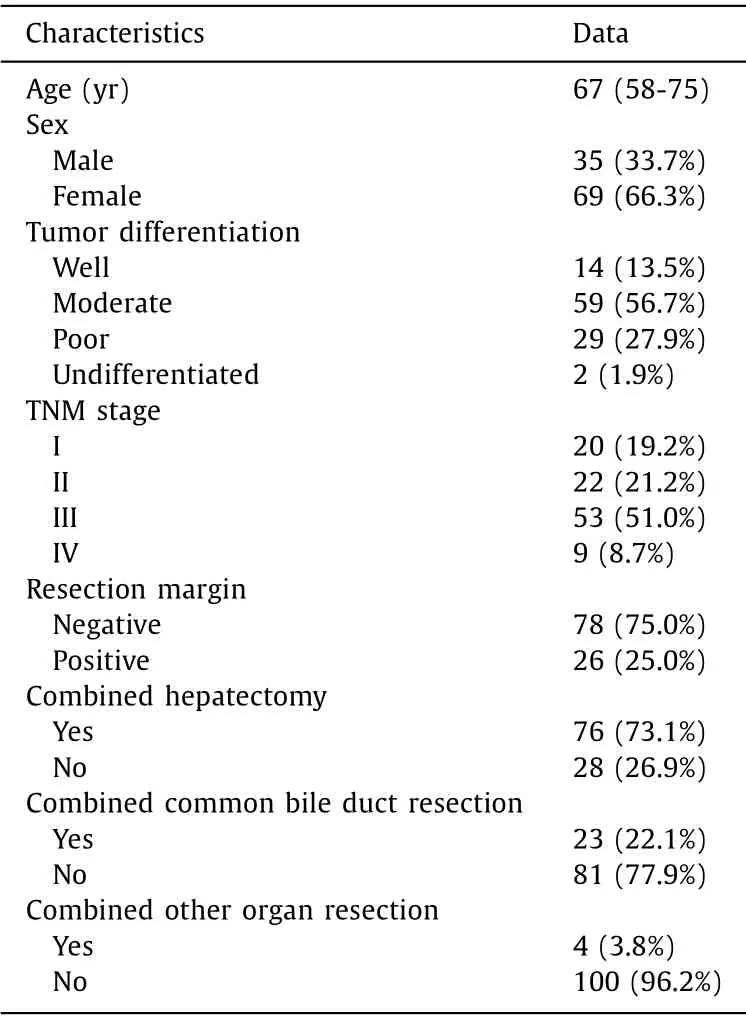
Table 1 Demographic and clinical characteristics of patients with gallbladder cancer undergoing resection ( n = 104).
Results
Patient characteristics
The study group included 35 men (33.7%) and 69 women(66.3%), with a median age of 67 years (IQR 58-75). Seventy-three(70.2%) patients had well or moderately differentiated cancer, and 31 (29.8%) had poorly or undifferentiated cancer. Of these patients,20 (19.2%) had stage I disease, 22 (21.2%) had stage II disease,53 (51.0%) had stage III disease and 9 (8.7%) had stage IV disease according to the TNM classification (8th edition) of the American Joint Committee on Cancer. Seventy-six (73.1%) patients received combined hepatectomy, 23 (22.1%) received combined common bile duct resection, and only 4 (3.8%) received extensive resection. The majority of patients had a negative resection margin(78, 75.0%), and 26 (25.0%) had positive margins ( Table 1 ). At the time of the last follow-up (June 2020), 11 (10.6%) patients were still alive. The median survival time was 16 months.
Preoperative LCR had higher accuracy in predicting the OS of GBC patients
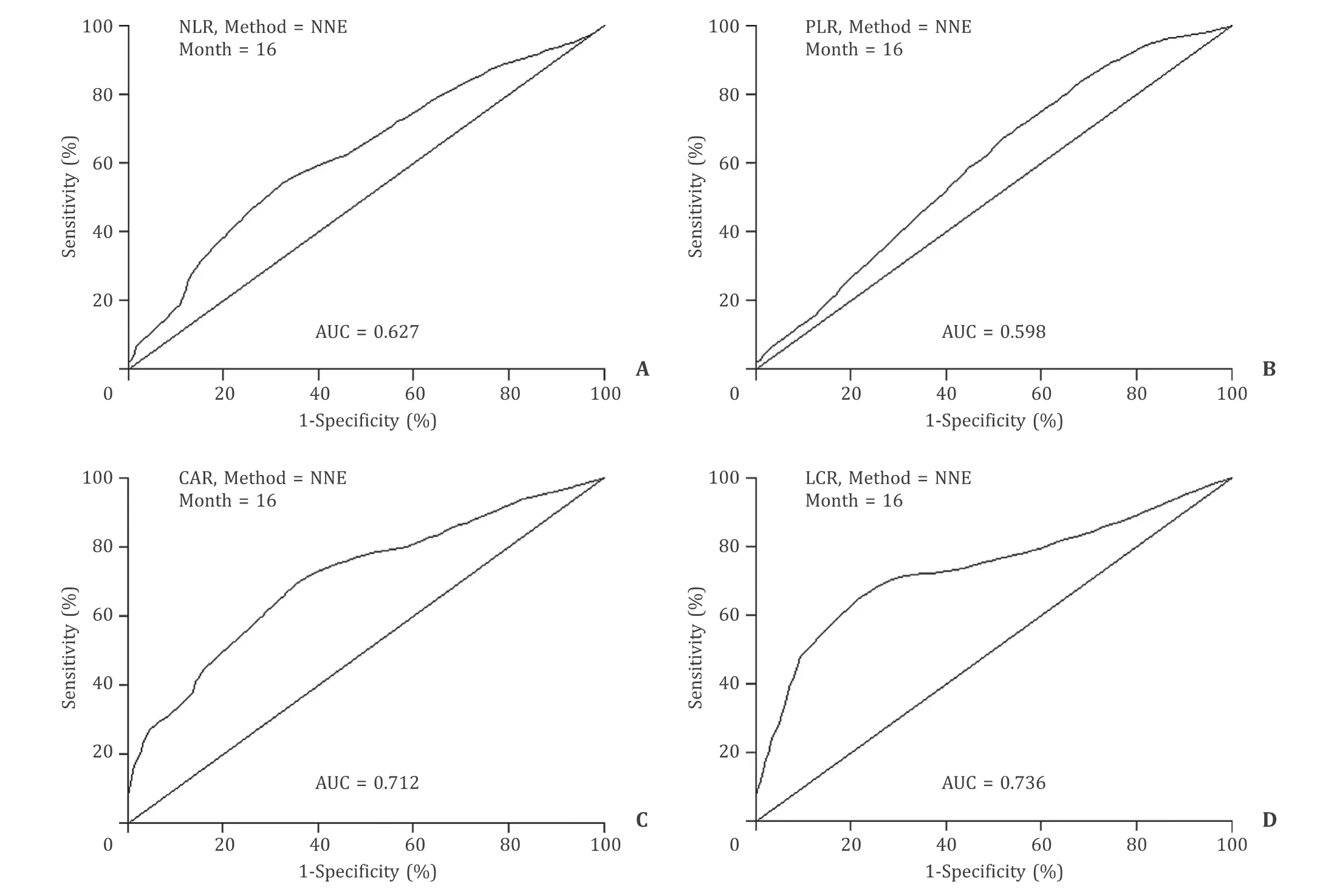
Fig. 1. Time-dependent receiver operating characteristic (ROC) curve analysis was used to evaluate the value of each combination for predicting overall survival (OS). The preoperative lymphocyte to CRP ratio (LCR) showed the highest accuracy for predicting the OS of GBC patients compared with other combinations of inflammatory factors,including neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and CRP to albumin ratio (CAR).
According to previous reports, five key inflammation-related factors (CRP, neutrophils, platelets, lymphocytes and albumin) and four combinations of these factors (LCR, NLR, PLR and CAR) were analyzed in this study. A time-dependent ROC curve for OS was constructed to estimate the prediction accuracy of these parameters. Compared with other combinations of inflammatory factors(the AUC of the NLR was 0.627, the AUC of the PLR was 0.598, and the AUC of the CAR was 0.712), the preoperative LCR showed the highest accuracy in predicting the OS of GBC patients (the AUC of the LCR was 0.736) ( Fig. 1 ).
Decreased preoperative LCR was related to advanced tumor stage
We compared the differences in the preoperative LCR in different GBC groups, and the results were shown in Table 2 . Significant differences in the preoperative LCR could be seen in patients stratified by pathological T category, lymph node metastasis and TNM stage, which were regarded as tumor progression markers. A lower preoperative LCR correlated with tumor invasion (P= 0.018), the presence of lymph node metastasis (P= 0.011) and advanced TNM stage (P= 0.022). These findings indicated that a low preoperative LCR was associated with an advanced tumor stage in GBC patients.
A low preoperative LCR was associated with a poor prognosis in GBC patients
To evaluate the prognostic value of the preoperative LCR in GBC patients, we generated Kaplan-Meier survival curves ( Fig. 2 ).The optimal cutoff value for the preoperative LCR was 145.5. It is obvious that patients with a low LCR (LCR ≤145.5) had muchlower OS than patients with a high LCR (LCR>145.5) (P<0.001).Then, we conducted univariate analyses, and the following variables were significantly correlated with OS: a low LCR (P<0.001)and increased platelet count (P= 0.005), CA19-9 (P= 0.002), CEA(P= 0.006), and CA125 (P<0.001). In addition, poor tumor differentiation (P<0.001), advanced pathological T category (P<0.001), the presence of lymph node metastasis (P<0.001), positive resection margin (P<0.001) and combined common bile duct resection (P= 0.013) were also associated with reduced OS in the univariate analyses. Based on this finding, we selected the risk factors described above for multivariate Cox regression analysis of survival. As shown in Table 3 , a low LCR (P<0.001) appeared to be an independent prognostic factor for OS in GBC patients, as did a high CA125 level (P= 0.019) and the presence of lymph node metastasis (P= 0.004). Therefore, the preoperative LCR was a significant marker for predicting the prognosis of GBC patients.
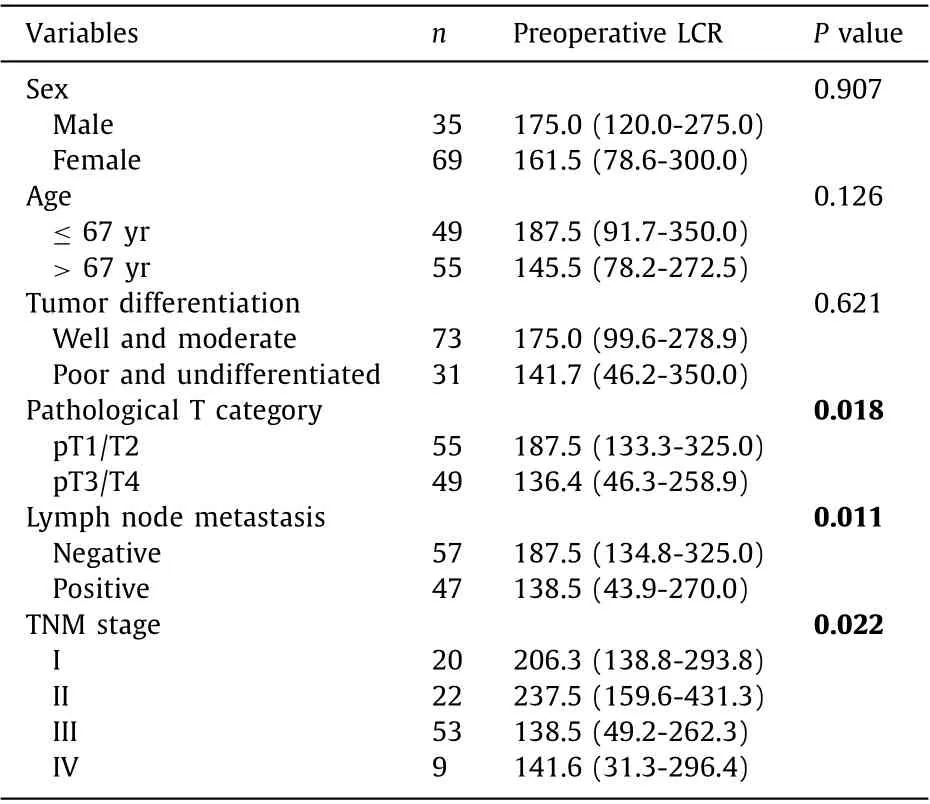
Table 2 Levels of preoperative LCR in different gallbladder cancer groups.
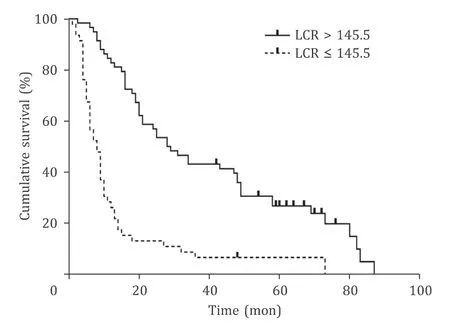
Fig. 2. Prognostic impact of LCR status on the OS of GBC patients. Patients with a low LCR (LCR ≤145.5) had much lower OS than patients with a high LCR (LCR >145.5) ( P < 0.001, log-rank test). LCR: lymphocyte to C-reactive protein ratio; OS:overall survival; GBC: gallbladder cancer.
Discussion
Systemic inflammation has been considered to be closely related to oncogenesis and tumor development over the last four decades, and accumulating studies [9-16] have demonstrated that various systemic inflammatory factors using peripheral blood examination play a reliable role in predicting the prognosis of human malignancies. These markers include neutrophils, lymphocytes, platelets, CRP, albumin and their combinations [12-22] . However, it remains unknown which is the best parameter for evaluating the postoperative outcomes in patients with resectable GBC. In this study, we evaluated the prognostic value of the preoperative LCR in patients with resectable GBC. Our results showed that the preoperative LCR had the highest accuracy in predicting OS compared with other markers. A lower preoperative LCR was significantly correlated with a more advanced GBC stage, represented by tumor invasion, the presence of lymph node metastasis and advanced TNM stage. Moreover, the preoperative LCR with our optimal cutoff threshold (LCR = 145.5) was an independent prognostic factor for OS in GBC patients undergoing curative surgery.
The mechanism by which preoperative LCR is associated with the prognosis of cancer patients remains unclear, but the relationship between cancer and inflammation prompts some possible hypotheses. Peripheral lymphocytes are considered playing a critical role in the antitumor immune response, and a decrease in lymphocytes may result in tumor progression [24] . A previous study showed that low preoperative lymphocyte alone was an independent predictor of a poor prognosis in resected pancreatic ductal adenocarcinoma [25] . On the other hand, serum CRP is the most representative marker of inflammation in clinical settings, and high levels of CRP indicate an enhanced inflammatory environment,which could promote tumor development and angiogenesis, induce DNA damage, and favor neoplastic spread and metastasis [26] .Growing evidences show that a high level of preoperative CRP is associated with a poor prognosis for adult solid tumors [ 15 , 27 , 28 ].Based on these considerations, the LCR could reflect both the immune status and inflammatory conditions of tumor patients, where lower levels of LCR represent a decline in the antitumor immune response and an enhancement of the inflammatory environment.
In our study, the preoperative LCR level appeared to be an independent risk factor for OS in GBC patients. Patients with a lower preoperative LCR had a poorer OS after surgery, which was consistent with a previous study [19] . Considering this evidence, we could regard the preoperative LCR as a new and practical prognostic indicator in GBC patients during the perioperative period.
Compared with TNM stage, our new measurement using preoperative LCR is more convenient and feasible; moreover, it can be evaluated before surgery, which could help us identify high risk populations early and decide appropriate therapeutic schedules for GBC patients. For patients with lower LCRs, we should carefully assess the benefit patients can obtain from surgery and weigh the pros and cons. Gene detection is also recommended after surgery for the identification of targets for additional treatments such as targeted therapies or immunotherapies. As mentioned above, a decreased preoperative LCR level may reflect a weak antitumor immune response and a strong inflammatory environment, and immunotherapies may achieve good efficacy in patients with a lower LCR. Further investigations are needed to validate this hypothesis.Additionally, multivariate analyses for OS in GBC patients suggest that a high level of CA125 (>35 U/mL) and the presence of lymph node metastasis were also independent risk factors for poor OS.CA125 is considered an important prognostic factor in ovarian cancer [ 29 , 30 ], and it is also reported to be an independent prognostic factor in some gastrointestinal cancers [ 31 , 32 ]; thus, the combination of these measurements may help us make a more reliable and accurate decision.
Our study is the first to investigate the relationship between the preoperative LCR and postoperative outcomes in GBC patients. Inevitably, there are some limitations we acknowledge for this study.Firstly, we only focused on five inflammatory factors, and not all the inflammatory factors were covered. Secondly, although the preoperative LCR showed excellent prognostic value, the reliability and accuracy of this measurement still require further validation.Thirdly, the patients enrolled were solely from one institution, and the sample size was relatively small. To overcome these hurdles,larger and prospective multicenter studies including broader inflammatory factor-based analyses and diverse ethnic populations may be needed to validate the prognostic value of the preoperative LCR for the identification of GBC patients with a high risk of poor postoperative outcomes.
In conclusion, our study identified the LCR as a new prognostic indicator in patients with resectable GBC. A low preoperative LCR has a strong association with advanced tumor stage and poor OS. Quantification of the preoperative LCR will help clinicians determine more appropriate perioperative strategies in GBC patients.
Acknowledgments
None.
CRediT authorship contribution statement
Wen-Yan Yao: Conceptualization, Data curation, Formal analysis, Investigation, Writing - original draft. Xiang-Song Wu: Conceptualization, Data curation, Formal analysis, Funding acquisition,Writing - original draft. Shi-Lei Liu: Data curation, Investigation,Writing - original draft. Zi-You Wu: Data curation, Formal analysis,Writing - original draft. Ping Dong: Conceptualization, Resources,asome data are missing. OS: overall survival; HR: hazard ratio; WBC: white blood cell count; CA19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; CA125:carbohydrate antigen 125; LCR: lymphocyte to C-reactive protein ratio.Writing - review & editing. Wei Gong: Conceptualization, Funding acquisition, Resources, Supervision, Writing - review & editing.
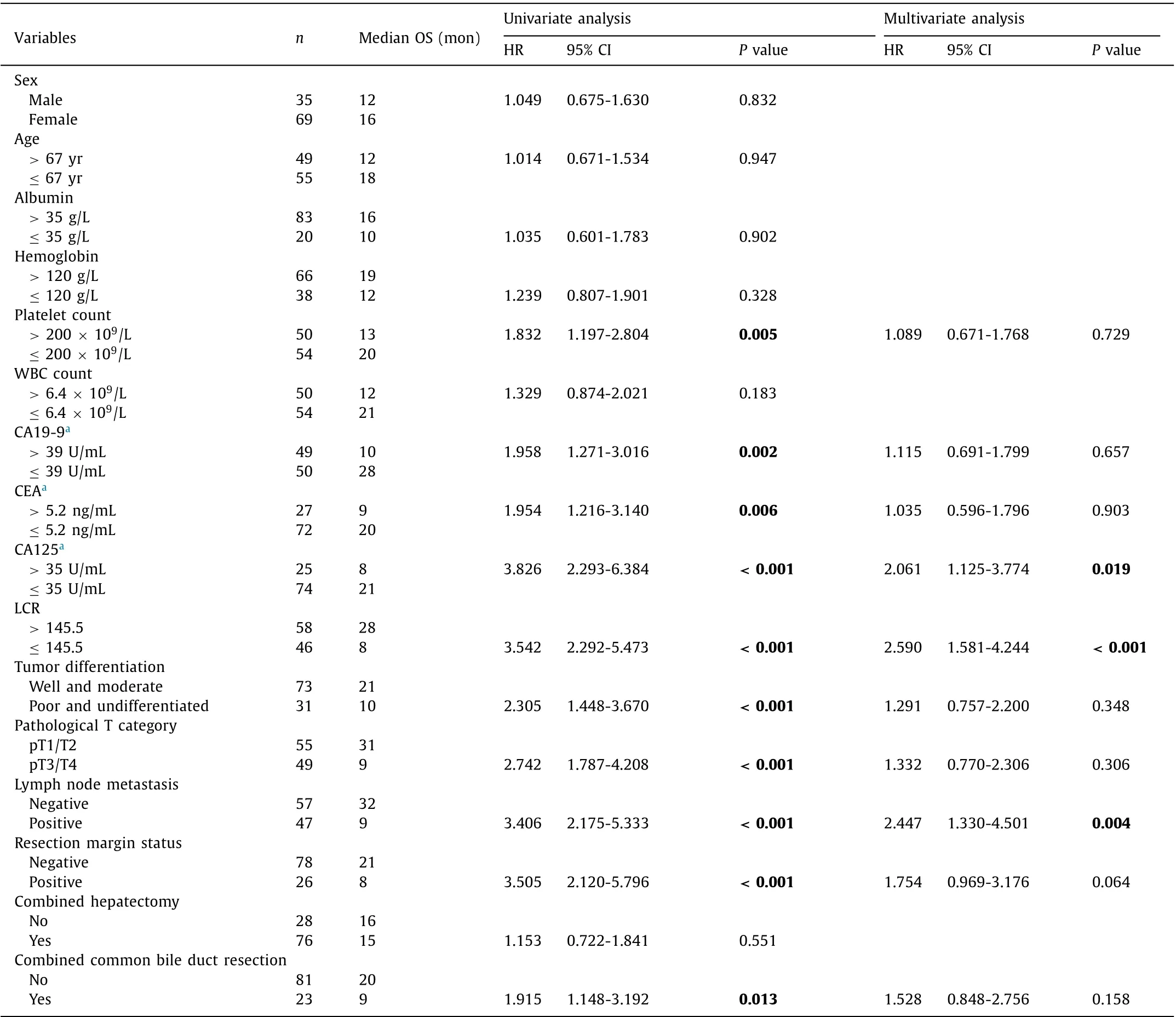
Table 3 Univariate and multivariate analysis for predictors of overall survival.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81974371), the Emerging Frontier Program of Hospital Development Center (SHDC12018107),the General Surgery Construction Program of Shanghai Municipal Health Commission (2017ZZ02011), the Project of Excellent Young Scholars from Shanghai Municipal Health and Family Planning Commission (2018YQ10), the Talent Development Fund from Shanghai Municipal Human Resources and Social Security Bureau(2018048), the Experiment Animal Program of Shanghai Science and Technology Committee Innovative (19140902700), Research Team of High-level Local Universities in Shanghai and Shanghai Key Laboratory of Biliary Tract Disease Research Foundation(17DZ2260200), the National Science and Technology Major Project(2019ZX09301-158).
Ethical approval
This study was approved by the Ethics Committee of the Institutional Review Board of Xinhua Hospital, Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-D-2019-122). Written informed consent was obtained from all participants.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Surgical exploration with non-resection in the setting of resectable,borderline and locally advanced pancreatic cancer
- Risk factors for post-endoscopic retrograde cholangiopancreatography(ERCP) abdominal pain in patients without post-ERCP pancreatitis
- On-table hepatopancreatobiliary surgical consults for difficult cholecystectomies: A 7-year audit
- Prognostic potential of the small GTPase Ran and its methylation in hepatocellular carcinoma
- Relief of jaundice in malignant biliary obstruction: When should we consider endoscopic ultrasonography-guided hepaticogastrostomy as an option?
- Hepatobiliary&Pancreatic Diseases International
